Cell based therapy offers hope to individuals that have lost their sight through retinal degenerations. Previous studies have found that photoreceptors from immature mice can be successfully transplanted to the mature mouse retina; however, rod photoreceptors from adult donors failed to integrate into the host retina following transplantation. The authors have found that rod photoreceptors from adult mice can be successfully transplanted to the mature retina and this provides a potentially new avenue for cell based therapy.
Abstract
Purpose.
Previous studies indicate that early postnatal mouse photoreceptors have the ability to integrate into the mature host retina after transplantation, while progenitors and fully differentiated photoreceptors do not. The authors sought to determine whether the decline in the ability of photoreceptors to integrate after transplantation with increasing age is related to a loss of migratory ability in the adult neurons or by a decrease in their survival.
Methods.
Dissociated retinal cells were transferred from green fluorescent protein–positive (GFP+) donor mice of ages ranging from embryonic day (E)12.5 to adults (>28 days postnatal [P]). Immunofluorescence was used to assess marker expression and the morphology of integrated cells. In vitro cultures of dissociated Nrl-GFP mice were used to assay survival.
Results.
It was confirmed in previous reports that neonatal rods integrate into adult hosts. However, in contrast to previous reports, the age of the donor cell was not as critical as previously reported, because it was found that donor cells older than P11 effectively integrated into adult host retina. Although fully adult photoreceptors (P28 and older) show a higher transplant failure rate than immature ones (P5), successful transplants had very similar numbers of integrated cells for both mature and immature donors. Integrated cells from all ages were indistinguishable from those of the host in morphology and marker expression.
Conclusions.
Fully mature photoreceptors retain the ability to integrate into the mature retina. The authors propose that their potential for integration is limited primarily by their decreased survival after dissociation.
Photoreceptor degeneration is the leading cause of blindness in the developed world, and therapies exist for only a small subset of cases.1–3 Transplantation of photoreceptors could replace rods and cones that are lost either because of genetic mutations or aging-related pathology.4,5 Primary retinal progenitors,6 photoreceptors from neonatal to adult ages,7–10 and retinal cells derived from neural progenitors11 or human embryonic stem cells12 have all been tested for their potential for integration into the retina. When transplanted into the subretinal space of normal animals, or those with photoreceptor degeneration, these various types of donor cells show differing ability to survive and integrate into the host retinas. However, for many years there was no consensus as to what types of cells would provide the best source for cell replacement therapy for retinal disease.
More recently, several groups10,13 have shown that transplantation of green fluorescent protein (GFP)-labeled photoreceptors into hosts with an intact outer nuclear layer results in integration of fully formed photoreceptors, with axons and synaptic terminals, as well as inner and outer segments. In a study by MacLaren et al.,10 the effectiveness of the donor cells to integrate into the host retina varied significantly as a function of age: postmitotic photoreceptor cells from neonatal mouse retina integrated at a rate of several hundred per transplanted eye, whereas donor cells from either embryonic retina (primarily progenitors), or cells from donors older than postnatal day (P)11, failed to integrate. They concluded from these results that the newly postmitotic rod photoreceptors are uniquely competent to integrate into a mature host retina, and this property is not present in either retinal progenitors or mature rod photoreceptors. An alternative possibility is that the older rod photoreceptors are sensitive to the dissociation protocols used for transplantation and that they have very limited survival potential when transplanted as dissociated cells. In this study, we have investigated whether the neonatal rod photoreceptors have a greater potential to survive after dissociation, and whether this property can explain their more limited integration after transplantation.
Materials and Methods
Animals
All experiments were performed with adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and in accordance with protocols approved by the University of Washington Institutional Animal Care and Use Committee. For transplant experiments, we used GFP mice that express enhanced GFP (EGFP) ubiquitously under the control of a chicken β-actin promoter and cytomegalovirus enhancer.14 Nrl-GFP mice15 were used for in vitro rod photoreceptor survival experiments. In these animals, rod-exclusive EGFP expression is driven by the neural retina leucine (Nrl) zipper promoter.
Donor Cell Preparation for Subretinal Transplantation
Donor cells were derived from GFP mice as follows: the donor mice were killed according to approved protocol, the eyes were removed, and the retinas were dissected in cold Hanks' balanced salt solution (BSA; Sigma, Saint Louis, MO). The retinas were placed in a papain solution (1 mL per 2 retinas; Worthington Biochemical, Lakewood, NJ) for 50 minutes at 37°C on a nutator. After this incubation, the retinas were triturated 10 times with a 1000-μL plastic pipette tip to dissociate them into single cells. The papain reaction was stopped with ovomucoid albumin inhibitor, and cells were collected by centrifuging at 300g for 4 minutes. The donor cells were resuspended in retinal culture media (see below) at a density of 100,000 cells per microliter and kept on ice until transplantation.
Subretinal Transplantation
Transplant recipients were adult (4–52 weeks old) wild-type C57/Bl6 mice. The transplant recipient mice were anesthetized with ketamine/xylazine and topical proparacaine, and placed in a stereotaxic head holder (mouse adaptor; Stoelting, Wood Dale, IL). Using a no. 11 scalpel, an incision was made into the superior sclera to expose a 0.2- × 0.2-mm area of retina while taking care not to puncture the retina. A micropipette was filled with donor cell suspension and mounted in the stereotaxic pipette holder (Stoelting). The pipette was inserted through the cut at a shallow angle to the scleral surface, and advanced 0.25 mm into the subretinal space. A 0.5-μL cell suspension was slowly injected with a manual plunger. The pipette was allowed to remain in place for 30 seconds and was then very slowly withdrawn to minimize efflux of the transplanted cell suspension. Bacitracin ointment was applied to the eye after surgery. The total time from dissection of the donor tissue until placement of the cells in the subretinal space was no longer than 2 hours.
Analysis of Transplants
Animals were killed 2 weeks after the subretinal transplantation. The eyes were removed from the host animals and dissected in PBS. The lens was removed, and the eyecup was fixed for 30 minutes in 4% paraformaldehyde. Then the retinas were dissected from the surrounding ocular tissues, embedded in 5% agar, and cut into 60-μm thick sections with a vibratome. Sections were incubated in 5% goat serum and 1% Triton-X in PBS for 60 minutes to reduce nonspecific labeling. Primary antibodies were applied overnight with 0.15% Triton-X and 2% goat serum. Antibodies used were chicken anti-GFP (1:500, Abcam ab13970, Cambridge, UK), rabbit anti-recoverin (1:750, Millipore ab5585, Billerica, MA), biotinylated goat anti-Otx2 (2.5 μg/mL, BAF1979; R&D Systems, Minneapolis, MN), mouse anti-Bassoon (1:500, VAM-P5003; Stressgen Bioreagents), and mouse anti-Rhodopsin 4D2 (1:1000, gift from Robert Molday, University of British Columbia). Sections were washed in PBS three times and again incubated overnight with fluorescent conjugated secondary antibodies (Invitrogen) or, for the otx2 antibody, with streptavidin conjugate (Invitrogen). Sections were counterstained with DAPI (Sigma). Integrated cells were counted under a fluorescent microscope (Axioplan 2; Zeiss, Göttingen, Germany), and images were acquired with a confocal microscope (A1; Nikon, Tokyo, Japan). Images were processed with commercially available software (Volocity; Perkin-Elmer, Waltham, MA and Photoshop; Adobe, San Jose, CA).
Dissociated Photoreceptor Survival Assay
Nrl-GFP mice aged P5 or 4 months were killed and their neural retinas were dissected in Hanks' balanced salt solution. Retinas were then dissociated with a papain kit (Worthington Biochemical) for 50 minutes at 37°C. Cells were collected by centrifuging for 4 minutes at 300g and were resuspended in retinal culture medium containing DMEM:F12, 100 U/mL penicillin, 100 μg/mL streptomycin, N2 supplement, 1% FBS (all from GIBCO), 5 mM Hepes, and 6 mg/mL glucose (both from Sigma). Cells were plated at a density of 300,000 total cells/cm2 on poly-d-lysine coated coverslips in 24-well plates. Cultures were incubated in 5% CO2 at 37°C, and were fixed after either 1 or 4 hours in vitro. Cells were immunolabeled for GFP (see above) and counterstained with DAPI, and GFP+ cells were counted manually in five random 20× fields on two coverslips per experiment.
Results
The Window of Integration Extends Beyond P11
We transplanted retinal cell suspensions from mice ubiquitously expressing EGFP under the control of the chicken β-actin promoter into adult (>30 day) wild type mice. Each transplant comprised 50,000 donor cells that were injected into the subretinal space. Transplant recipients were killed 14 days after the injection.
Transplants from donors that were in their first postnatal week (P1–P7) showed robust integration of cells with rod photoreceptor morphology in the outer nuclear layer (Fig. 1a; Figs. 2a, 2b), confirming previous results.10,13 The numbers of integrated cells varied substantially among the transplants, but on average, successful transplants from donors aged P1 to P7 had several hundred integrated rod photoreceptor cells in each retina. Consistent with the findings of others, <1% of the total transplanted cells integrated.10,13 Some of the losses can be accounted for by reflux around the injection needle, and a large number of cells likely do not survive the transplant process. In most cases, many cells remained in the subretinal space (Fig. 2a), but in some cases only a few cells remained in the subretinal space after the 2-week survival period. The transplanted GFP+ cells had typical rod photoreceptor morphology, again confirming earlier reports.10,13
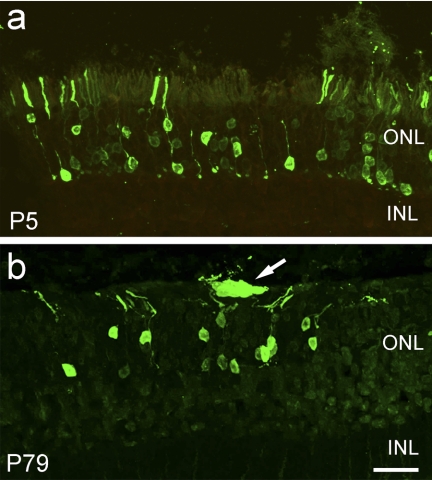
Figure 1.
Rods from neonatal and mature donors can integrate after transplantation. After transplantation into the subretinal space of adult host mice, P5 (a) and P79 (b) GFP-labeled dissociated retinal cells integrate into the unlabeled host retina with approximately equal efficiency. ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar: (a) 20 μm; (b) 20 μm.
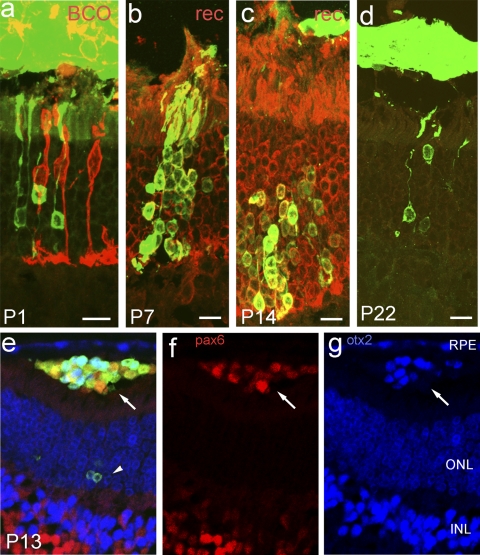
Figure 2.
Donor photoreceptors older than P11 integrate into the adult host retina. GFP+ donor photoreceptors from P1 (a), P7 (b), P14 (c), and P22 (d) donors integrate into the ONL of adult hosts, which is outlined by recoverin (rec) immunolabeling. All the integrated cells express recoverin (b, c), but not blue cone opsin (BCO) (a). BCO is dispersed throughout the cell body because of retinal detachment at the transplant site. A few cells in the subretinal space express BCO (e–g). Transplanted cells that remain in the subretinal space (arrow) also express pax6 (f) and otx2 (g). ONL, outer nuclear layer; INL, inner nuclear layer; RPE, retinal pigmented epithelium. Scale bar, 10 μm.
To define the best time for integration after transplantation, we also harvested donor cells from progressively older mice. Previous studies had failed to see any integration beyond P11.10 However, when we tested donor cells derived from animals at P14, P21 (Figs. 2c, 2d), or even fully mature adult donors P79 (Fig. 1b), we found that photoreceptors were able to effectively integrate into the outer nuclear layer. In all cases, we observed only recoverin-positive rod photoreceptors in the outer nuclear layer; by contrast, cells remaining in the subretinal space expressed markers of amacrine cells, bipolar cells and photoreceptors (Figs. 2e, 2f, 2g). These results indicate that mature rod photoreceptors do not lose the ability to migrate into the mature retina.
Mature Photoreceptors Can Integrate into the Host Outer Nuclear Layer
To determine whether there are quantitative differences in the integration ability of adult donor rod photoreceptors compared to that of neonatal rods, we conducted a series of transplantations in which immature and mature donor cells were subjected to identical dissociation conditions and transplanted into littermates on the same day. This allowed us to eliminate much of the variability between experiments. Donor cells were found in the subretinal space of more than 80% of the transplants, which indicates an excellent success rate.
P5 was chosen as the immature reference age because this was previously reported as the peak of integration.5 The adult donor comparison group comprised donor animals from P28 to P290. Again, we transplanted enzymatically dissociated retinal cell suspensions prepared from GFP mice into the subretinal space of wild type adult recipient mice (4–52 weeks), and 14 days later assessed transplant integration.
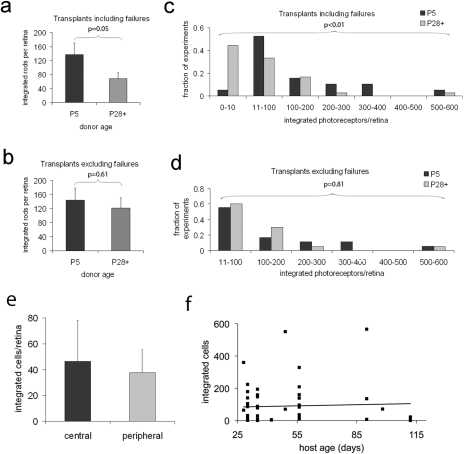
Transplanted rods from donors aged 28 to 290 days integrated at an average rate of 76 ± 22 cells per retina, compared to 136 ± 33 cells for P5 donors (P = 0.05; Fig. 3a). This difference can largely be accounted for by the much higher failure rate of old transplants (Fig. 3c). Failures were defined as cases where 10 or fewer cells integrated, and occurred in 44.4% (16 of 36 cases) for donor ages over 28 days, compared to only 5.2% (1 of 19 cases) in P5 donors. The distribution of cases is significantly different (P = 0.008; Mann-Whitney U test) when all transplants, including those that failed, are accounted for. When failures are excluded, however, the integration rates are much more similar: 144 ± 34.2 cells for P5 and 121 ± 28.7 cells for donors P28 and older (P = 0.61; Fig. 3b), and the distributions largely overlap (P = 0.81; Mann-Whitney U test; Fig. 3d). These data suggest that the mature rod photoreceptors are nearly as effective as the P5 cells at integrating into wild type host retinas; however, there is more variability in the success rate of the transplant.
Figure 3.
Mature photoreceptors integrate into the adult retina. (a, c) The average number of integrated cells per retina for P5 and P28+ donors, respectively (N = 19 for P5, N = 36 for P28+; P = 0.05; Student's t-test). These experiments are binned by integrated cells per retina in (c) and show significantly different distributions (P < 0.01; Mann-Whitney U test). (b, d) The average number of integrated cells per retina when failures (transplants with <10 integrated rods) are excluded (N = 18 for P5, N = 20 for P28+; P = 0.6; Student's t-test). In (f), these experiments are again binned by integrated cell number, and distributions of P5 and P28+ cells are no longer significantly different when failures are excluded (P = 0.81; Mann-Whitney U test). (e) Central and peripheral retinal cells from a 290-day-old mouse show similar integration. (f) Summary of transplanted cells into host animals from 30 days to >100 days of age to show the lack of a relationship between host age and the number of integrated cells.
The periphery of the mammalian retina retains some immature characteristics into adulthood, albeit much less so than the ciliary margin zone of the bird and fish.16,17 We asked whether some property of peripheral rod photoreceptors makes them more suitable for transplantation and whether peripheral cells account for the integration that we have observed. To test this, we cut the retina of a 290-day-old donor mouse into central and peripheral sections, and separately dissociated and transplanted the cells from each section. The central donor cells integrated at a similar rate as the peripheral ones (central 47 ± 31 cells/retina, N = 2; peripheral 38 ± 18 cells/retina, N = 4; Fig. 3e). Although the number of integrated cells in this experiment was lower than the average integration rate of 121 ± 29 cells, the results fall within the overall distribution, where 10 to 100 integrated cells were seen in more than 50% of cases (Fig. 3d). We also tested whether the age of the host was important for the successful integration of the transplanted cells. We transplanted cells into host animals from a range of ages (30 to >100 days of age). There was no clear relationship between the age of the host and the number of integrated cells (Fig. 3f).
Integrated Adult Photoreceptors Re-elaborate Morphology and Gene Expression of Native Photoreceptors
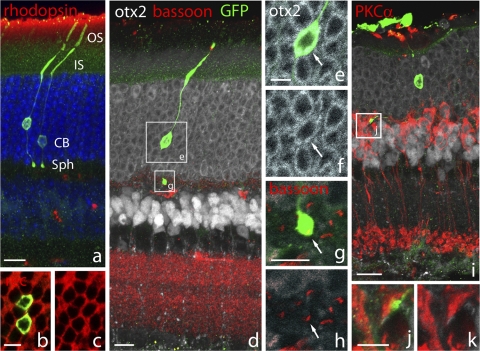
We next characterized the morphology and marker expression of integrated photoreceptors from donors older than P28. Transplanted cells integrated into the outer nuclear layer (ONL) of the host and assumed morphology that was indistinguishable from host rods (Fig. 4a). This is despite the fact that donor cells lose all their processes on enzymatic dissociation. Mature rod photoreceptors therefore retain the capability to regenerate their cellular structure after transplantation into mature retinas. Integrated adult rods elaborated an inner and outer segment, as well as a basal axon with a synaptic ending akin to a rod spherule (Figs. 4a, 4d). The rod photopigment rhodopsin is shuttled to the outer segments in mature, fully functional rod photoreceptors, whereas in immature and damaged cells the protein can be found throughout the cell body.18–20 The fact that rhodopsin is located only in the outer segments of the transplanted cells (Fig. 4a) suggests that they have fully matured in their new location. The cell bodies of the integrated rod photoreceptors exhibit a characteristic ring-like nuclear staining pattern for the photoreceptor transcription factor Otx2,21,22 which contrasts with the more diffuse localization of Otx2 in the bipolar nuclei (Figs. 4d–f). This difference in Otx2 distribution is likely caused by the unique chromatin configuration of the rodent rod,23 which develops as photoreceptors mature. We also tested for expression of components of the phototransduction cascade, such as recoverin, which is a calcium-binding protein that enhances the photoresponse.24 Recoverin is found in the cytoplasm of integrated rods at the same expression level as in neighboring host rods (Figs. 4b, 4c).
Figure 4.
Integrated mature rod photoreceptors elaborate complete morphology. Transplanted +, shown in green throughout) that integrated into the ONL make inner (IS) and outer segments (OS), an axon, and a spherule synapse (Sph) (a, Z-stack). The outer segments of integrated rods express rhodopsin (a), the cell body is positive for recoverin (b, c), and Otx2 (d, Z-stack; e, f, 1-μm optical section). Note that the photoreceptors exhibit a ring-like nuclear distribution of Otx2, while the bipolars (arrow) have a more diffuse distribution (d, i). The spherule synapse of integrated cells has a single ribbon that labels for bassoon (g, h, arrow; 1-μm optical section). Synaptic terminals appose directly to rod bipolar processes immunolabeled for PKCa (i, Z-stack; j, k, 1-μm optical section). Scale bars: (a, d, i) 10 μm); (b, c, e, f–h, j, k) 5 μm.
The integrated cells extend axons toward the outer plexiform layer, where photoreceptors synapse with bipolar cells (Fig. 4d). The axon terminals have the morphology of a rod spherule and possess a single synaptic ribbon identified by immunolabeling for the ribbon synapse component bassoon (Figs. 4g, 4h). This is consistent with the structure of native rod ribbon synapses.25 The axon terminals of the integrated rods appose directly to rod bipolar dendrites labeled with PKCα (Figs. 4i–k). In summary, all the rod photoreceptor markers we tested labeled all integrated photoreceptors in a distribution indistinguishable from that in host rods.
In Vitro Survival of Mature Photoreceptors is Less Than That of Immature Ones
Although we observed similar rates of integration for P5 and adult rod photoreceptors, the failure rate of adult transplants was much higher. We therefore tested whether a difference in survival of the dissociated donor cells can account for this disparity. We dissociated retinas from P5 and 4-month-old Nrl-GFP mice, which express GFP exclusively in rods,15 and compared the number of surviving rod photoreceptors 4 hours after plating on coverslips. At P5, the number of GFP-identified rods remained stable. In contrast, the number of rods from 4-month-old retinas declined sharply, with only 18% ± 5% surviving 4 hours after plating (Fig. 5b; P < 0.001, N = 3). The ability to survive during the dissociation and transplantation procedures declines substantially with age of the photoreceptors and may account for the higher rate of failure in the transplantation of older cells in our experiments and those of others.
Figure 5.
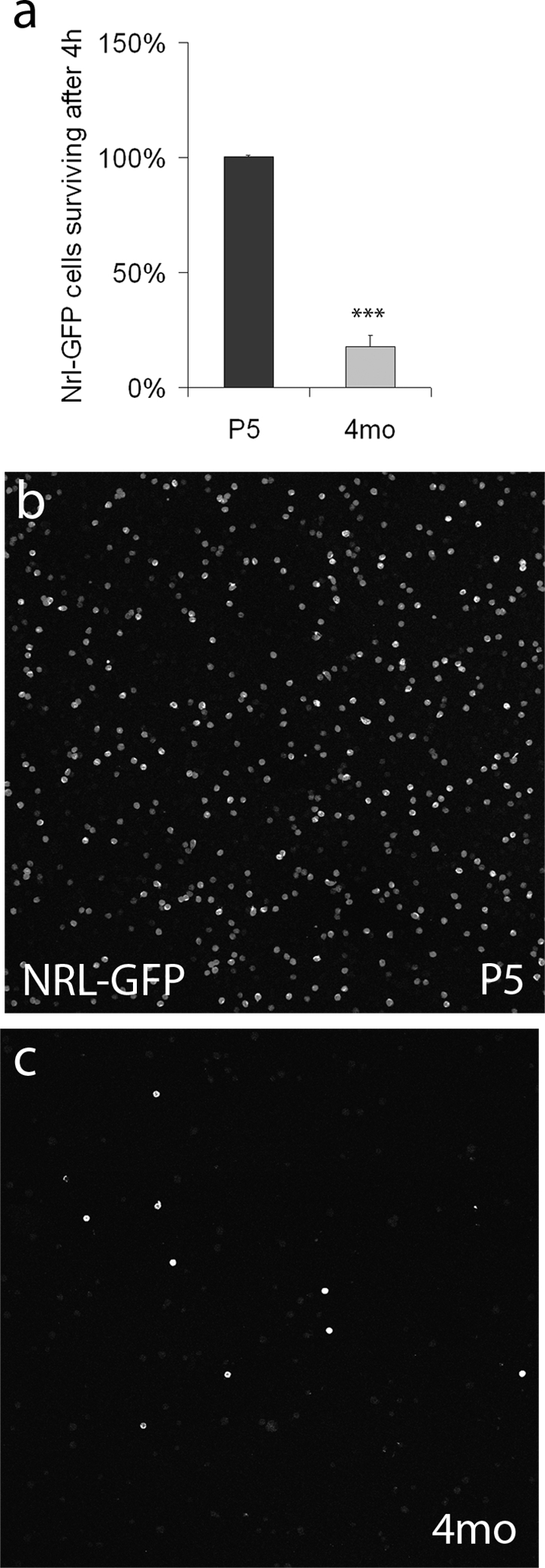
In vitro survival of immature and mature rods. (a) In vitro survival of dissociated Nrl-GFP rods. The y-axis shows the percentage of Nrl-GFP cells present at 4 hours in vitro compared to the time of plating. ***P < 0.001, Student's t-test, N = 3. (b, c) Representative fields comparing the number of surviving Nrl-GFP+ rod cells after 4 hours in vitro. P5, postnatal day 5; 4mo, 4 months.
Discussion
We have shown for the first time that mature rod photoreceptors can integrate into the adult host retina and elaborate morphology and marker expression consistent with a mature photoreceptor. This stands in contrast to recent reports that have found an inability of mature photoreceptors to integrate into the outer nuclear layer.10 On the other hand, a study carried out almost 20 years ago reported that 1- to 2-month-old donor photoreceptors could survive up to 1 month in the subretinal space and develop an outer segment and synapse-like morphology in C3H (rd/rd) mice.8
Our results show that mature rods are capable of integrating into the retina at a rate comparable to immature cells, but that the transplant failure rate is much higher. The higher failure rate for transplantation success may explain why previous groups were not able to successfully transplant cells from donors older than 1 week of age. There is a clear difference in the ability of neonatal and adult photoreceptors to survive the dissociation and isolation protocol; early postnatal rods have excellent tolerance for dissociation, while the majority of adult rods die within a few hours after isolation. Together, these results suggest that the higher failure rate in the transplantation of mature rods may be related to reduced survival of the adult donor cells during the transplantation procedures.
The fact that we find comparable numbers of integrated cells in mature and neonatal donors is surprising in light of the dramatic differences in cell survival between these groups found from the in vitro experiments. One possible explanation for this apparent discrepancy is that the integration of cells into the outer nuclear layer might re-establish trophic support to the transplanted cells, perhaps by placing them back in contact with the Müller glia.27 Overall, however, the numbers of integrated cells in both young and old donors is low; this has been observed in other similar studies where <1% of the total transplanted cells integrated.10,13 There are several possible explanations for the low efficiency of integration, including efflux of the transplanted cells at the time of injection, barriers to migration of the rods past the outer limiting membrane,27 and death of the transplanted cells caused by immune rejection.28
We have shown that mature photoreceptors are able to regenerate their complete cellular structure when grafted into the intact host retina. Compared to other neurons, photoreceptors may have a greater potential for cellular remodeling because their outer segment discs are shed and regenerated daily.29,30 In addition to regenerating their mature structure, the transplanted rod photoreceptors also make apparently normal synaptic connections with the host retinal circuitry. This is consistent with previous studies showing that dissociated adult salamander retinal cells can re-establish synapses in vitro.31 In addition, in vivo imaging studies have shown that selective axonal sprouting, accompanied by synapse formation and elimination, continues in the adult mammalian central nervous system.32–34 Experience-dependent dendritic remodeling has also been shown by in vivo imaging of the adult visual cortex.35,36 This suggests that synaptic remodeling of the adult central nervous system is more ubiquitous than previously thought, and may also occur in seemingly static circuits like those of the outer plexiform layer.
Although we presume that the majority of the cells we transplant that integrate into the ONL and differentiate as rod photoreceptors were actually rod photoreceptors in the donor retinas, there is a possibility that Müller glia, which were also transplanted, transdifferentiate into photoreceptors after transplantation. Several groups have shown that mammalian Müller glia can re-enter the cell cycle.37 However, in the adult mouse in vivo, these differentiated into amacrine cells but not into photoreceptors.38 Furthermore, MacLaren et al.10 have used BrdU injections after transplantation and found that integrated photoreceptors from early postnatal donors had all been postmitotic at the time of transplant. To our knowledge, no study has found that Müller glia can give rise to cells with the expression of mature photoreceptor markers and morphology like that shown in this or other transplantation studies. Therefore, we conclude that the adult integrated cells are most likely to be postmitotic photoreceptors from the donor retina.
We have shown that the lower integration potential of adult photoreceptors is not related to a lack of plasticity but is more likely caused by a decline in the survival of cells dissociated for transplantation. These findings lead us to the hypothesis that improved survival should increase integration of transplanted adult photoreceptors. To test this hypothesis, new methods for rescuing photoreceptors are needed. Novel protective agents can be developed by high-throughput screening,39 provided that an assay can be developed that is both naturalistic and easy to scale. Such screens may not only identify novel photoreceptors rescue compounds, but also further elucidate the mechanisms of death and survival of photoreceptors.
Acknowledgments
The authors thank members of the Reh, Wong, and Bermingham-McDonogh laboratories for technical assistance, as well as helpful discussion and constructive comments on the manuscript; and Anand Swaroop for the Nrl-GFP mice.
Footnotes
Supported by National Institutes of Health (NIH) Grants T32EY07031 (JG), 1 PO1 GM081619-01 (TAR), and TA-CBT-0608-0464-UWA-WG (TAR), and from the Foundation Fighting Blindness (Wynn/Gund Translational Award (TAR).
Disclosure: J. Gust, None; T.A. Reh, None.
References
- 1. Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vedula SS, Krzystolik MG. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2008;2:CD005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. West EL, Pearson RA, MacLaren RE, Sowden JC, Ali RR. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009;175:3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu G, Seiler MJ, Mui C, et al. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res. 2005;80:515–525 [DOI] [PubMed] [Google Scholar]
- 7. Aramant R, Seiler M, Turner JE. Donor age influences on the success of retinal grafts to adult rat retina. Invest Ophthalmol Vis Sci. 1988;29:498–503 [PubMed] [Google Scholar]
- 8. Gouras P, Du J, Kjeldbye H, Kwun R, Lopez R, Zack DJ. Transplanted photoreceptors identified in dystrophic mouse retina by a transgenic reporter gene. Invest Ophthalmol Vis Sci. 1991;32:3167–3174 [PubMed] [Google Scholar]
- 9. Gouras P, Du J, Kjeldbye H, Yamamoto S, Zack DJ. Reconstruction of degenerate rd mouse retina by transplantation of transgenic photoreceptors. Invest Ophthalmol Vis Sci. 1992;33:2579–2586 [PubMed] [Google Scholar]
- 10. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207 [DOI] [PubMed] [Google Scholar]
- 11. Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16:197–205 [DOI] [PubMed] [Google Scholar]
- 12. Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartsch U, Oriyakhel W, Kenna PF, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86:691–700 [DOI] [PubMed] [Google Scholar]
- 14. Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319 [DOI] [PubMed] [Google Scholar]
- 15. Akimoto M, Cheng H, Zhu D, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci U S A. 2006;103:3890–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210 [DOI] [PubMed] [Google Scholar]
- 17. Kubota R, Hokoc JN, Moshiri A, McGuire C, Reh TA. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002;134:31–41 [DOI] [PubMed] [Google Scholar]
- 18. Hicks D, Barnstable CJ. Different rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J Histochem Cytochem. 1987;35:1317–1328 [DOI] [PubMed] [Google Scholar]
- 19. Rex TS, Fariss RN, Lewis GP, Linberg KA, Sokal I, Fisher SK. A survey of molecular expression by photoreceptors after experimental retinal detachment. Invest Ophthalmol Vis Sci. 2002;43:1234–1247 [PubMed] [Google Scholar]
- 20. Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci U S A. 2005;102:3301–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishida A, Furukawa A, Koike C, et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263 [DOI] [PubMed] [Google Scholar]
- 22. Fossat N, Le Greneur C, Beby F, et al. A new GFP-tagged line reveals unexpected Otx2 protein localization in retinal photoreceptors. BMC Dev Biol. 2007;7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solovei I, Kreysing M, Lanctot C, et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–368 [DOI] [PubMed] [Google Scholar]
- 24. Sampath AP, Strissel KJ, Elias R, et al. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–420 [DOI] [PubMed] [Google Scholar]
- 25. Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29 [DOI] [PubMed] [Google Scholar]
- 26. Bringmann A, Iandiev I, Pannicke T, et al. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–451 [DOI] [PubMed] [Google Scholar]
- 27. Pearson RA, Barber AC, West EL, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and Z0–1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19:487–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. West EL, Pearson RA, Barker SE, et al. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28:1997–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda). 2010;25:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacLeish PR, Townes-Anderson E. Growth and synapse formation among major classes of adult salamander retinal neurons in vitro. Neuron. 1988;1:751–760 [DOI] [PubMed] [Google Scholar]
- 32. De Paola V, Holtmaat A, Knott G, et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875 [DOI] [PubMed] [Google Scholar]
- 33. Nishiyama H, Fukaya M, Watanabe M, Linden DJ. Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron. 2007;56:472–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658 [DOI] [PubMed] [Google Scholar]
- 35. Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hubener M. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci. 2008;11:1162–1167 [DOI] [PubMed] [Google Scholar]
- 36. Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105:19508–19513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. An WF, Tolliday N. Cell-based assays for high-throughput screening. Mol Biotechnol. 2010;45:180–186 [DOI] [PubMed] [Google Scholar]