Notch proteins are a family of transmembrane receptors that coordinate binary cell fate decisions and terminal differentiation. This study demonstrates that biosynthesis of the cell surface-associated mucin MUC16 is posttranscriptionally regulated by Notch signaling at early stages of epithelial cell differentiation, suggesting that Notch signaling plays an important role in maintaining a wet-surface phenotype at the ocular surface.
Abstract
Purpose.
Notch proteins are a family of transmembrane receptors that coordinate binary cell fate decisions and differentiation in wet-surfaced epithelia. We sought to determine whether Notch signaling contributes to maintaining mucosal homeostasis by modulating the biosynthesis of cell surface-associated mucins in an in vitro model of human corneal (HCLE) and conjunctival (HCjE) epithelial cell differentiation.
Methods.
HCLE and HCjE cells were grown at different stages of differentiation, representing nondifferentiated (preconfluent and confluent) and differentiated (stratified) epithelial cultures. Notch signaling was blocked with the γ-secretase inhibitor dibenzazepine (DBZ). The presence of Notch intracellular domains (Notch1 to Notch3) and mucin protein (MUC1, -4, -16) was evaluated by electrophoresis and Western blot analysis. Mucin gene expression was determined by TaqMan real-time polymerase chain reaction.
Results.
Here we demonstrate that Notch3 is highly expressed in undifferentiated and differentiated HCLE and HCjE cells, and that Notch1 and Notch2 biosynthesis is enhanced by induction of differentiation with serum-containing media. Inhibition of Notch signaling with DBZ impaired MUC16 biosynthesis in a concentration-dependent manner in undifferentiated cells at both preconfluent and confluent stages, but not in postmitotic stratified cells. In contrast to protein levels, the amount of MUC16 transcripts were not significantly reduced after DBZ treatment, suggesting that Notch regulates MUC16 posttranscriptionally. Immunoblots of DBZ-treated epithelial cells grown at different stages of differentiation revealed no differences in the levels of MUC1 and MUC4.
Conclusions.
These results indicate that MUC16 biosynthesis is posttranscriptionally regulated by Notch signaling at early stages of epithelial cell differentiation, and suggest that Notch activation contributes to maintaining a mucosal phenotype at the ocular surface.
Notch proteins are a family of 4 single-pass transmembrane receptors (Notch1 to Notch4) involved in cell fate decisions and terminal differentiation in multicellular organisms. Notch-mediated intracellular signaling is triggered from direct cell-to-cell contact after binding of Notch to transmembrane ligands (Delta and Jagged) on adjacent cells.1 This binding elicits a γ-secretase proteolytic event leading to the release of a Notch intracellular domain fragment that translocates to the nucleus and activates transcription factors important to cell differentiation and morphogenesis.
On mucosal surfaces, Notch signaling controls cell differentiation in a tissue-specific manner. Inhibition of Notch signaling in the mouse small intestine by the γ-secretase inhibitor dibenzazepine (DBZ) or conditional removal of the Notch pathway transcriptional factor CSL/RBP-J results in a rapid conversion of proliferative crypt cells into postmitotic goblet cells.2 On the other hand, inactivation of Notch1 in mice using a tissue-specific, inducible, gene-targeting approach results in extensive hyperplasia and keratinization of corneal epithelium.3 The presence of Notch1 and Notch2, and their ligands Delta1 and Jagged1, has been demonstrated in human corneal epithelium, where they contribute to the regulation of cell proliferation and cytokeratin expression.4 Notch1 to Notch3, and their ligands Delta1 and Jagged1 are present in human conjunctival epithelium,5 but their contribution to cell differentiation remains unclear.
Mucins are a group of high molecular weight glycoproteins implicated in maintaining a wet-surface phenotype on mucosal surfaces due to their hydrophilic character.6,7 The stratified ocular surface epithelia express at least 3 cell surface-associated mucins, MUC1, MUC4, and MUC16.8 Studies on the regulation of mucins in human cells have been facilitated by the development of in vitro systems such as telomerase-immortalized corneal and conjunctival epithelial cell lines.9 Using these cell lines, it has become clear that individual cell surface-associated mucins are independently regulated during cell differentiation.10,11 We hypothesize that Notch signaling plays an important role in maintaining a wet-surface phenotype by regulating mucin biosynthesis. The purpose of this study was to investigate the effect of Notch signaling on the biosynthesis of cell surface-associated mucins in human corneal (HCLE) and conjunctival (HCjE) epithelial cells during cell differentiation and stratification.
Materials and Methods
Cell Culture
Telomerase-immortalized human corneal-limbal (HCLE) and conjunctival (HCjE) epithelial cells were plated at a seeding density of 5 × 104 cells/cm2 on six-well plates (Costar Corning, Inc., Corning, NY) and maintained at 37°C in 5% CO2. Derivatization and mucin profile of HCLE and HCjE cell cultures, as well as their distinct patterns of cytokeratin expression, have been previously reported.9 HCLE and HCjE cultures were grown in a medium optimized for proliferation of keratinocytes (keratinocyte serum-free medium; Gibco-Invitrogen Corp.; Carlesbad, CA) to achieve confluence. After reaching confluence, cells were switched to stratification medium containing DMEM/F12 (Gibco-Invitrogen Corp.) with 1 mM CaCl2 and 10 ng/mL EGF (Hyclone, Logan, UT) and 10% calf serum (Gibco-Invitrogen Corp.) for 7 days to promote stratification and optimal biosynthesis of cell surface-associated mucins.9,10
Inhibition of Notch Signaling
Cell cultures at different stages of growth (preconfluent, confluent, and stratified) were treated with 10 μM and 25 μM of the γ-secretase inhibitor DBZ (Calbiochem; Darmstadt, Germany) in DMSO, or with DMSO alone as control. DBZ is a potent inhibitor of the Notch signaling pathway that abrogates proteolytic processing of the intracellular domain, both in cell culture and in vivo.2,12,13 Mucin analyses were performed at 4, 24, and 48 hours after addition of DBZ.
Electrophoresis and Western Blot Analysis
Protein from cell cultures was extracted using RIPA buffer (150 μM NaCl, 50 μM Tris, pH 8.0, 1% NP 40, 0.5% deoxycholate, 0.1% SDS) plus a protease inhibitor (cOmplete Protease Inhibitor Cocktail; Roche Biochemical; Indianapolis, IN). Protein concentration was determined using a commercially available assay kit (Pierce BCA Protein Assay Kit; Thermo Scientific; Rockford, IL). Notch receptors were resolved under reducing conditions on 4% stacking and 7.5% separating SDS-PAGE gels, followed by transfer onto nitrocellulose membranes. Detection was carried out using rabbit polyclonal IgG raised against Notch1 (clone H-131), Notch2 (clone 25-255) and Notch3 (clone M-134) (Santa Cruz Biotechnology; Santa Cruz, CA). Clones H-131, 25-255, and M-134 have been shown to recognize cleaved Notch transmembrane intracellular domains of 100, 120, and 2 bands of approximately 80 and 85 kDa, respectively.14–16 Cell surface-associated mucins (25 μg total protein) were separated by 1% agarose gel electrophoresis and transferred onto nitrocellulose membranes by vacuum blotting. Membranes were blocked with 5% nonfat dry milk in PBS overnight, then incubated with antibodies M11 (Neo Markers, Fremont, CA) to MUC16, 214D4 (Upstate; Lake Placid, NY) to MUC1, and 8G7 (Santa Cruz Biotechnology) to MUC4. Binding of GAPDH antibody (Santa Cruz Biotechnology) was used as loading control. After incubation with the appropriate peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology), positive binding was detected using a chemiluminescent substrate (SuperSignal West Pico Chemiluminescent Substrate; Pierce, Rockford, IL). MUC4 in HCLE cells was detected using an enhanced and more sensitive substrate (SuperSignal West Femto Maximum Sensitivity Substrate; Pierce), which enables detection of low femtogram amounts of protein. Band intensities were quantified by densitometry (ImageJ software; National Institutes of Health; Bethesda, MD; in the public domain available at http://rsb.info.nih.gov/nih-image).
RNA Isolation and cDNA Synthesis
Total RNA was extracted from HCLE and HCjE cells using extraction reagent (TRIzol; Gibco-Invitrogen Corp.) according to the manufacturer's protocol. Residual genomic DNA in the RNA preparation was eliminated by digestion with amplification-grade DNase I (Gibco-Invitrogen Corp.). Reverse transcription of 2 μg total RNA was performed with random hexamer primers and reverse transcriptase (SuperScript II; Gibco-Invitrogen Corp.), according to the manufacturer's protocol.
Real-time Polymerase Chain Reaction (PCR)
The relative amounts of MUC16 mRNA in HCLE and HCjE cells were determined by real-time PCR using a sequence detection system (ABI Prism 7900HT; PE Applied Biosystems; Foster City, CA). PCR amplification was performed in the presence of double-labeled fluorogenic probes (TaqMan Probes; Applied Biosystems), which allow the relative quantitation of gene expression in real time. The primers and probes used for MUC16 and GAPDH have been published.17 The average threshold cycle (Ct) values for GAPDH were used as an endogenous reference to correct for differences in the amount of total RNA added to each reaction. Therefore, the amount of target gene in each sample was normalized to the endogenous control by subtracting the Ct of GAPDH from that of the MUC16. The comparative Ct(2 − ΔΔCt) method18 was used for relative quantitation of the number of MUC16 transcripts, with the relative MUC16 mRNA levels in confluent cells treated with DMSO for 24 hours selected as the calibrator. Samples were assayed in triplicate in a total volume of 50 μL, using thermal cycling conditions comprised of 2 minutes at 50°C, 10 minutes at 95°C followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. No template controls were run in each assay to confirm lack of DNA contamination in reagents used for amplification.
Results
Notch Intracellular Domains were Differentially Expressed During In Vitro Differentiation of Human Corneal and Conjunctival Epithelial Cells
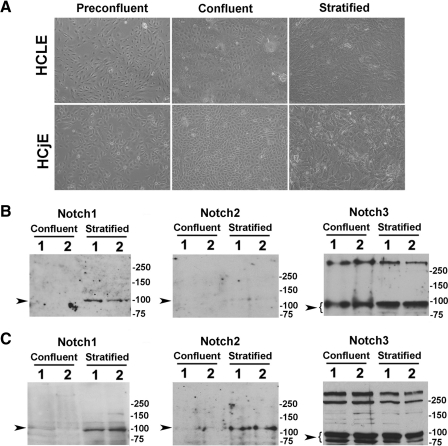
The role of Notch signaling on mucin biosynthesis was evaluated in HCLE and HCjE cells at 3 different stages of culture: preconfluent, confluent in the absence of serum, and stratified cells after 7 days of serum supplementation (Fig. 1A). These stages of growth represent 3 different stages of differentiation—proliferating, nondifferentiated epithelial cells; confluent, nondifferentiated epithelial cells; and stratified, partially differentiated epithelial cells.9,19
Figure 1.
Notch receptors in human corneal and conjunctival epithelial cell cultures. (A) Representative phase contrast micrographs showing HCLE and HCjE cells at three stages of culture: preconfluent, confluent in the absence of serum, and stratified after 7 days of serum supplementation. (B) By Western blot analyses, the intracellular domain of Notch3, but not Notch1 or Notch2, was present in undifferentiated confluent HCLE cells. The Notch3 antibody bound to a major band in the 80 to 90 kDa region corresponding to the intracellular domain, as previously described.14 Induction of cell differentiation and stratification resulted in binding of the Notch1 antibody to the 100 kDa intracellular domain. Weak binding of the Notch2 antibody was observed after induction of cell differentiation. (C) The intracellular domains of Notch1 to Notch3 were detected in HCjE cells, primarily after induction of cell differentiation and stratification. Experiments were performed in duplicate. Sample lanes were loaded with 50 μg (Notch1), 100 μg (Notch2), and 20 μg (Notch3) total protein. Arrowheads indicate the position of the Notch transmembrane intracellular domain.
To analyze the expression of Notch at the protein level, we performed Western blot analyses of HCLE and HCjE cells before and after induction of cell differentiation. We found that levels of the Notch3 intracellular domain were robustly expressed in undifferentiated and differentiated HCLE cells, and that protein levels corresponding to Notch1 intracellular domain were induced after 7 days of serum supplementation (Fig. 1B). Weak binding of the Notch2 antibody was observed after induction of cell differentiation in HCLE cells. Similarly, undifferentiated and differentiated HCjE cells also synthesized the Notch3 intracellular domain (Fig. 1C). Induction of differentiation and stratification in HCjE cells resulted in enhanced appearance of all Notch1 to Notch3 proteins, as observed in human conjunctival tissue.5
Notch Signaling Modulated MUC16 Biosynthesis during Corneal and Conjunctival Epithelial Cell Differentiation
Having established the presence of Notch intracellular domains in HCLE and HCjE cells during differentiation, we tested whether γ-secretase inhibition of Notch signaling affected the biosynthesis of cell surface-associated mucins. For these experiments, epithelial cells grown at different stages of differentiation were incubated with 0 (DMSO control), 10, and 25 μM DBZ for 4, 24, or 48 hours.
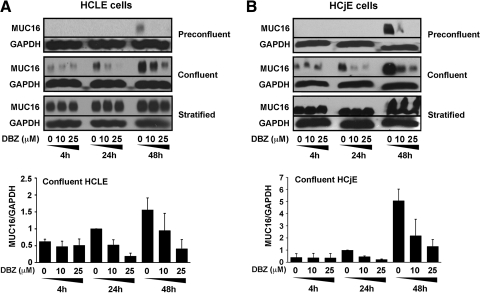
As shown in Figure 2, the levels of MUC16 in control cells were, as previously demonstrated, dependent on the stage of epithelial differentiation—absent during cell proliferation, but increasing as cells reached confluence and stratified in the presence of serum.10,19,20 Inhibition of Notch signaling with DBZ impaired MUC16 biosynthesis in undifferentiated HCLE cells, both at preconfluent and confluent stages, in a concentration-dependent manner (Fig. 2A). Notch/γ-secretase inhibition in preconfluent HCLE cells resulted in complete abrogation of MUC16 biosynthesis at 48 hours. In confluent cells treated with 25 μM DBZ, MUC16 biosynthesis decreased by 72% to 90% at 24 hours, and by 64% to 89% at 48 hours. Interestingly, DBZ did not affect MUC16 biosynthesis in stratified, differentiated HCLE cells, supporting the concept that Notch signaling is a dominant force in controlling cell fate during early cell differentiation.
Figure 2.
Effect of γ-secretase inhibition on MUC16 biosynthesis during corneal and conjunctival epithelial cell differentiation. (A) By Western blot analyses, inhibition of Notch signaling with DBZ markedly reduced the levels of MUC16 protein, in a concentration-dependent manner, in preconfluent and confluent HCLE cells. Densitometric analyses revealed that treatment of confluent cells with 25 μM DBZ for 24 and 48 hours decreased MUC16 antibody binding up to 90% and 89%, respectively. (B) Similarly to that of HCLE cells, inhibition of Notch signaling with DBZ markedly reduced MUC16 biosynthesis in preconfluent and confluent HCjE cells. By densitometry, treatment of confluent HCjE cells with 25 μM DBZ for 24 and 48 hours decreased MUC16 antibody binding up to 80% and 82%, respectively. Treating HCLE and HCjE cells with DBZ for 4 hours did not affect MUC16 biosynthesis under any culture conditions. Levels of MUC16 biosynthesis in densitometric analyses are relative to DMSO control at 24 hours. Error bars are SEM values based on 2 separate experiments.
A marked reduction in MUC16 biosynthesis was also observed in HCjE cells after Notch/γ-secretase inhibition during preconfluent and confluent stages (Fig. 2B). In preconfluent cells, levels of MUC16 were completely abrogated at 48 hours when cells were treated with 25 μM DBZ, whereas in confluent cells, MUC16 decreased by 73% to 80% at 24 hours, and by 69% to 82% at 48 hours. Similarly to HCLE cells, DBZ did not affect MUC16 biosynthesis in stratified, differentiated HCjE cells.
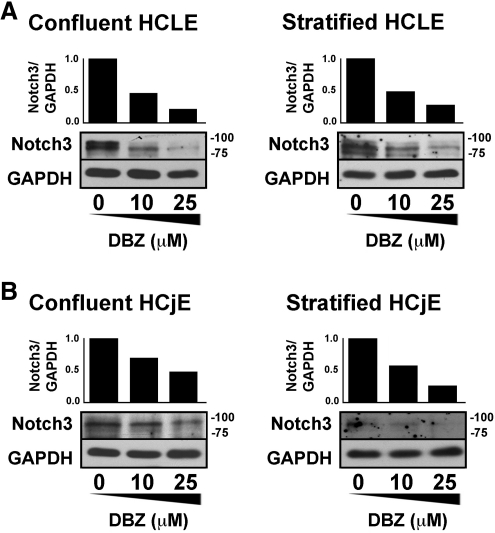
The effect of DBZ on Notch inhibition in HCLE and HCjE cells was demonstrated in control experiments that evaluated the proteolytic processing of Notch3, the most abundant receptor both under confluent and stratified cell culture conditions. A remarkable concentration-dependent decrease in Notch3 antibody binding was observed after Notch/γ-secretase inhibition in both cell lines under confluent and stratified conditions (Fig. 3), indicating that DBZ blocks the processing of Notch3 intracellular domain in vitro during corneal and conjunctival epithelial cell differentiation.
Figure 3.
Effect of γ-secretase inhibition on Notch processing in corneal and conjunctival epithelial cells. Incubation of HCLE and HCjE cells with DBZ for 48 hours markedly reduced the levels of Notch3 intracellular domain in a concentration-dependent manner. (A) Densitometric analyses revealed that 25 μM DBZ reduced Notch3 antibody binding in confluent and stratified HCLE cells by 78% and 72%, respectively. (B) In confluent and stratified HCjE cells, the decrease observed was 52% and 74%, respectively.
MUC16 Biosynthesis Was Posttranscriptionally Regulated by Notch
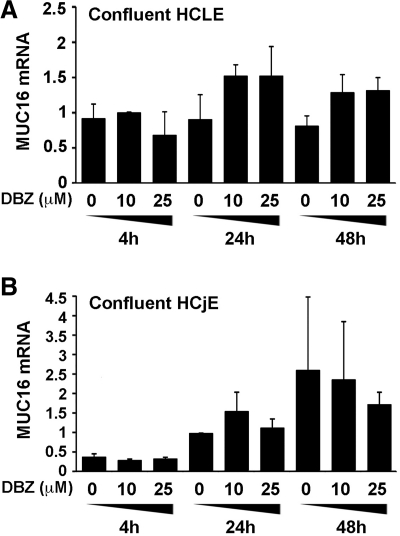
A key step in Notch signaling is the γ-secretase proteolytic event that mediates release of the Notch intracellular domain, which then enters the nucleus to participate directly in the transcriptional regulation of nuclear target genes.1 To determine whether Notch/γ-secretase inhibition suppresses the level of MUC16 biosynthesis through a transcriptional mechanism, we performed real-time PCR experiments in confluent cultures treated with DBZ or DMSO alone. HCLE and HCjE cells were harvested after 4, 24, or 48 hour treatments, total RNA was isolated, and 2 μg of total RNA was subjected to reverse transcription and real-time PCR amplification. The number of MUC16 transcripts in HCLE cells treated with DBZ at different concentrations and time points was not inferior to that of control cells treated with DMSO alone (Fig. 4A). Similarly, the number of MUC16 transcripts in DBZ-treated HCjE cells was similar to those in DMSO control (Fig. 4B). These results indicate that the level of MUC16 protein does not correspond to the level of MUC16 transcript in confluent cells after γ-secretase inhibition, and point to a posttranscriptional mechanism of regulation for MUC16 in ocular surface epithelial cells.
Figure 4.
Real-time PCR analysis of MUC16 expression in confluent HCLE and HCjE cells incubated with increasing concentrations of DBZ during 4, 24, and 48 hours. In contrast with the marked decrease of MUC16 found at the protein level (Fig. 2), the level of MUC16 transcripts in DBZ-treated cells was not significantly reduced at any time point compared with DMSO control. Y-axis values were calculated using the 2 − ΔΔCt method. Levels of MUC16 transcripts in cells treated with DMSO for 24 hours were used as the calibrator. Error bars are SEM values based on 2 separate experiments.
Notch Signaling Differentially Regulated Cell Surface-Associated Mucins
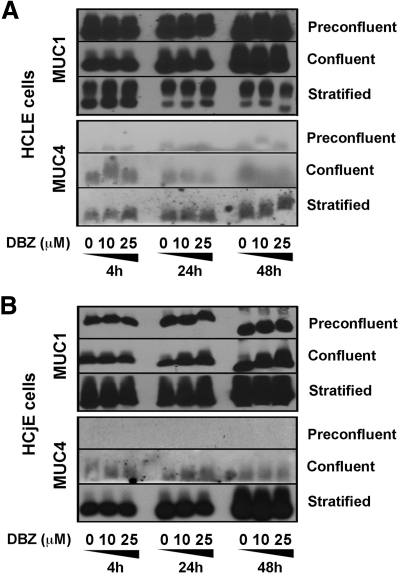
In addition to MUC16, human corneal epithelium expresses MUC1 and low levels of MUC4, whereas the conjunctival epithelium expresses both MUC1 and MUC4.21 Because cell surface-associated mucins can be differentially regulated at the ocular surface both in vivo and in vitro,10,11,22 we sought to determine whether Notch/γ-secretase inhibition in HCLE and HCjE cell cultures regulated the biosynthesis of MUC1 and MUC4 in a manner similar to that of MUC16. Immunoblot analyses of DBZ-treated corneal and conjunctival epithelial cells showed no differences in levels of MUC1 and MUC4 compared with that of DMSO control in cells grown at different stages of differentiation (Fig. 5). In these experiments, MUC1 was present in proliferating as well as in differentiated cells, whereas MUC4 was predominantly present in differentiated cells, particularly in conjunctival epithelial cells, as described in human tissue.8
Figure 5.
Effect of γ-secretase inhibition on MUC1 and MUC4 biosynthesis during corneal and conjunctival epithelial cell differentiation. (A) By Western blot analyses, treatment of HCLE cells with increasing concentrations of DBZ for 4, 24, and 48 hours did not alter the levels of MUC1 and MUC4 compared with DMSO control at any stage of cell differentiation. Due to low levels of MUC4 in human corneal epithelium, amounts of MUC4 protein in HCLE cells were detected using an enhanced chemiluminescent substrate (SuperSignal West Femto Maximum Sensitivity Substrate). (B) Similarly to that of HCLE cells, no change was detected for MUC1 and MUC4 in HCjE cells cultured with DBZ.
Discussion
MUC16, originally known as the ovarian tumor cell marker CA125, plays a multifunctional role at the ocular surface, exhibiting the classical mucin-associated function of maintaining hydration of the epithelial surface, as well as forming a disadhesive barrier that provides boundary lubrication and prevents pathogen adhesion.23 Regulation of MUC16 is, therefore, of paramount importance to the maintenance of a healthy, wet ocular surface phenotype. Here we demonstrate that MUC16 biosynthesis is regulated by Notch signaling at early stages of human corneal and conjunctival epithelial cell differentiation and provide evidence of a new posttranscriptional mechanism of MUC16 regulation.
Canonical Notch signaling has numerous effects on normal mucosal epithelia. Early studies on the function of Notch signaling in secretory cell lineages revealed that Notch signaling is involved in the modulation of genes known to specify gut differentiation.12,24 In intestinal crypts, Notch/γ-secretase inhibition induces rapid conversion of proliferative cells into postmitotic, mucin-producing, goblet cells.2,25 Interestingly, conditional expression of an activated Notch mutant in postmitotic intestinal epithelial cells also increases the number of goblet cells, indicating that the composition of the epithelium is not solely determined by progenitor cells.26 Here, we used a γ-secretase inhibitor to block the Notch cascade in an in vitro model of human corneal and conjunctival epithelial cell differentiation. Our results show that inhibition of Notch signaling abrogates the induction of MUC16 biosynthesis in proliferating and confluent epithelial cells, but not in postmitotic stratified cells, supporting the concept that Notch signaling controls binary cell fate choices during early cell differentiation at the ocular surface.
Abnormal cell differentiation occurs in Notch1-null mouse corneas.3 Histologic examination of these mice showed extensive keratinization of the corneal epithelium, caused by the inability of Notch1-deficient cells to regenerate the tissue through a vitamin A mechanism.27 Our finding that Notch signaling regulates MUC16 biosynthesis further supports the notion that hydrophilic mucins are important to the prevention of keratinization and maintenance of a wet-surface phenotype. This concept is reinforced by findings showing that induction of keratinization by depletion of retinoic acid in rats results in downregulation of the cell surface mucin rMuc4 and the goblet cell mucin rMuc5ac.22 Interestingly, in the latter study, rMuc1 was not affected by vitamin A deficiency, even after severe keratinization. Similarly, in our study, we found that Notch signaling regulated MUC16 biosynthesis, but not that of MUC1 and MUC4, suggesting that individual mucins have specific roles on mucosal surfaces, supporting previous data indicating that surface mucins are differentially regulated.10,11
A partial reduction (of approximately 50%) in the expression of Notch receptors and ligands has been recently described in conjunctival cells obtained by impression cytology from patients with non-Sjögren's dry eye.5 Unexpectedly, this reduction in Notch mRNA levels does not seem to correlate with loss of MUC16 protein in dry eye patients—analyses of protein from both tears and conjunctival epithelial cells collected via impression cytology revealed that MUC16 levels in dry eye patients did not significantly differ from those found in normal subjects.28 As most cells collected by impression cytology are apical cells, it is possible to speculate that the reduction of Notch signaling observed in patients with dry eye occurs in postmitotic, stratified cells that are already differentiated and, therefore, not susceptible to alteration of MUC16 biosynthesis by a Notch-mediated mechanism. Alternatively, the remaining Notch levels in the nonkeratinized conjunctival epithelium of dry eye patients may be sufficient to direct MUC16 biosynthesis in these patients. Additional research in patients with severe keratinizing disorders such as cicatricial pemphigoid or Stevens-Johnson syndrome should shed more light onto the role of Notch signaling in maintaining the mucosal phenotype of the ocular surface.
Several structural studies have shown that upon ligand-induced Notch activation, the released Notch intracellular domain enters the nucleus and interacts with the DNA-binding CSL protein and the coactivator Mastermind to form a transcriptionally active complex that recruits general transcription factors that promote chromatin acetylation and expression of Notch target genes.1,29 To investigate whether Notch activation directly modulates MUC16 protein biosynthesis at the transcriptional level, we analyzed the relative amount of MUC16 transcripts in conditions where Notch/γ-secretase inhibition suppresses MUC16 protein biosynthesis. When incubated with DBZ, however, no decrease in the number of MUC16 transcripts were observed in confluent HCLE and HCjE cells, supporting a posttranscriptional regulatory mechanism of MUC16 biosynthesis. It is possible to speculate that the posttranscriptional regulation of MUC16 biosynthesis could result from the deficient expression of genes involved in the posttranslational modification of the mucin, such as glycosyltransferases, or genes involved in the biosynthesis of the appropriate nucleotide sugars, which would result in targeting of the nascent MUC16 polypeptide toward degradation. For example, MUC1 is known to be rapidly degraded in glycosylation-defective (ldlD) cells that lack the epimerase to make UDP-Gal/GalNAc from UDP-Glc/GlcNAc.30 Examples of posttranscriptional mechanisms of regulation of cell surface-associated mucins have been reported in the literature. rMuc4 protein, but not transcript, levels are significantly reduced when normal mammary epithelial cells are cultured in Matrigel by a mechanism that involves TGF-ß.31,32 Posttranscriptional regulation of rMuc4 has also been demonstrated in cornea, where its precursor is synthesized in all layers of the epithelium, but it is posttranscriptionally degraded in basal and intermediate layers by a proteosomal mechanism that is partly dependent on TGF-ß.33 Our data indicate that, in addition to rMuc4, posttranscriptional regulation of MUC16 by Notch signaling constitutes an additional mechanism of regulation of mucin gene expression in ocular surface epithelial cells.
In summary, in this study, we evaluated the role of Notch/γ-secretase inhibition in an in vitro model of human corneal and conjunctival epithelial cell differentiation. We found that MUC16 biosynthesis is posttranscriptionally regulated by Notch signaling at early stages of epithelial cell differentiation, suggesting that Notch signaling plays an important role in maintaining a wet-surface phenotype at the ocular surface.
Footnotes
Supported by Massachusetts Lions Eye Research Fund and NIH/NEI Grant R01 EY014847 (PA).
Disclosure: L. Xiong, None; A.M. Woodward, None; P. Argüeso, None
References
- 1. Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647 [DOI] [PubMed] [Google Scholar]
- 2. van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963 [DOI] [PubMed] [Google Scholar]
- 3. Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421 [DOI] [PubMed] [Google Scholar]
- 4. Ma A, Boulton M, Zhao B, Connon C, Cai J, Albon J. A role for Notch signaling in human corneal epithelial cell differentiation and proliferation. Invest Ophthalmol Vis Sci. 2007;48:3576–3585 [DOI] [PubMed] [Google Scholar]
- 5. Mantelli F, Schaffer L, Dana R, Head SR, Argueso P. Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci. 2009;50:2666–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bansil R, Stanley E, LaMont JT. Mucin biophysics. Annu Rev Physiol. 1995;57:635–657 [DOI] [PubMed] [Google Scholar]
- 7. Rose MC. Mucins: structure, function, and role in pulmonary diseases. Am J Physiol. 1992;263:L413–L429 [DOI] [PubMed] [Google Scholar]
- 8. Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506 [DOI] [PubMed] [Google Scholar]
- 10. Hori Y, Spurr-Michaud S, Russo CL, Argueso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004;45:114–122 [DOI] [PubMed] [Google Scholar]
- 11. Hori Y, Spurr-Michaud SJ, Russo CL, Argueso P, Gipson IK. Effect of retinoic acid on gene expression in human conjunctival epithelium: secretory phospholipase A2 mediates retinoic acid induction of MUC16. Invest Ophthalmol Vis Sci. 2005;46:4050–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milano J, McKay J, Dagenais C, et al. Modulation of Notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358 [DOI] [PubMed] [Google Scholar]
- 13. Menke V, van Es JH, de Lau W, et al. Conversion of metaplastic Barrett's epithelium into post-mitotic goblet cells by gamma-secretase inhibition. Dis Model Mech. 2010;3:104–110 [DOI] [PubMed] [Google Scholar]
- 14. Jia L, Yu G, Zhang Y, Wang MM. Lysosome-dependent degradation of Notch3. Int J Biochem Cell Biol. 2009;41:2594–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mine T, Matsueda S, Li Y, et al. Breast cancer cells expressing stem cell markers CD44+ CD24 lo are eliminated by Numb-1 peptide-activated T cells. Cancer Immunol Immunother. 2009;58:1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosati E, Sabatini R, Rampino G, et al. Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood. 2009;113:856–865 [DOI] [PubMed] [Google Scholar]
- 17. Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–2495 [DOI] [PubMed] [Google Scholar]
- 18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 19. Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sumiyoshi M, Ricciuto J, Tisdale A, Gipson IK, Mantelli F, Argueso P. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010;90:655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tei M, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Vitamin A deficiency alters the expression of mucin genes by the rat ocular surface epithelium. Invest Ophthalmol Vis Sci. 2000;41:82–88 [PubMed] [Google Scholar]
- 23. Perez BH, Gipson IK. Focus on molecules: human mucin MUC16. Exp Eye Res. 2008;87:400–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Searfoss GH, Jordan WH, Calligaro DO, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278:46107–46116 [DOI] [PubMed] [Google Scholar]
- 25. Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zecchini V, Domaschenz R, Winton D, Jones P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005;19:1686–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vauclair S, Majo F, Durham AD, Ghyselinck NB, Barrandon Y, Radtke F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13:242–253 [DOI] [PubMed] [Google Scholar]
- 28. Caffery B, Joyce E, Heynen ML, et al. MUC16 expression in Sjogren's syndrome, KCS, and control subjects. Mol Vis. 2008;14:2547–2555 [PMC free article] [PubMed] [Google Scholar]
- 29. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689 [DOI] [PubMed] [Google Scholar]
- 30. Altschuler Y, Kinlough CL, Poland PA, et al. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol Biol Cell. 2000;11:819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price-Schiavi SA, Carraway CA, Fregien N, Carraway KL. Post-transcriptional regulation of a milk membrane protein, the sialomucin complex (Ascites sialoglycoprotein (ASGP)-1/ASGP-2, rat muc4), by transforming growth factor beta. J Biol Chem. 1998;273:35228–35237 [DOI] [PubMed] [Google Scholar]
- 32. Price-Schiavi SA, Zhu X, Aquinin R, Carraway KL. Sialomucin complex (rat Muc4) is regulated by transforming growth factor beta in mammary gland by a novel post-translational mechanism. J Biol Chem. 2000;275:17800–17807 [DOI] [PubMed] [Google Scholar]
- 33. Lomako J, Lomako WM, Carothers Carraway CA, Carraway KL. Regulation of the membrane mucin Muc4 in corneal epithelial cells by proteosomal degradation and TGF-beta. J Cell Physiol. 2010;223:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]