Contradictory roles have been attributed to IL-1 in uveal melanoma progression. Inhibiting IL-1 with IL-1ra in experimental uveal melanoma inhibited tumor growth and modified tumor stroma including periodic acid-Schiff loops. Myeloid suppressor cells decreased.
Abstract
Purpose.
In contrast to many malignancies showing evidence that interleukin-1 (IL-1) promotes progression through effects on tumor vascularity and myeloid suppressor cell populations, in uveal melanoma there is evidence that IL-1 can inhibit progression.
Methods.
The effects of the IL-1 receptor antagonist IL-1ra against the aggressive/invasive MUM2B and the nonaggressive/noninvasive OCM1 uveal melanoma models were examined in vitro and in vivo in mouse xenografts. Vascularity and myeloid suppressor cell populations and their regulators were assessed.
Results.
In vitro, IL-1, and IL-1ra did not affect the proliferation of the uveal melanoma cells or their production of IL-1, IL-6, transforming growth factor (TGF) β, or VEGF. In vivo, IL-1ra treatment resulted in substantial growth inhibition of MUM2B tumors; less inhibition was observed against OCM1 tumors. Periodic acid-Schiff loops and CD11b+ macrophages within the tumor stroma decreased in vivo; CD31+ blood vessels were not altered. IL-1ra treatment in vivo did not affect tumor-derived IL-1, IL-6, TGF-β, or VEGF. In contrast, host IL-1β, IL-6, and tumor necrosis factor decreased. Host VEGF was not altered. Intratumoral IL-12(p40) and CXCL10, markers of host M1 polarization, increased, and intratumoral arginase and CD206, markers of myeloid-derived suppressor cells (MDSC) and M2 macrophage polarization, decreased. IL-1ra treatment in vivo also reduced splenic CD11b+Gr1+ MDSC.
Conclusions.
IL-1 may play a role in promoting uveal melanoma progression. Inhibiting IL-1 with IL-1ra inhibits tumor growth in vivo but not in vitro. Tumor stroma is modified, myeloid suppressor cells are reduced, and M1 macrophage polarization is increased in vivo.
Metastatic disease will develop in as many as 40% of patients with uveal melanoma, the prognosis of which is poor; the median survival is approximately 6 months. Chemotherapeutics used to treat cutaneous melanoma rarely produce durable responses in patients with uveal melanoma, and new systemic treatments are needed.1 Interleukin (IL)-1, an endogenous mediator of acute and chronic inflammatory conditions, has demonstrated antitumor activity, including the ability to enhance antitumor immune responses and chemotherapy cytotoxicity and to inhibit tumor migration and invasion.2–4 There is accumulating evidence, however, that both IL-1 isoforms (IL-1α and IL-1β) may play roles in tumor development, invasion, angiogenesis, and metastases, either directly or indirectly through the induction of other cytokines (see Ref. 5 for review). IL-1 may also contribute to the ability of tumors to escape immune surveillance by promoting M2-polarized macrophages and myeloid-derived suppressor cells (MDSC).6–9
Although several studies support inhibiting IL-1 in cancer therapy, little information is available regarding the role of IL-1 in uveal melanoma. During inflammatory responses in the eye, IL-1 is produced by macrophages and corneal cells and promotes a number of processes that may be tumor promoting, including angiogenesis.10 Among the transcripts found to be upregulated in the progression from intraocular to metastatic uveal melanoma was that of the IL-1 receptor.11 There is also evidence that providing IL-1 may be therapeutic in uveal melanoma. IL-1 has been shown to inhibit uveal melanoma migration and invasion.4 Gene expression for the IL-1 receptor accessory protein essential for IL-1 signaling is underexpressed in uveal melanoma manifesting monosomy 3, which is associated with metastasis.12 Moreover, there is evidence that IL-1 also does not promote, but rather abrogates, the immune-privileged nature of the ocular environment implicated in protecting uveal melanoma from destruction by immune effectors.13
A naturally occurring IL-1 antagonist, IL-1ra, competitively blocks IL-1α and IL-1β at the receptor level. A human recombinant IL-1ra is used clinically to treat patients with rheumatoid arthritis, and its application to many ocular inflammatory diseases is also under investigation.14–16 Human recombinant IL-1ra has been shown to inhibit the development and growth of metastases in several animal tumor models, including mouse B16 melanoma and human cutaneous melanoma xenografts.9,17–20 Anti-inflammatory drugs are being tested to lessen the toxicity of plaque radiotherapy in patients with uveal melanoma.21 Given this and the potential contradictory roles of IL-1, we examined the effects of IL-1ra in two established uveal melanoma models, the highly invasive/aggressive MUM2B model and the poorly invasive/aggressive OCM1 model (see Ref. 22 for review). We focused on the modulation of tumor vascularity and myeloid suppressor cell populations.
Materials and Methods
Cell Lines and Animals
MUM2B and OCM1 human uveal melanoma cell lines were maintained in Dulbecco's modified Essential medium (DMEM) with 10% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin (Mediatech, Herndon, VA).23 The cultures were grown at 37°C in 5% CO2 to confluence, passaged by treatment with 0.05% trypsin in EDTA at 37°C, and washed in media before being centrifuged at 200g for 10 minutes to form a pellet. Athymic NCR male nu/nu mice 4 to 6 weeks of age were obtained from Taconic Farms (Hudson, NY) and were fed a commercial diet and water ad libitum. All experimental procedures on the animals were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The animal use and care protocol was approved by the Institutional Animal Use and Care Committee.
Reagents
Human recombinant IL-1ra (Amgen, Thousand Oaks, CA), IL-1β (eBiosciences, Inc., San Diego, CA), and temozolomide (Schering Corporation, Kenilworth, NJ) were purchased.
Tumor Model
Tumors were established by injecting 107 tumor cells in 100 μL serum-free DMEM subcutaneously into a flank. IL-1ra was administered intraperitoneally. Tumor size was measured bidimensionally with calipers every 2 to 3 days, and tumor volume was calculated by the formula (length × width2) ÷ 2. Mice were euthanatized when tumors reached 2000 mm3.
Cell Proliferation
A colorimetric assay (CelTiter 96 AQueous Non-Radioactive Cell Proliferation Assay; Promega, Madison, WI) was used to examine cell proliferation in vitro. Inner salt (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium [MTS]) was added to 96-well cell cultures, and absorbance was read at 492 nm as recommended by the manufacturer. Data represent mean percentage proliferation ([experimental absorbance − background absorbance]/[absorbance of control cultures − background absorbance] × 100%]). Controls of drug vehicle alone (dimethyl sulfoxide) did not exhibit proliferation changes compared with cells in culture medium alone.
Flow Cytometry
Cells were washed twice in PBS/1% bovine serum albumin plus 0.05% sodium azide and stained for 30 minutes on ice with phycoerythrin-conjugated monoclonal antibodies specific for CD11b and Gr1 (BD Biosciences, San Jose, CA). Cells were first preincubated for 20 minutes with rabbit IgG blocking buffer to mask nonspecific binding sites and then further incubated with the indicated antibodies or an isotype control antibody for 30 minutes at 4°C. The cells were subsequently washed with PBS containing 2% FBS and fixed with 1% buffered formalin in PBS. Cells were then analyzed by two-color flow cytometry using a flow cytometer (EPICS Altra; Beckman Coulter, Fullerton, CA), as previously described.23
Tumor RNA
RNA from cell lines in vitro was obtained using a purification method (RNeasy; Qiagen, Valencia, CA) according to the manufacturer's instructions. Dissected tumors grown in vivo were placed in tissue storage reagent (RNAlater; Ambion, Austin, TX) and stored at 4°C. RNA was then extracted (RNeasy; Qiagen) and stored at −80°C.
Quantitative Real-Time Polymerase Chain Reaction
RNA was analyzed with a PCR system (ABI 7500 Fast Real-Time PCR; Applied Biosystems, Foster City, CA) as previously described.23 Prestandardized primers and probes (TaqMan; Applied Biosystems) for human IL-1β, IL-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, and vascular endothelial growth factor (VEGF) and for mouse IL-1β, IL-6, IL-12(p40), TNF-α, VEGF, arginase (Arg1), CD206 (Mrc1), and CXCL10 were used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), human or mouse, was used as the endogenous control. Reverse transcription and PCR were accomplished using a one-step protocol and a mix (TaqMan Universal Master Mix; Applied Biosystems). Ct values were determined, and the relative number of copies of mRNA (RQ) was calculated using the ΔΔCt method. Differences in RQ of 0.5 log were considered significant.
Enzyme-Linked Immunosorbent Assay
ELISA kits (R&D Systems, Minneapolis, MN) were used to assay the production of IL-1β, IL-6, TGF-β, TNF-α, and VEGF according to the methods recommended by the manufacturer.
Immunohistochemistry
Standard techniques using antibodies to mouse CD11b, CD31, CD140b, (BD Biosciences), α-smooth muscle actin (ASMA; Invitrogen, Carlsbad, CA), and biotinylated secondary rabbit antibody (anti-rat IgG), followed by streptavidin-biotin peroxidase complex, were used.23 Periodic acid Schiff (PAS) staining was performed using published methods that involve staining tumor sections with PAS reagent without hematoxylin staining.24 The intensity of immunohistochemical reactions was determined with a digital imaging system (Image Analysis System; Leica, Wetzlar, Germany), and the results were quantified (Image Pro, version 6.2; Media Cybernetics, Bethesda, MD), as previously described.23 Images of 10 representative fields were captured with a charge-coupled device camera (Retiga EXi Fast 1394; QImaging, Surrey, BC) connected to a microscope (DMI 4000B; Leica) at a magnification of ×100 and saved as TIFF files (QWin Plus version 3 software; Leica). Images were analyzed (Image-Pro Plus version 6.2 software; Media Cybernetics) using the count/size function. Positive staining in each image was measured, and its ratio to total area of each image was calculated and expressed as mean relative stain area ± SD.
Statistical Analysis
Standard error of the mean (SEM) or standard deviation (SD) for each set of measurements was calculated and represented as y-axis error bars on each graph. Tumor volume data were analyzed using ANOVA. Dose-effect graphs were generated using dose effect analyzer software (CalcuSyn; Biosoft, Ferguson, MO). Synergy or antagonism was also determined (CalcuSyn; Biosoft), quantified by the combination index (CI).25 CI = 1 indicates an additive effect, CI < 1 indicates synergy, and CI > 1 indicates antagonism. Results are reported for the mutually exclusive assumption of modes of activity; however, applying the alternative assumption showed the same results. Other parameters were analyzed with two-sided Student's t-test. P < 0.05 was considered statistically significant.
Results
IL-1ra Does Not Directly Affect Uveal Melanoma Proliferation or Cytokine Production In Vitro but Does Inhibit Growth In Vivo
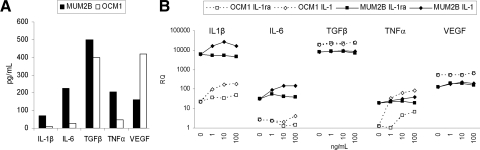
IL-1 affects tumor vascularity and myeloid suppressor cells both directly and indirectly through the induction of several cytokines.5,8 The expression of IL-1 and other cytokines implicated in the development of tumor vascularity and myeloid suppressor cells was examined first (Fig. 1). The aggressive MUM2B cell lines produced more IL-1β as well as IL-6, TGF- β, and TNF-α. In contrast, the nonaggressive OCM1 cells produced more VEGF (Fig. 1A). Exposure to IL-1ra in vitro had no effects on the production of these cytokines. As a control for these studies, tumor cells were also exposed to IL-1 in vitro. This resulted in up to log-fold increases in IL-6 and TNF-α in both cell lines; VEGF expression was not altered (Fig. 1B).
Figure 1.
Effects on cytokine production in vitro. (A) Baseline production by MUM2B and OCM1 cells of cytokines as determined by ELISA. Data represent means for duplicate samples. (B) MUM2B and OCM1 cells were cultured in vitro for 24 hours without and with 1, 10, and 100 ng/mL IL-1 or IL-1ra. Relative cytokine mRNA expression was assessed by qRT-PCR. Data represent means of two different cultures.
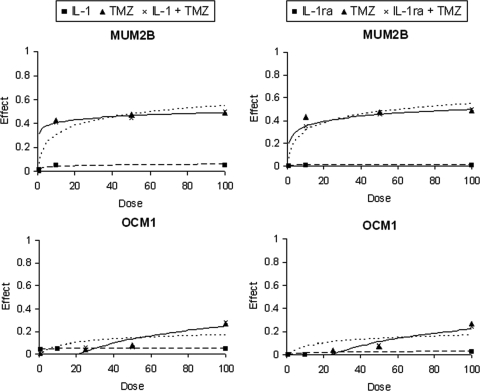
Cells were cultured with a range of concentrations of IL-1 and IL-1ra in vitro to examine effects on proliferation (Fig. 2). IL-1 has been shown to sensitize tumors to the cytotoxic effects of several cytotoxic chemotherapeutics in vitro,3 and cells were also cultured with the combination of IL-1 or IL-1ra and the chemotherapeutic, temozolomide. IL-1 and IL-1ra did not manifest antiproliferative activity against either cell line. As had been previously observed, only MUM2B, which did not express O6-methylguanine-DNA-methyltransferase, demonstrated sensitivity to temozolomide.23 CI values were approximately 1, indicating that neither IL-1 nor IL-1ra altered the activity of temozolomide.
Figure 2.
(left) Effects on proliferation in vitro. MUM2B and OCM1 cells were cultured in vitro for 72 hours with 0 to 100 ng/mL IL-1 (■, dashed line), 0 to 100 μg/mL temozolomide (TMZ; ▴, dotted line), or IL-1 and TMZ at these concentrations at a constant ratio (×, solid line) and (right) with IL-1ra (■, dashed line), TMZ (▴, dotted line), or IL-1ra and TMZ at a constant ratio (×, solid line). Proliferation of triplicate samples was assessed with MTS. Dose-effect plots, where the effect is the fractional inhibition of proliferation, are displayed and represent means for two different cultures.
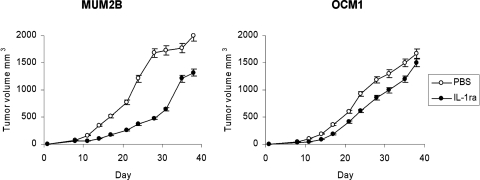
The effects of IL-1ra on tumor growth in vivo were then examined (Fig. 3). A dose and schedule of IL-1ra that is effective in mouse arthritis models and that mimics current clinical application were administered.26 Substantial MUM2B tumor growth inhibition was observed and compared with that in untreated controls (P = 0.001). OCM1 tumor growth was inhibited compared with that in untreated controls (P = 0.02), but not to the extent seen in MUM2B (P = 0.02).
Figure 3.
Effects on tumor growth in vivo. MUM2B and OCM1 cells were implanted subcutaneously on day 1 into mice. Groups of mice were treated with PBS or with IL-1ra at 1 mg/mouse/d on days 1 to 7. Data represent mean ± SEM; n = 10 mice per group.
IL-1ra Modifies Uveal Melanoma Stroma In Vivo
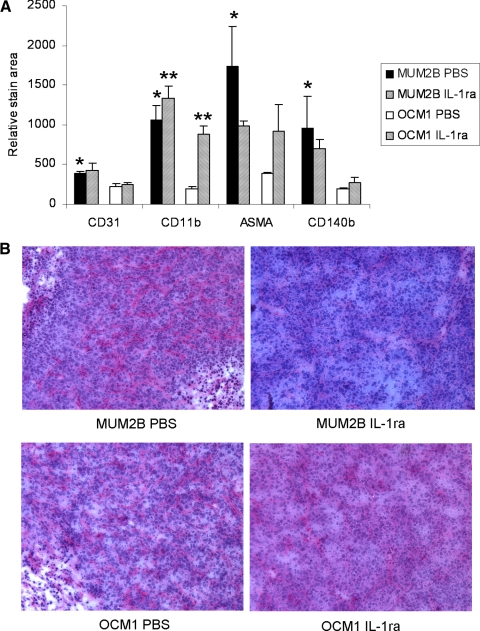
Given the lack of direct antitumor activity in vitro, studies were performed to examine the possibility that antitumor activity of IL-1ra in vivo was indirect and mediated by effects on tumor stroma. Standard immunohistochemical techniques were used to examine the effects in vivo of IL-1ra on the cellular infiltrate of uveal melanoma tumors (Fig. 4A). The stroma of MUM2B and OCM1 tumors varied with MUM2B, demonstrating greater infiltration of CD11b+ macrophages, CD31+ blood vessels, CD140b+ fibroblasts and pericytes, and ASMA+ pericytes, which are cells implicated in sustaining tumor growth (all P < 0.05). The frequency of CD11b+ cells significantly decreased intratumorally in both MUM2B and OCM1 tumors in response to IL-1ra (P < 0.01). Decreases in other cell types were not significant. As had been previously noted, on PAS staining MUM2B xenografts exhibited randomly oriented staining with many cross-linking parallel channels and some loops (Fig. 4B).27 IL-1ra treatment decreased this staining pattern. The OCM1 tumors showed no PAS staining or some perivascular staining around normal vessels, but IL-1ra had no effect on this staining pattern.
Figure 4.
Effects on cellular infiltrate in vivo. MUM2B and OCM1 cells were implanted subcutaneously on day 1 into mice. Groups of mice were treated with IL-1ra at 1 mg/mouse/d or with PBS on days 6 to 10. Tumors were harvested on day 10. (A) Infiltrating cell phenotypes were assessed by immunohistochemistry. Data represent mean ± SD; n = 4 mice per group. *P < 0.05 MUMB2B PBS versus OCM1 PBS. **P < 0.05 IL-1ra versus PBS. (B) PAS staining of tumors.
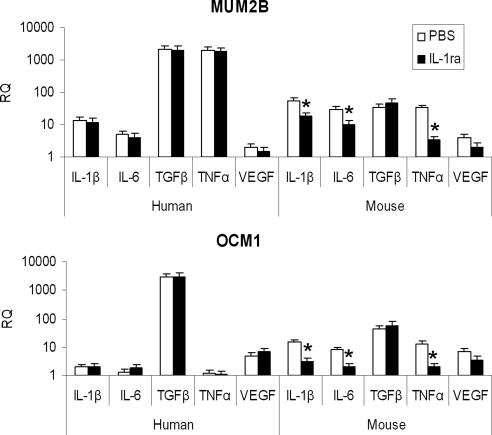
The effects of IL-1ra on the expression of intratumoral cytokines in vivo were examined (Fig. 5). The dose and schedule of IL-1ra applied as described were used, and the human, tumor-derived cytokines were examined in vitro (Fig. 1) were evaluated. The stroma of the xenografts studied was mouse-derived; to examine the changes in the stroma, mouse IL-1β, IL-6, TGF-β, TNF-α, and VEGF production were also examined. Expression of tumor-derived cytokine mRNA in vivo paralleled those observed in vitro. MUM2B tumors were characterized by >0.5 log more (human) IL-1, IL-6, TGF-β, and TNF-α transcripts. OCM1 tumors were characterized by >0.5 log more (human) VEGF. Although in vitro IL-1ra had no direct effect on human uveal melanoma cell cytokine production (Fig. 1), in vivo it decreased the expression of mouse IL-1β, IL-6, and TNF-α (Fig. 5; all P < 0.05). VEGF levels, however, were not significantly altered.
Figure 5.
Effects on intratumoral cytokines in vivo. MUM2B and OCM1 cells were implanted subcutaneously on day 1 into mice. Groups of mice were treated with IL-1ra at 1 mg/mouse/d or with PBS on days 6 to 10. Tumors were harvested on day 10. Human and mouse relative cytokine mRNA levels (RQ) were assessed by qRT-PCR. Data represent mean ± SD; n = 4 mice per group. *P < 0.05, IL-1ra versus PBS.
IL-1ra Decreases Uveal Melanoma–Associated Myeloid Suppressor Cells
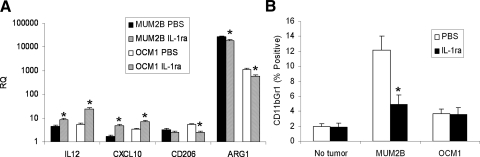
The effects of IL-1ra on intratumoral myeloid suppressor cell activity in vivo were examined using a PCR-based method. (Fig. 6A). Both the decreases in arginase, a product of MDSC and M2-polarized macrophage,28,29 and CD206, a marker of M2-polarization,30 and the increases in IL-12(p40) and in CXCL10, markers of M1-polarization,30 observed in response to IL-1ra (all P < 0.05) were consistent with a decrease in MDSC and M2 macrophage polarization and an increase in M1 polarization. Whether IL-1ra could modulate systemic CD11b+Gr1+ MDSC was also examined. The initial studies were performed using spleens harvested at day 10 from mice with MUM2B and OCM1 tumors established as described. These spleens did not manifest a significant increase in the frequency of MDSC, and IL-1ra treatment, using the same dose and the 5-day schedule, had no effect (data not shown). A similar study was then performed harvesting tumors at day 17 after the 5-day course of IL-1ra, when tumors were larger, with volumes >1000 mm3 (Fig. 6B). Increases in splenic MDSC were observed, but only in mice bearing MUM2B tumors. The frequency of splenic MDSC decreased with IL-1ra (P < 0.001).
Figure 6.
Effects on myeloid suppressor cell populations in vivo. MUM2B and OCM1 cells were implanted subcutaneously into mice. Groups of mice were treated with IL-1ra at 1 mg/mouse/d on days on days 13 to 17, and spleens were harvested on day 17. Spleens from age-matched mice not bearing tumors were also harvested (No tumor). (A) Relative intratumoral arginase (Arg), CD206, IL-12(p40) (IL-12), and CXCL10 mRNA levels were assessed by qRT-PCR. Data represent mean ± SD; n = 4 mice per group. (B) Splenic CD11b+Gr1+ MDSC frequencies were assessed by flow cytometry. Data represent mean ± SD; n = 4 mice per group. *P < 0.05, IL-1ra versus PBS.
Discussion
IL-1 has been shown to play a central role in metastatic tumor growth in cutaneous melanoma.17–20 Furthermore, serum concentrations of both isoforms are elevated in patients with high-risk cutaneous melanoma.31 In uveal melanoma, there is evidence suggesting that IL-1 can both promote and inhibit tumor progression.4,10–12 Although a case report did indicate high levels of IL-1β in a patient, the clinical significance of IL-1 production in uveal melanoma is not known.32 We studied uveal melanoma models and found that IL-1 may play a role in tumor progression. Inhibiting IL-1 with IL-1ra inhibits uveal melanoma tumor growth, modifies the tumor stroma and immune response (myeloid suppressor cell populations in particular), and may have a role in treatment.
We found that the more aggressive and metastatic uveal melanoma cells (MUM2B) produced more IL-1 than the less aggressive primary cells (OCM1). MUM2B also produced more IL-6 and TNF-α, other cytokines implicated in the progression of uveal melanoma.33 IL-1ra had no effect on the production of these cytokines by the tumor cells, either in vitro (Fig. 1) or in vivo (Fig. 5). Unexpectedly, OCM1 cells and tumors, which were less vascular than MUM2B, produced more VEGF, another cytokine implicated in uveal melanoma progression.34 Levels of VEGF were not altered by IL-1ra. IL-1 may serve as an autocrine growth factor for some tumors, and IL-1ra has been shown to directly inhibit the proliferation of a mouse skin carcinoma.35,36 In contrast, IL-1ra has been shown to block the IL-1–mediated reduction in growth in vitro of human prostatic and hepatic carcinoma and glioblastoma cells.37–39 We did not observe any direct effects of IL-1ra on uveal melanoma cell proliferation in vitro. These results parallel a previous report evaluating two anti-inflammatory steroids, triamcinolone acetonide and anecortave acetate, which also had no effect on the growth or production of VEGF by human uveal melanoma cell lines in vitro.40 Although inactive in vitro, IL-1ra did manifest antitumor activity in vivo. This was most evident in the MUM2B model, which produced more IL-1. It should be noted that there is extensive sequence homology between mouse and human IL-1, and mouse and human IL-1 show cross-species activities.41,42
IL-1ra decreased PAS+ loops, networks lacking endothelium termed vasculogenic mimicry, that have previously been identified in uveal melanoma, including MUM2B tumors, and other cancer types. IL1B was one of the genes found to be upregulated in uveal melanoma-forming networks, which are associated with a poor prognosis, in a gene array study.43 Inhibitors of matrix metalloproteinase and cadherin have been shown to inhibit the formation of these loops in uveal melanoma models.44–47 In an in vitro breast cancer model, the anti-inflammatory cyclo-oxygenase (COX)-2 inhibitor celecoxib was shown to inhibit PAS+ vasculogenic mimicry.48 We did not observe significant changes in the blood vessels of macroscopic tumors. When applied in the setting of microscopic tumors, IL-1ra has been shown to reduce vascularization.19 We did not observe effects on tumor blood vessel density or in tumor-infiltrating fibroblasts and pericytes when IL-1ra was applied in the setting of established, macroscopic tumor. Furthermore, we did not observe the decrease in tumor VEGF production in vivo previously reported with IL-1ra.20
Although proinflammatory, IL-1 also promotes M2-polarization and the development of MDSC, which suppress the immune response by a variety of mechanisms.7,8 The infiltration of CD11b+CD68+ macrophages in patients with uveal melanoma has been associated with an unfavorable prognosis.49–51 An immunosuppressive role of tumor-associated CD11b+ cells has been suggested in transplantable uveal melanoma models in mice.52 It has recently been reported that in aged mice, the outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages.53 MDSC have recently been shown to expand in the blood of patients with uveal melanoma.54 We found that MUM2B tumors were associated with increases in myeloid suppressor cell populations. We observed a decrease in M2 macrophage polarization and MDSC with IL-1ra treatment. This was evident systemically in the MUM2B model, with the decrease in splenic CD11b+Gr1+ MDSC, and intratumorally in both MUM2B and OCM1 models, with the reduced infiltration of CD11b+ cells, the reduced levels of arginase and CD206, and the increased levels of IL-12(p40) and CXCL10. The ability of IL-1ra to decrease levels of other cytokines implicated in the generation of myeloid suppressor cell populations, such as IL-6, and TNF-α, might have contributed to these alterations. Although the expansion and function of MDSC does not require T cells,55 these xenograft studies do have limitations, and further study in other models will be necessary.
IL-1ra has demonstrated benefits in experimental models of keratitis, corneal neovascularization, conjunctivitis, uveitis, and corneal graft rejection.14–16,56–58 The results of our studies support the testing of IL-1ra in patients with uveal melanoma. Direct antitumor effects and indirect antitumor effects on stroma and immune suppressor mechanisms could potentially be exploited. Other anti-inflammatory drugs, including the nonselective COX inhibitor nepafenac, which is used clinically as an adjunct to cataract surgery, have demonstrated antitumor activity in other uveal melanoma models.59 Anti-inflammatory drugs are currently being applied clinically in patients with uveal melanoma to lessen the macular edema and retinitis that complicate plaque radiotherapy.21 IL-1 appears to act relatively “upstream” in the molecular cascade, leading to inflammation and to tumor promotion, and its inhibition by IL-1ra may have clinical applications in patients with uveal melanoma.
Acknowledgments
The authors thank Susan Achberger for excellent technical assistance.
Footnotes
Supported in part by National Institutes of Health Grant R01CA118660.
Disclosure: P.L. Triozzi, None; W. Aldrich, None; A. Singh, None
References
- 1. Singh AD, Borden EC. Metastatic uveal melanoma. Ophthalmol Clin North Am. 2005;18:143–150 [DOI] [PubMed] [Google Scholar]
- 2. Neville ME, Pezzella KM. Anti-tumour effects of interleukin 1 beta: in vivo induction of immunity to B16 melanoma, a non-immunogenic tumour. Cytokine. 1994;6:310–317 [DOI] [PubMed] [Google Scholar]
- 3. Johnson CS. Interleukin-1: therapeutic potential for solid tumors. Cancer Invest. 1991;11:600–608 [DOI] [PubMed] [Google Scholar]
- 4. Woodward JK, Elshaw SR, Murray AK, et al. Stimulation and inhibition of uveal melanoma invasion by HGF, GRO, IL-1alpha and TGF-beta. Invest Ophthalmol Vis Sci. 2002;43:3144–3152 [PubMed] [Google Scholar]
- 5. Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;2:387–408 [DOI] [PubMed] [Google Scholar]
- 6. Song X, Krelin Y, Dvorkin T, et al. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8208 [DOI] [PubMed] [Google Scholar]
- 7. Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290 [DOI] [PubMed] [Google Scholar]
- 8. Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Triozzi PL, Aldrich W. Effects of interleukin-1 receptor antagonist and chemotherapy on host-tumor interactions in established melanoma. Anticancer Res. 2010;30:345–354 [PubMed] [Google Scholar]
- 10. Oh H, Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1891–1898 [PubMed] [Google Scholar]
- 11. Marshall JC, Nantel A, Blanco P, Ash J, Cruess SR, Burnier MN., Jr Transcriptional profiling of human uveal melanoma from cell lines to intraocular tumors to metastasis. Clin Exp Metastasis. 2007;24:353–362 [DOI] [PubMed] [Google Scholar]
- 12. Tschentscher F, Hüsing J, Hölter T, et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res. 2003;63:2578–2584 [PubMed] [Google Scholar]
- 13. Dana MR, Dai R, Zhu S, Yamada S, Streilein JW. Interleukin-1 receptor antagonist suppresses Langerhans cell activity and promotes ocular immune privilege. Invest Ophthalmol Vis Sci. 1998;39:70–77 [PubMed] [Google Scholar]
- 14. Keane-Myers AM, Miyazaki D, Liu G, Dekaris I, Ono S, Dana MR. Prevention of allergic eye disease by treatment with IL-1 receptor antagonist. Invest Ophthalmol Vis Sci. 1999;40:3041–3046 [PubMed] [Google Scholar]
- 15. Lim WK, Fujimoto C, Ursea R, et al. Suppression of immune-mediated ocular inflammation in mice by interleukin 1 receptor antagonist administration. Arch Ophthalmol. 2005;123:957–963 [DOI] [PubMed] [Google Scholar]
- 16. Rosenbaum JT, Boney RS. Activity of an interleukin 1 receptor antagonist in rabbit models of uveitis. Arch Ophthalmol. 1992;110:547–549 [DOI] [PubMed] [Google Scholar]
- 17. McKenzie RC, Oran A, Dinarello CA, Sauder DN. Interleukin-1 receptor antagonist inhibits subcutaneous B16 melanoma growth in vivo. Anticancer Res. 1996;16:437–441 [PubMed] [Google Scholar]
- 18. Voronov E, Shouval DS, Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavi G, Voronov E, Dinarello CA, Apte RN, Cohen S. Sustained delivery of IL-1 Ra from biodegradable microspheres reduces the number of murine B16 melanoma lung metastases. J Control Release. 2007;123:123–130 [DOI] [PubMed] [Google Scholar]
- 20. Elaraj DM, Weinreich DM, Varghese S, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–1096 [DOI] [PubMed] [Google Scholar]
- 21. Horgan N, Shields CL, Mashayekhi A, et al. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: a randomized controlled trial. Ophthalmology. 2009;116:1383–1390 [DOI] [PubMed] [Google Scholar]
- 22. Folberg R, Kadkol SS, Frenkel S, et al. Authenticating cell lines in ophthalmic research laboratories. Invest Ophthalmol Vis Sci. 2008;49:4697–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Triozzi PL, Aldrich W, Dombos C. Differential effects of imatinib mesylate against uveal melanoma in vitro and in vivo. Melanoma Res. 2008;18:420–430 [DOI] [PubMed] [Google Scholar]
- 24. Folberg R, Pe'er J, Gruman LM, et al. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: a matched case-control study. Hum Pathol. 1992;23:1298–1305 [DOI] [PubMed] [Google Scholar]
- 25. Chou T-C, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454 [Google Scholar]
- 26. Inoue K, Masuko-Hongo K, Okamoto M, Nishioka K. Efficacy of daily compared to intermittent administration of IL-1Ra for protection against bone and cartilage destruction in collagen-challenged mice. Clin Exp Rheumatol. 2003;21:33–39 [PubMed] [Google Scholar]
- 27. Braun RD, Abbas A. Orthotopic human choroidal melanoma xenografts in nude rats with aggressive and nonaggressive PAS staining patterns. Invest Ophthalmol Vis Sci. 2006;47:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654 [DOI] [PubMed] [Google Scholar]
- 29. Mills CD, Shearer J, Evans R, Caldwell MD. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol. 1992;149:2709–2714 [PubMed] [Google Scholar]
- 30. Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–1144 [DOI] [PubMed] [Google Scholar]
- 31. Yurkovetsky ZR, Kirkwood JM, Edington HD, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13:2422–2428 [DOI] [PubMed] [Google Scholar]
- 32. Omulecki W, Damato BE, Sekundo W, Lee WR, Toczyska-Rozentryt E, Omulecka A. Bilateral uveal melanoma presenting simultaneously. Ger J Ophthalmol. 1994;3:228–231 [PubMed] [Google Scholar]
- 33. Ijland SA, Jager MJ, Heijdra BM, Westphal JR, Peek R. Expression of angiogenic and immunosuppressive factors by uveal melanoma cell lines. Melanoma Res. 1999;9:445–450 [DOI] [PubMed] [Google Scholar]
- 34. Yang H, Jager MJ, Grossniklaus HE. Bevacizumab suppression of establishment of micrometastases in experimental ocular melanoma. Invest Ophthalmol Vis Sci. 2010;51:2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolf JS, Chen Z, Dong G, et al. IL (interleukin)-1 promotes nuclear factor-B and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res. 2001;7:1812–1820 [PubMed] [Google Scholar]
- 36. La E, Rundhaug JE, Fischer SM. Role of intracellular interleukin-1 receptor antagonist in skin carcinogenesis. Mol Carcinog. 2001;30:218–223 [DOI] [PubMed] [Google Scholar]
- 37. Hsieh TC, Chiao JW. Growth modulation of human prostatic cancer cells by interleukin-1 and interleukin-1 receptor antagonist. Cancer Lett. 1995;95:119–123 [DOI] [PubMed] [Google Scholar]
- 38. Yamada Y, Karasaki H, Matsushima K, Lee GH, Ogawa K. Expression of an IL-1 receptor antagonist during mouse hepatocarcinogenesis demonstrated by differential display analysis. Lab Invest. 1999;79:1059–1067 [PubMed] [Google Scholar]
- 39. Oelmann E, Kraemer A, Serve H, et al. Autocrine interleukin-1 receptor antagonist can support malignant growth of glioblastoma by blocking growth-inhibiting autocrine loop of interleukin-1. Int J Cancer. 1997;71:1066–1076 [DOI] [PubMed] [Google Scholar]
- 40. El Filali M, Homminga I, Maat W, van der Velden PA, Jager MJ. Triamcinolone acetonide and anecortave acetate do not stimulate uveal melanoma cell growth. Mol Vis. 2008;14:1752–1759 [PMC free article] [PubMed] [Google Scholar]
- 41. Huang JJ, Newton RC, Rutledge SJ, et al. Characterization of murine IL-1 beta: isolation, expression, and purification. J Immunol. 1988;140:3838–3843 [PubMed] [Google Scholar]
- 42. Libert C, Brouckaert P, Shaw A, Fiers W. Induction of interleukin 6 by human and murine recombinant interleukin 1 in mice. Eur J Immunol. 1990;20:691–694 [DOI] [PubMed] [Google Scholar]
- 43. Demou ZN, Hendrix MJ. Microgenomics profile the endogenous angiogenic phenotype in subpopulations of aggressive melanoma. J Cell Biochem. 2008;105:562–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seftor RE, Seftor EA, Kirschmann DA, Hendrix MJ. Targeting the tumor microenvironment with chemically modified tetracyclines: inhibition of laminin 5 gamma2 chain promigratory fragments and vasculogenic mimicry. Mol Cancer Ther. 2002;1:1173–1179 [PubMed] [Google Scholar]
- 45. Hess AR, Seftor EA, Seftor RE, Hendrix MJ. Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase (MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res. 2003;63:4757–4762 [PubMed] [Google Scholar]
- 46. Zhang S, Li M, Gu Y, et al. Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma. J Exp Clin Cancer Res. 2008;27:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cong R, Sun Q, Yang L, Gu H, Zeng Y, Wang B. Effect of Genistein on vasculogenic mimicry formation by human uveal melanoma cells. J Exp Clin Cancer Res. 2009;28:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P. Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res. 2005;7:R422–R435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Waard-Siebinga I, Hilders CG, Hansen BE, van Delft JL, Jager MJ. HLA expression and tumor-infiltrating immune cells in uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1996;234:34–42 [DOI] [PubMed] [Google Scholar]
- 50. Makitie T, Summanen P, Tarkkanen A, Kivela T. Tumor-infiltrating macrophages (CD68+ cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421 [PubMed] [Google Scholar]
- 51. Maat W, Ly LV, Jordanova ES, de Wolff-Rouendaal D, Schalij-Delfos NE, Jager MJ. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:505–510 [DOI] [PubMed] [Google Scholar]
- 52. McKenna KC, Kapp JA. Accumulation of immunosuppressive CD11b+ myeloid cells correlates with the failure to prevent tumor growth in the anterior chamber of the eye. J Immunol. 2006;177:1599–1608 [DOI] [PubMed] [Google Scholar]
- 53. Ly LV, Baghat A, Versluis M, et al. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol. 2010;185:3481–3488 [DOI] [PubMed] [Google Scholar]
- 54. McKenna KC, Beatty KM, Bilonick RA, Schoenfield L, Lathrop KL, Singh AD. Activated CD11b+ CD15+ granulocytes increase in the blood of patients with uveal melanoma. Invest Ophthalmol Vis Sci. 2009;50:4295–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J Immunol. 2001;167:4293–4302 [DOI] [PubMed] [Google Scholar]
- 56. Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea. 1998;17:403–409 [DOI] [PubMed] [Google Scholar]
- 57. Stapleton WM, Chaurasia SS, Medeiros FW, Mohan RR, Sinha S, Wilson SE. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp Eye Res. 2008;86:753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamada J, Zhu SN, Streilein JW, Dana MR. Interleukin-1 receptor antagonist therapy and induction of anterior chamber-associated immune deviation-type tolerance after corneal transplantation. Invest Ophthalmol Vis Sci. 2000;41:4203–4208 [PubMed] [Google Scholar]
- 59. Marshall JC, Fernandes BF, Di Cesare S, et al. The use of a cyclooxygenase-2 inhibitor (Nepafenac) in an ocular and metastatic animal model of uveal melanoma. Carcinogenesis. 2007;28:2053–2058 [DOI] [PubMed] [Google Scholar]