This study is the first to demonstrate the humoral and cellular immune response in humans to corneal endothelial cells from genetically engineered pigs. The results suggest that pig corneas may provide an acceptable alternative to human corneas for clinical transplantation.
Abstract
Purpose.
To compare the in vitro human humoral and cellular immune responses to wild-type (WT) pig corneal endothelial cells (pCECs) with those to pig aortic endothelial cells (pAECs). These responses were further compared with CECs from genetically engineered pigs (α1,3-galactosyltransferase gene-knockout [GTKO] pigs and pigs expressing a human complement-regulatory protein [CD46]) and human donors.
Methods.
The expression of Galα1,3Gal (Gal), swine leukocyte antigen (SLA) class I and class II on pCECs and pAECs, with or without activation by porcine IFN-γ, was tested by flow cytometry. Pooled human serum was used to measure IgM/IgG binding to and complement-dependent cytotoxicity (CDC) to cells from WT, GTKO, and GTKO/CD46 pigs. The human CD4+ T-cell response to cells from WT, GTKO, GTKO/CD46 pigs and human was tested by mixed lymphocyte reaction (MLR).
Results.
There was a lower level of expression of the Gal antigen and of SLA class I and II on the WT pCECs than on the WT pAECs, resulting in less antibody binding and reduced human CD4+ T-cell proliferation. However, lysis of the WT pCECs was equivalent to that of the pAECs, suggesting more susceptibility to injury. There were significantly weaker humoral and cellular responses to the pCECs from GTKO/CD46 pigs compared with the WT pCECs, although the cellular response to the GTKO/CD46 pCECs was greater than to the human CECs.
Conclusions.
These data provide the first report of in vitro investigations of CECs from genetically engineered pigs and suggest that pig corneas may provide an acceptable alternative to human corneas for clinical transplantation.
Although corneal transplantation is readily available in the United States and certain other regions of the developed world, the worldwide need for human donor corneas far exceeds the supply.1 The shortage is particularly severe in Asia.1,2 Furthermore, in some countries (e.g., South Africa), the shortage has been exacerbated by the high incidence of infection with the human immunodeficiency virus in the population, making donation unsafe.3 Even in the developed world, the increasing popularity of refractive surgery is likely to reduce the supply of human corneas4,5; current Eye Bank Association of America standards do not allow the use of corneas that have been subjected to surgery for full-thickness corneal transplantation (penetrating keratoplasty).
Pig corneas could provide an alternative source, because the anatomic and biomechanical properties of human and pig corneas are similar.1 The immune-privileged environment of the cornea appears to provide corneal xenogeneic grafts with some degree of protection.6,7 Indeed, corneas transplanted from wild-type (WT, i.e., unmodified) pigs into monkeys have been reported to survive for several months (>3 months) if corticosteroid is applied locally.8
Immune-mediated destruction of corneal allografts and xenografts is primarily CD4+ T-cell-mediated and targets the corneal endothelial cell (CEC),9–13 although keratocytes have also been suggested as important targets of corneal graft rejection.14,15 CD8+ T cells and NK T cells may play a role in rejection when CD4+ T cells are absent or their function is impaired.16 The immune response to corneal xenografts appears to occur almost exclusively by the indirect pathway.17 There is a resident myeloid corneal dendritic cell population that is normally MHC class II–negative, but can readily upregulate class II expression during inflammation.18 Thus, it is likely that a population of passenger leukocytes in xenogeneic corneas is involved in direct xenoantigen presentation to host T cells as well as in the alloimmune response,19 especially if a corneal graft is placed into a high-risk patient (e.g., with a neovascularized and/or inflamed host corneal bed).
The role of cytotoxic anti-donor antibodies in corneal graft rejection remains a matter of discussion.20–23 Clinical studies suggest that, in some instances, antibodies may contribute to corneal allograft failure if a high-risk recipient has been sensitized to donor alloantigens24 or if the donor-recipient combination is ABO-incompatible.23,25 Similarly, sensitization to xenoantigens has been detrimental to graft survival in rodent models of xenotransplantation.13,26,27 This effect is, at least in part, a byproduct of the T cell- and macrophage-mediated response generated to the graft.
With the current speed of advances in the genetic engineering of pigs,28,29 it is increasingly likely that these immune responses will be overcome by the transplantation of corneas from genetically engineered pigs.
The primary purpose of the present study was to compare in vitro human humoral and cellular immune responses to pig CECs (pCECs) with those to pig aortic endothelial cells (pAECs), which are the target in vascularized solid organ xenografts, and to explore whether the effect of these immune responses is reduced when CECs from genetically engineered pigs are tested. This study is the first in which CECs from such pigs have been investigated. Our results demonstrated that the human humoral and cellular immune responses to genetically engineered pCECs were greatly reduced compared with those to WT pCECs, but were not comparable to those of human CECs (hCECs).
Materials and Methods
Sources of Human Serum and Peripheral Blood Mononuclear Cells
Sera from six healthy human volunteers (including all ABO blood types) were pooled to form a single human serum reagent. The samples were obtained in accordance with the Declaration of Helsinki, with the informed consent of the subjects. Blood was immediately centrifuged at 4°C to preserve complement activity. Serum was stored at −80°C until use. Participants gave informed consent per the guidelines of the Institutional Review Board of the University of Pittsburgh. Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats from multiple human donors (Institute for Transfusion Medicine, Pittsburgh, PA).
Sources of Pig Corneas and Aortas
Corneas and thoracic aortas were excised from (1) WT pigs, (2) α1,3-galactosyltransferase gene-knockout (GTKO) pigs (that do not express the Galα1,3Gal [Gal] antigen that is the major target for human anti-pig antibodies),30 and (3) GTKO pigs transgenic for the human complement-regulatory protein CD46 (GTKO/CD46 pigs),31,32 all provided by Revivicor, Inc. (Blacksburg, VA). Three or four pigs from each type were used in these experiments. They were all of non-A blood type (O). All animal care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources of the National Institutes of Health.
Human Corneas
Corneas from deceased human subjects that were not usable for transplantation were provided by the Pittsburgh Center for Organ Recovery and Education with the approval of the University of Pittsburgh Committee for Oversight of Research Involving the Dead and in accordance with the guidelines of the Declaration of Helsinki for research involving the use of human tissue.
Corneal and Vascular Endothelial Cell Cultures
Freshly enucleated pig eyes were rinsed in PBS (Invitrogen, Carlsbad, CA) including antibiotic-antimycotic (Invitrogen). Corneoscleral rims were dissected and placed (endothelial side up) in the center-well organ culture dish (BD Biosciences, San Diego, CA). Epithelial and stromal cells were isolated and cultured as previously described.33,34
pCECs were peeled off by incubating with 0.25% trypsin-EDTA (Invitrogen) for 30 min at 37°C. pCECs were washed with RPMI medium (Invitrogen) containing 10% heat-inactivated bovine serum (Invitrogen) and cultured in medium 199 (Invitrogen) containing 10% heat-inactivated FBS (Sigma-Aldrich, St. Louis, MO), antibiotic-antimycotic, and endothelial growth factor (30 μg/mL, BD Biosciences). hCECs were isolated according to the method of Zhu and Joyce35 and cultured with reduced-serum medium (Opti-MEM I; Invitrogen) containing calcium chloride (200 μg/mL; Sigma-Aldrich), 0.08% chondroitin sulfate (Sigma-Aldrich), ascorbic acid (20μg/mL; Sigma-Aldrich), bovine pituitary extract (100 μg/mL; Biomedical Technologies, Stoughton, MA), 8% heat-inactivated FBS, antibiotic-antimycotic, and endothelial growth factor.
pAEC culture was carried out as previously described.32 All cells were cultured in collagen-I-coated 25- or 75-cm2 tissue culture flasks (BD Biosciences). Activation of subconfluent pAECs and pCECs and of hCECs was performed by culture for 48 hours in recombinant porcine IFN-γ (40 ng/mL; Serotec, Raleigh, NC) and human IFN-γ (50 ng/mL; Serotec), respectively.
Flow Cytometric Analysis
Cells were diluted to 105 cells per tube in FACS buffer (PBS [Invitrogen] containing 1% BSA and 0.1% NaN3). Surface expression of Gal, hCD46, and swine leukocyte antigen (SLA) class I and II antigens was detected by flow cytometer (BD LSR II; BD Biosciences), as previously described.32 The expression of B7 molecules (CD80/86) on the cornea was also tested by using hamster anti-mouse CD80 monoclonal antibody (clone 16–10A1; BD Biosciences), which has been reported to cross-react with pig CD8036 and human CTLA4-Ig (R&D Systems, Minneapolis, MN) followed by FITC-labeled goat anti-human IgG-Fc polyclonal antibody (Bethyl Laboratories, Montgomery, TX).
Fluorescence Microscopy
To investigate the distribution of Gal epitopes and hCD46 on the pig cornea (epithelial, stromal, and endothelial cell layers), conjunctiva and sclera, corneas from WT and genetically engineered pigs were embedded in OCT compound (Tissue-Tek; Miles Laboratories, Naperville, IL) and stored at −80°C. Frozen sections were cut in 5-μm thickness, air dried, and fixed with cold acetone for 10 min at −20°C. After acetone-dried slides were washed with PBS, the slides were blocked with serum-free protein block (Dako, Carpinteria, CA), followed by incubation with FITC-conjugated BSI-B4 lectin (5 μg/mL; Sigma-Aldrich) or FITC-conjugated anti-human CD46 antibody (Serotec) for 20 minutes at room temperature. After washing with PBS, 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) was used for nuclear staining. The distribution of Gal and hCD46 were observed under fluorescence microscopy (Nikon, Elgin, IL).
Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from the cells (TRIzol; Invitrogen) followed by DNase I treatment (Applied Biosystems, Inc. [ABI], Foster, CA), according to the manufacturer's instructions. The amount of total RNA was measured by spectrophotometry (Victor3 Multilabel Counter; Perkin Elmer, Covina, CA). Total RNA (500 ng) was subjected to first-strand cDNA synthesis in a volume of 20 μL (High Capacity cDNA Reverse Transcription Kit; ABI, RNase OUT; Invitrogen), according to the manufacturer's instructions.
PCR was performed (TaKaRa Ex Taq Hot Start Version; Invitrogen) under the following conditions: denaturation at 98°C for 10 seconds, primer annealing/elongation at 68°C for 1 minute at 35 cycles. The PCR primer sequences used and the estimated length of the PCR products were as follows: porcine class II transactivator (CIITA) (200-bp), forward 5′-GACACGGACACCATCAACTG-3′, reverse: 5′-ACCTCCACGCTCTCACTGAT-3′, and porcine GAPDH (133-bp), forward: 5′-GGGCATGAACCATGAGAAGT-3′, reverse: 5′-TGTGGTCATGAGTCCTTCCA-3′. RT-PCR products were visualized on 2% agarose gels.
Binding of Human Serum IgM and IgG to pAECs and pCECs
Binding of human xenoreactive antibodies to pig cells was measured using relative mean fluorescence intensity (MFI), as previously described.32
Complement-Dependent Cytotoxicity Assay with Chromium51
The complement-dependent cytotoxicity assay was performed as previously described.32,37 Briefly, 104 51Cr-labeled target cells suspended in RPMI medium containing 10% heat-inactivated bovine serum were loaded into each well and incubated with a non–heat-inactivated pooled human (including antibodies and complement) at various dilutions for 60 minutes at 37°C. After incubation, lysis of the target cells was detected by measuring the release of 51Cr radioactivity into the cell supernatant. Cell killing was calculated as follows: percent cytotoxicity = [(A − C)/(B − C)] × 100, where A represents the experimental release (counts per million in the supernatant from target cells incubated with serum), B is the maximum release (counts per million released from target cells lysed with 4% Triton), and C is the minimum release (counts per million in the supernatant from target cells incubated with medium only). CDC values at the varying serum concentrations were calculated, and a curve was generated for each sample.
Mixed Lymphocyte Reaction
Human CD4+ T cells as responders were isolated from PBMCs by negative selection with the CD4+ T-cell isolation kit (Kit II; Miltenyi Biotec, Auburn, CA) as previously described.38 CD4+ T-cell purity was >98% by flow cytometric analysis. Human CD4+ T cells as responders (2 × 105 cells/well) were co-cultured with irradiated (2500 cGy) pAECs, pCECs, or hCECs with/without stimulation by pIFN-γ or hIFN-γ (at responder-stimulator ratios 10:1) for 5 to 6 days. All assays were performed in serum-free medium (AIM V; Invitrogen). The optimal conditions for the MLR were determined in preliminary experiments using different stimulator:responder cell ratios and different incubation times. The cells were cultured at 37°C in 5% CO2, and [3H]thymidine (1 μCi/well) was added to each well during the last 16 hours of incubation. The cells were harvested on glass-fiber filter mats with a cell harvester and were analyzed by β-scintillation counting on a liquid scintillation counter (Perkin Elmer). The mean of triplicate results were expressed as [3H]thymidine uptake.
Statistical Methods
The statistical significance of differences was determined by Student's t- or nonparametric tests, as appropriate (Prism ver. 4; GraphPad Software, San Diego, CA). Values are presented as mean ± SEM. Differences were considered to be significant at P < 0.05.
Results
Expression of Gal and hCD46 on Cells from Pigs and Humans
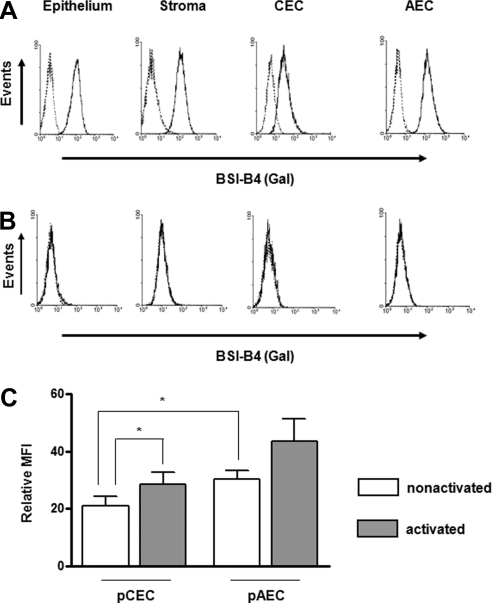
By flow cytometry, all cultured WT pig corneal cell layers including epithelium, stroma, and endothelium, as well as the pAECs, expressed Gal on their surfaces (Fig. 1A). The expression of Gal on the pCECs was lower than on other corneal cells. In contrast to WT, cultured corneal cells from GTKO pigs did not express Gal (Fig. 1B). Although the expression of Gal on the pCECs was significantly lower than on the pAECs when the cells were quiescent (not activated), there was significantly increased expression when the pCECs were activated with pIFN-γ (Fig. 1C). In contrast to the pCECs, the expression of Gal on the pAECs was not significantly increased after activation. These results indicated that Gal expression on cultured WT pCECs is low, but may be inducible under various pathologic conditions of the host bed.
Figure 1.
Expression of Gal on pCECs was significantly lower than on pAECs. For flow cytometry, cultured corneal cells (epithelium, stroma, and endothelium), and AECs from (A) WT and (B) GTKO pigs were stained with BSI-B4 to detect Gal. Dotted traces: unstained control. Results are representative of three independent experiments. (C) The expression of Gal on quiescent WT pCECs and pAECs was compared with that on activated cells (n = 6; *P < 0.05).
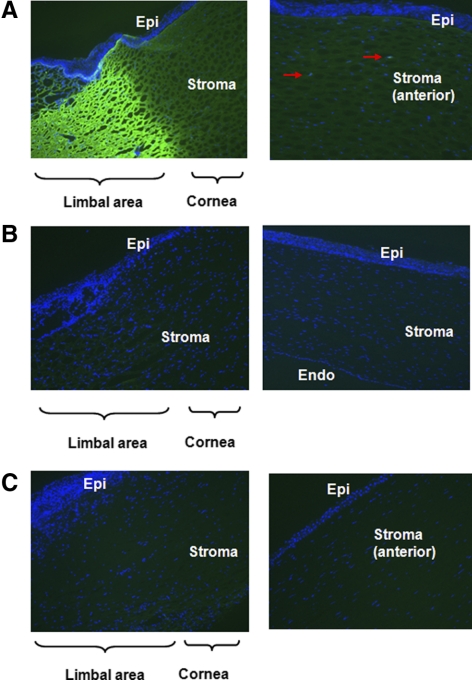
By fluorescent staining, although strong expression of Gal was found in the limbal area of the WT pig corneas, this expression gradually diminished in the central cornea (Fig. 2A, left), with minimal Gal expression in the keratocytes of the anterior stroma (Fig. 2A right). The corneal epithelium and endothelium showed no expression of Gal (not shown). Both GTKO pig (Fig. 2B) and human (Fig. 2C) corneas demonstrated an absence of Gal expression.
Figure 2.
Minimum expression of Gal in WT pig corneas. Fluorescence staining for Gal (BSI-B4-FITC: green) and nuclei (DAPI: blue) in corneas from (A) WT pigs, (B) GTKO pigs, and (C) humans was determined by fluorescence microscopy (original magnification, ×200). In WT pigs, Gal expression was mainly seen in the limbal area and gradually diminished toward the central cornea. Weakly Gal-positive cells were recognized in keratocytes (red arrows) in the anterior stroma. In contrast to WT pig corneas, there was no expression of Gal in GTKO pig and human corneas. Results are representative of three independent experiments.
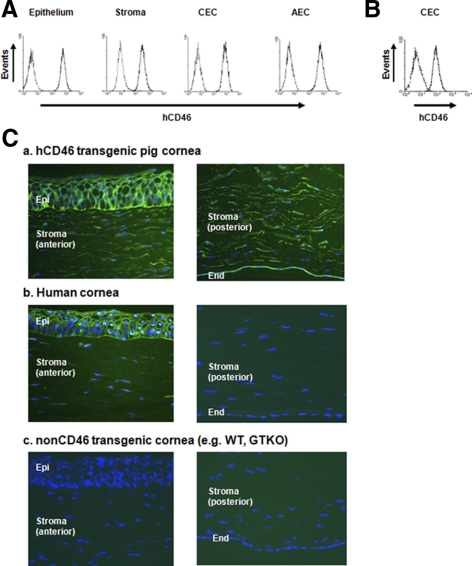
By flow cytometry, cultured corneal cells from the hCD46 pigs showed significant expression of hCD46 in all corneal cell types and the pAECs (Fig. 3A). The expression of hCD46 on the pCECs was greater than on the hCECs (Fig. 3B). There was no expression of hCD46 in any corneal cells from WT pigs (not shown).
Figure 3.
Significant expression of human CD46 on cultured corneal cells and corneal tissues from hCD46-transgenic pigs than human corneal cells. Cultured cells from hCD46 transgenic pigs and humans were stained for CD46. Dotted traces: isotype control. (A) CD46-transgenic pigs expressed high levels of CD46 on epithelium, stroma, and CECs, as well as on AECs. (B) Expression of hCD46 on hCECs. Expression of hCD46 on hCD46-transgenic pigs was considerably greater than on hCECs. (C) Staining for hCD46 (FITC: green) and nuclei (DAPI: blue) in corneas from (a) hCD46 pig, (b) human, and (c) WT pig was determined by fluorescence microscopy (original magnification, ×200). The hCD46 pig expressed hCD46 in all corneal layers including the endothelium, in which there is no expression in human and WT pig corneas. Results are representative of at least two independent experiments.
Fluorescent staining showed that the hCD46 pigs expressed high levels of hCD46 in all corneal layers, including the CECs (Fig. 3Ca). Human corneas expressed hCD46 in the epithelial and anterior stromal areas, with minimal expression in the posterior stroma and no expression in endothelium (Fig. 3Cb). The WT pig corneas showed no expression of hCD46 (Fig. 3Cc) in any corneal layer.
Human IgM/IgG Binding to, and Complement-Dependent Cytotoxicity of, WT pCECs and pAECs
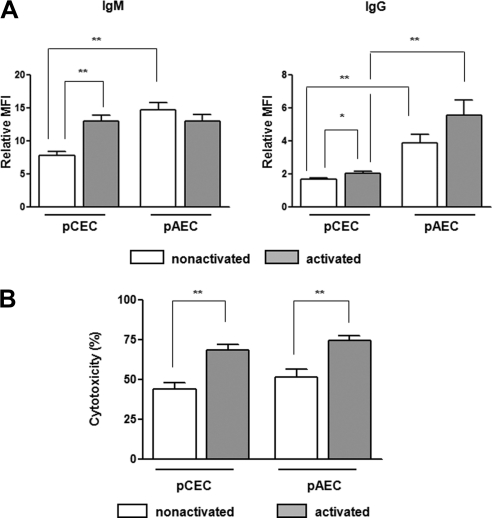
Human IgM and IgG binding to the WT pCECs was significantly lower than to the WT pAECs (Fig. 4A), correlating with the lower expression of Gal on the pCECs. However, when the cells were activated, both IgM and IgG binding to the pCECs were increased. In contrast to the pCECs, there was no significant difference in IgM and IgG antibody binding between the quiescent and activated pAECs. There was no significant difference in IgM binding to the pCECs and to the pAECs, although there was still significantly less binding of IgG to the pCECs. We anticipated that lower IgM/IgG binding to the pCECs would be associated with less lysis compared with that of the pAECs, but there was no significant difference in complement-mediated cell lysis between the pCECs and pAECs, whether the cells were quiescent or activated (Fig. 4B), suggesting that pCECs may be less protected against complement-mediated lysis.
Figure 4.
Less human IgM/IgG antibody binding to WT pCECs than to pAECs, but no difference in lysis. (A) IgM and IgG binding to WT pCECs and pAECs was measured using pooled human serum at 10% concentration. When cells were quiescent (nonactivated), IgM and IgG binding to pCECs was significantly lower than to pAECs (P < 0.01). After activation, there was increased binding to pCECs (P < 0.01), but not to pAECs. There remained less IgG (but not IgM) binding to pCECs than to pAECs (P < 0.01; n = 6; *P < 0.05, **P < 0.01). (B) The lysis caused by 25% pooled human serum (n = 6) of the WT pCECs before and after activation was compared with lysis of the pAECs. There was no significant difference in complement-dependent cytotoxicity of the pCECs and the pAECs (**P < 0.01).
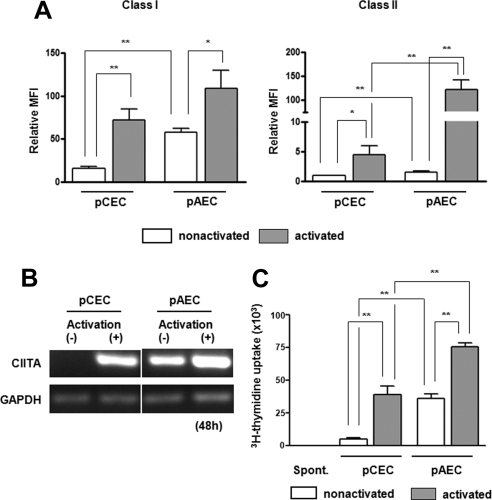
Human Cellular Response to WT pCECs and pAECs
Quiescent and activated WT pCECs and pAECs were tested for SLA class I and II expression (Fig. 5A). SLA class I and II expression on the pCECs was significantly lower than that on the pAECs and was significantly upregulated when the cells were activated. Although there was no significant difference in SLA class I expression between activated pCECs and pAECs (P = 0.20), there was still significantly lower expression of SLA class II on the pCECs than on the pAECs after activation. The increased expression of SLA class II on both the pCECs and the pAECs was through a class II transactivator (CIITA)–dependent mechanism (Fig. 5B). To investigate the human T-cell response to the pCECs and pAECs, we co-cultured isolated human CD4+ T cells with quiescent or activated pCECs or pAECs in MLR. The human CD4+ T-cell direct response to the pCECs was significantly weaker than to the pAECs, when the cells were quiescent or activated (P < 0.01; Fig. 5C), correlating with lower expression of SLA class II on the pCECs. This suggested that less immunosuppressive therapy may be necessary to suppress the T-cell response to a pig corneal graft than to a vascularized pig organ graft.
Figure 5.
Significantly weaker human CD4+ T-cell response to the WT pCECs than to pAECs, which was related to reduced expression of SLA class II on the WT pCECs, pCECs, and pAECs from a WT pig were activated with pIFN-γ (40 ng/mL) for 48 hours. (A) SLA class I and II expression were measured by flow cytometry. Expression of SLA class I and II was lower on quiescent pCECs than on pAECs (P < 0.01). After activation, there was greater upregulation of SLA class I and II on the pCECs than on the pAECs. Although there was no significant difference in expression of SLA class I between the pCECs and pAECs after activation, there was higher expression of SLA class II on the pAECs than on the pCECs (P < 0.01, n = 6; *P < 0.05, **P < 0.01). (B) RT-PCR was used to compare the effect of pIFN-γ on CIITA mRNA expression on the WT pCECs and pAECs. The pCECs and pAECs were activated with pIFN-γ for 48 hours. The expression of CIITA mRNA was measured by RT-PCR, with GAPDH being used as an internal control. pAEC constitutively expressed CIITA, and it was upregulated when cells were activated. There was no expression of CIITA in the nonactivated pCEC; however, it was upregulated when cells were activated. Results are representative of three independent experiments. (C) Human CD4+ T cells were co-cultured with irradiated quiescent or activated the WT pCECs or pAECs. There was a weaker response to quiescent pCECs than to pAECs (P < 0.01). Although the human CD4+ T-cell proliferative responses to the pCECs and pAECs were both increased when the cells were activated (P < 0.01), there remained a significantly weaker response to pCECs than to pAECs (P < 0.01, n = 5; **P < 0.01).
Human Humoral and Cellular Responses to Genetically Modified Pig CECs
We investigated whether the human humoral and cellular immune responses to pCECs from genetically engineered pigs are reduced compared with those from WT pigs.
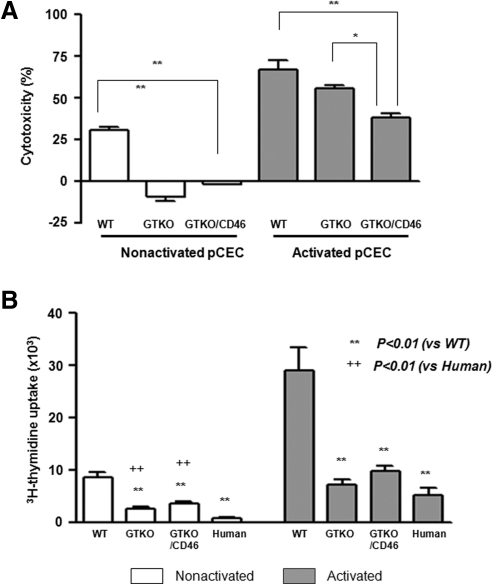
Although lysis of the quiescent GTKO and GTKO/CD46 pCECs was minimal or none and was significantly less than that of the WT pCECs, lysis increased after activation (Fig. 6A). There was no significant difference in lysis between the WT and the GTKO-activated pCECs. However, the GTKO/CD46 pCECs demonstrated significant resistance to lysis (Fig. 6A). These results suggest that even corneas from GTKO/CD46 pigs require further genetic modification to protect them from human serum cytotoxicity, if the pCECs become activated.
Figure 6.
Significant reduction of the human humoral and cellular responses to genetically engineered pCECs. (A) A complement-dependent cytotoxicity assay showed lysis of the WT, GTKO, and GTKO/CD46 pCECs before and after activation (by pIFN-γ 40ng/mL for 48 hours) by pooled human serum at 12.5% concentration. In contrast to the WT pCECs, there was no lysis of the quiescent GTKO and GTKO/CD46 pCECs. After activation, although there was increased lysis of the pCECs from all types of pig, there was significantly less lysis of the GTKO/CD46 pCECs than of the pCECs from other pigs (n = 4; *P < 0.05, **P < 0.01). (B) Human CD4+ T cells were co-cultured with the WT, GTKO, GTKO/CD46 pig or human stimulator CECs before and after activation for 6 days. MLR showed significantly less proliferation of human CD4+ T cells to GTKO and GTKO/CD46 pCECs and to hCECs than to the WT pCECs. There was significantly less proliferation of human CD4+ T cells to the quiescent hCECs than to the GTKO and GTKO/CD46 pCECs. After activation, however, there was no difference in human CD4+ T-cell proliferation to the GTKO and GTKO/CD46 pCECs or to the hCECs (n = 4; **P < 0.01 vs. WT, ++ P < 0.01 vs. human).
The human CD4+ T-cell responses to the GTKO and GTKO/CD46 pCECs were significantly weaker than those to the WT pCECs, both before and after activation of the cells (P < 0.01; Fig. 6B), with no significant difference between the response to GTKO and GTKO/CD46 pCECs. (These observations correlate with our previous observations regarding the human cellular response to GTKO PBMCs38 and pAECs.39).
Although the human CD4+ T-cell responses to GTKO and GTKO/CD46 pCECs were significantly greater than that to hCECs before activation of the cells (P < 0.01; Fig. 6B), there was no significant difference in the human CD4+ T-cell response to the human or pig CECs after activation (Fig. 6B).
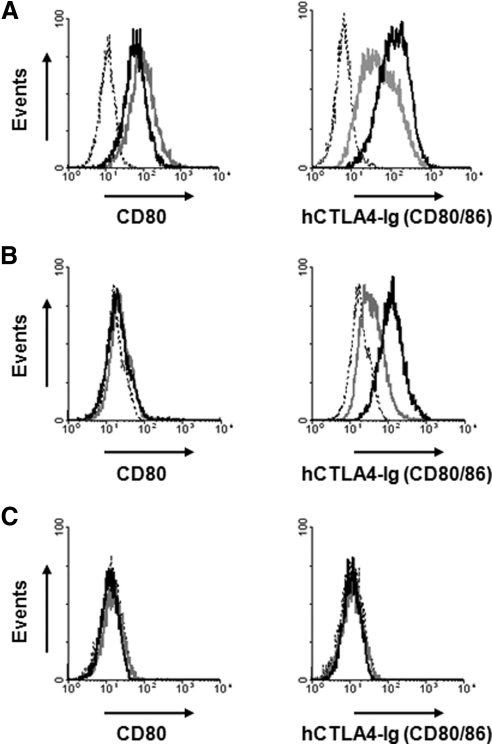
Expression of the Co-stimulatory Molecules CD80/86
Differences in expression of the co-stimulatory molecules CD80/86 on CECs between human and pig were assessed by flow cytometry, to investigate whether greater proliferation of human CD4+ T-cells to pCECs is associated with higher expression of CD80/86 on the pCECs than on the hCECs. The pAECs constitutively expressed CD80/86 molecules; CD86 expression was upregulated when the cells were activated (Fig. 7A). Although there was no expression of CD80 on the quiescent or activated pCECs, constitutive expression of CD80/86 was confirmed on the pCECs (Fig. 7B), indicating that pCECs constitutively express CD86. CD86 expression was upregulated after activation. In contrast to the pCECs, there was no expression of CD80 or CD86 on the quiescent or activated hCECs (Fig. 7C).
Figure 7.
pCECs constitutively expressed B7 molecules (CD80/CD86). The expression of B7 molecules (CD80/CD86) on the cells was documented with an anti-CD80 monoclonal antibody and hCTLA4-Ig. (A) There was constitutive expression of CD80 and CD86 on the pAECs. (B) Although there was no expression of CD80 on either the quiescent or activated pCECs, there was constitutive expression of CD80/86, suggesting constitutive expression of CD86. On activation, CD86 expression was upregulated. (C) There was no expression of CD80 or CD86 on hCECs, even after activation. Dotted lines: isotype control or secondary antibody only; gray lines: quiescent; black lines: activated. Results are representative of three independent experiments.
Discussion
Because the cornea is an immune-privileged tissue,40 its success as a xenograft may be greater than that of solid organ xenografts. Organ transplantation from a WT pig into a nonimmunosuppressed primate results in hyperacute rejection, which is associated with vascular occlusion and is commonly seen within minutes to hours.41,42 WT pig organs express Gal, the major pig antigen against which nonhuman primates and humans have preformed (natural) antibodies.43–47 Binding of anti-Gal antibodies to pig cells in vitro results in rapid complement-mediated destruction of the cells.32 In contrast, since the cornea is an avascular tissue, hyperacute rejection is not seen in small and large animal models of xenotransplantation.13 In general, corneal xenografts are rejected faster than allografts.26,48 Although the precise mechanism of corneal xenograft rejection remains uncertain, it is likely to be related to natural, preformed antibody binding and complement activation.13
Therefore, we first investigated whether the human humoral response to pig corneal cells, especially CECs, which are a main target of corneal graft rejection,9 is different from that to pAECs, which are the target in a vascularized organ xenograft. WT pig corneas express Gal, mainly in the anterior stromal keratocytes, but not in the epithelium and endothelium of the central cornea, as reported by others.49,50 We speculate that the reason Gal is expressed in the limbal area, but not centrally, may be associated with the blood supply. The limbal area has a better blood supply and therefore receives more nutrition (e.g., oxygen and glucose), which may be associated with an increased metabolic rate in the cells of the area. In contrast, the central cornea is relatively avascular and not well-supplied with nutrients. (Tears are the main source of nutrition, and these do not contain glucose.) We believe this proposed mechanism is supported by our observation that, although quiescent central corneal endothelial cells do not express much Gal, when the CECs are cultured, increased Gal expression is observed.
All corneal cells, including endothelial, express Gal when they are cultured in vitro. Although the level of Gal expression on the pCECs was significantly lower than on the pAECs, the expression of Gal on the pCECs was more significantly increased when the cells were activated. Lee et al.50 demonstrated that Gal expression is gradually induced on corneal stroma and cells during in vitro culture of the cornea and after pig-to-rat corneal transplantation. In experimental organ xenotransplantation, graft injury can be delayed by intensive immunosuppressive therapy, but this would not be an option with corneal grafts. If a corneal graft is implanted in a high-risk patient (e.g., with a neovascularized and inflamed host bed), preformed anti-pig antibodies, particularly anti-Gal antibodies, will gain immediate access to the graft and will almost certainly reduce graft survival. In the long term, therefore, the transplantation of corneas from WT pigs is likely to be problematic, even under cover of topical steroids and/or systemic immunosuppression.
Recently, the transplantation of cultivated CECs has been suggested as an approach to the treatment of corneal endothelial dysfunction.51 pCECs may be an alternative source of CECs for clinical transplantation because of the shortage of human donors and the variable quality of their CECs. However, our data suggest that CECs from WT pigs express significant levels of Gal after culture. In contrast, GTKO pCECs may have potential for this approach and would reduce antibody-mediated cell lysis.
Surprisingly, the lower expression of Gal with associated reduced antibody binding to the pCECs did not result in reduced lysis of the pCECs compared with that of the pAECs. Activation of cells by cytokines (e.g., IFN-γ) can trigger or amplify apoptosis.52 Indeed, our results indicated that even when there was no significant difference in IgM and IgG antibody binding to quiescent and activated pAECs, lysis of activated cells was significantly higher than that of quiescent cells.
Strong expression of complement regulatory proteins, such as CD46, CD55, and CD59, has been demonstrated in human corneal epithelium, but not endothelium.53 CECs are therefore highly susceptible to lysis by complement-fixing antibody.54,55 As a result of both reduced antibody binding and increased resistance to complement-mediated injury, GTKO/CD46 pCECs showed significantly reduced lysis. In contrast to our previous study in which lysis of GTKO/CD46 pAECs by pooled human sera was minimal, even if the cells were activated, there was still significant lysis of GTKO/CD46 pCECs after activation, suggesting that pCECs may be more susceptible to complement-mediated injury.
Further cytoprotection of pCECs may therefore be necessary for long-term pig corneal xenograft survival.56,57 Expression of a second human complement-regulatory protein (e.g., CD5558), and/or anti-inflammatory and anti-apoptotic genes (e.g., A2059), or hemoxygenase (HO)-I,60 may be necessary in GTKO/CD46 pigs. Pigs also express other (nonGal) antigens, against which there is a weaker, antibody-dependent, complement-mediated response when exposed to human serum.32,61 The nature of the nonGal antigens present on pig corneas is unknown, although the presence of N-glycolylneuraminic acid is likely.62,63
We next investigated whether the human cellular response to pCECs is different from that to pAECs. Lower expression of SLA class I and minimal to no expression of class II was documented on the pCECs compared with expression on the pAECs. Although both SLA class I and class II on the pCECs was upregulated with an inflammatory cytokine (e.g., IFN-γ), there remained lower expression of SLA class II on the pCECs compared with that on the pAECs. This was associated with a significantly weaker human CD4+ T-cell response. The CIITA has been termed a master regulator for MHC class II.64,65 Under normal circumstances, the nonvascular endothelial cells of the cornea display no MHC class II, and the genes encoding these molecules are silent. In a mouse model, exposure of these cells to IFN-γ failed to result in upregulation of MHC class II genes.66 CECs expresses only MHC class II when stimulated simultaneously with IFN-γ and TNF-α, and this expression is independent of CIITA.66 However, expression of SLA class II on pig corneal endothelium could be induced by IFN-γ only through the CIITA. Therefore, regulation of CIITA in the pig may be important to reduce the human CD4+ T-cell response to the pig cornea.67 Pigs with a mutant CIITA in which upregulation of class II is greatly inhibited are currently available.68
The absence of Gal expression on pig target cells reduces the human cellular response.38,39,69–71 Although the pCECs expressed a low level of SLA class II compared with the pAECs, the absence of Gal on the pCECs may also reduce the human CD4+ T-cell response. Indeed, the human CD4+ T-cell responses to CECs from GTKO and GTKO/CD46 pigs were significantly weaker than to WT CECs. There was no significant difference in SLA class II expression between WT, GTKO, and GTKO/CD46 pCECs (not shown).
The human CD4+ T-cell response to the hCECs was significantly weaker than to the pCECs when CECs were not activated, but not when the CECs were activated. One of the costimulatory interactions occurs between CD28 (on the surface of T cells) and the B7 ligands CD80 and CD86 (on antigen-presenting cells). pAECs, unlike human endothelium, constitutively express CD80 and CD86 and are fully capable of stimulating a human T-cell response through the direct pathway, providing the potential for full human T-cell activation at the donor endothelial cell surface.72 hCECs showed no expression of B7 molecules (CD80/CD86) even when activated (Fig. 7C); in contrast, pCECs constitutively expressed CD86 but not CD80 (Fig. 7B). These results correlate with a significantly greater proliferation of human CD4+ T cells to pCECs than to hCECs. The transplantation of corneas from pigs transgenic for the porcine costimulatory blockade agent porcine CTLA4-Ig73 or treatment of the donor tissue ex vivo before grafting (e.g., incubation with CTLA4-Ig or single administration of a viral vector expressing CTLA4-Ig74), which provides local immunosuppression against the T-cell response, is likely to reduce the human T-cell response.
In regard to coagulation disparities, with a graft as small as a corneal xenograft—and particularly as corneal grafts are vascularized by host vessels—it is unlikely that coagulation dysfunction will prove problematic. Therefore, expression of human “anticoagulant” or “antithrombotic” transgenes is unlikely to be necessary.
In conclusion, the present in vitro study suggests that corneal xenotransplantation may be more successful than vascularized organ xenotransplantation since there is a weaker human immune response. However, the use of corneas from pigs with multiple genetic modifications will be required to fully overcome the primate immune response. Corneal xenotransplantation may be a field in which xenotransplantation can move relatively rapidly into the clinic.
Acknowledgments
The authors thank Janice C. Anderson, Theodore Castellanos, and Karen Brown from the Pittsburgh Center for Organ Recovery and Education (CORE) for providing human corneas for these studies.
Footnotes
Supported by Eye Bank Association of America Grant 705721 (HH). Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by National Institutes of Health Grants U01 (AI068642) (DKCC) and R21 (A1074844) (DKCC); Olympus America Inc. Grant 2115 (HH); and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
Disclosure: H. Hara, None; N. Koike, None; C. Long, None; J. Piluek, None; D.S. Roh, None; N. SundarRaj, None; J.L. Funderburgh, None; Y. Mizuguchi, None; K. Isse, None; C.J. Phelps, Revivicor, Inc. (F, E); S.F. Ball, Revivicor, Inc. (F, E); D.L. Ayares, Revivicor, Inc. (F, E); D.K.C. Cooper, None.
References
- 1. Hara H, Cooper DK. Xenotransplantation: the future of corneal transplantation? Cornea. 2011;30:371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coster DJ, Williams KA. Donor cornea procurement: some special problems in Asia. Asia-Pacific J Ophthalmol. 1992;4:7–12 [Google Scholar]
- 3. Makgotloe AM, Carmichael TR. Plummeting corneal donations at the Gauteng Cornea and Eye Bank. S Afr Med J. 2009;99:797. [PubMed] [Google Scholar]
- 4. Michaeli-Cohen A, Lambert AC, Coloma F, Rootman DS. Two cases of a penetrating keratoplasty with tissue from a donor who had undergone LASIK surgery. Cornea. 2002;21:111–113 [DOI] [PubMed] [Google Scholar]
- 5. Mifflin M, Kim M. Penetrating keratoplasty using tissue from a donor with previous LASIK surgery. Cornea. 21:537–538, 2002; author reply 538–539 [DOI] [PubMed] [Google Scholar]
- 6. Tanaka K, Yamada J, Joyce N, Streilein JW. Immunobiology of xenogeneic cornea grafts in mouse eyes. I. Fate of xenogeneic cornea tissue grafts implanted in anterior chamber of mouse eyes. Transplantation. 2000;69:610–616 [DOI] [PubMed] [Google Scholar]
- 7. Tanaka K, Streilein JW. Immunobiology of xenogeneic cornea grafts in mouse eyes. II. Immunogenicity of xenogeneic cornea tissue grafts implanted in anterior chamber of mouse eyes. Transplantation. 2000;69:616–623 [DOI] [PubMed] [Google Scholar]
- 8. Pan Z, Sun C, Jie Y, Wang N, Wang L. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation. 2007;14:603–611 [DOI] [PubMed] [Google Scholar]
- 9. Takano T, Williams KA. Mechanism of corneal endothelial destruction in rejecting rat corneal allografts and xenografts: a role for CD4+ cells. Transplant Proc. 1995;27:260–261 [PubMed] [Google Scholar]
- 10. Qian Y, Dana MR. Molecular mechanisms of immunity in corneal allotransplantation and xenotransplantation. Expert Rev Mol Med. 2001;3:1–21 [DOI] [PubMed] [Google Scholar]
- 11. Williams KA, Coster DJ. The immunobiology of corneal transplantation. Transplantation. 2007;84:806–813 [DOI] [PubMed] [Google Scholar]
- 12. Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32:1005–1016 [DOI] [PubMed] [Google Scholar]
- 13. Hara H, Cooper DK. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17:338–349 [DOI] [PubMed] [Google Scholar]
- 14. Larkin DF, Alexander RA, Cree IA. Infiltrating inflammatory cell phenotypes and apoptosis in rejected human corneal allografts. Eye. 1997;11(Pt 1):68–74 [DOI] [PubMed] [Google Scholar]
- 15. Kim MK, Oh JY, Lee HI, et al. Susceptibility of porcine keratocytes to immune-mediated damage in xeno-related rejection. Transplant Proc. 2008;40:564–569 [DOI] [PubMed] [Google Scholar]
- 16. Higuchi R, Streilein JW. CD8+ T-cell-mediated delayed rejection of orthotopic guinea pig cornea grafts in mice deficient in CD4+ T cells. Invest Ophthalmol Vis Sci. 2003;44:175–182 [DOI] [PubMed] [Google Scholar]
- 17. Tanaka K, Sonoda K, Streilein JW. Acute rejection of orthotopic corneal xenografts in mice depends on CD4(+) T cells and self-antigen-presenting cells. Invest Ophthalmol Vis Sci. 2001;42:2878–2884 [PubMed] [Google Scholar]
- 18. Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173:4464–4469 [DOI] [PubMed] [Google Scholar]
- 20. Batchelor JR, Casey TA, Werb A, et al. HLA matching and corneal grafting. Lancet. 1976;1:551–554 [DOI] [PubMed] [Google Scholar]
- 21. The Collaborative Corneal Transplantation Studies Research Group The collaborative corneal transplantation studies (CCTS): effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403 [PubMed] [Google Scholar]
- 22. Fink N, Stark WJ, Maguire MG, et al. Effectiveness of histocompatibility matching in high-risk corneal transplantation: a summary of results from the Collaborative Corneal Transplantation Studies. Cesk Oftalmol. 1994;50:3–12 [PubMed] [Google Scholar]
- 23. Inoue K, Tsuru T. ABO antigen blood-group compatibility and allograft rejection in corneal transplantation. Acta Ophthalmol Scand. 1999;77:495–499 [DOI] [PubMed] [Google Scholar]
- 24. Hahn AB, Foulks GN, Enger C, et al. The association of lymphocytotoxic antibodies with corneal allograft rejection in high risk patients. The Collaborative Corneal Transplantation Studies Research Group. Transplantation. 1995;59:21–27 [DOI] [PubMed] [Google Scholar]
- 25. Borderie VM, Lopez M, Vedie F, Laroche L. ABO antigen blood-group compatibility in corneal transplantation. Cornea. 1997;16:1–6 [PubMed] [Google Scholar]
- 26. Ross JR, Howell DN, Sanfilippo FP. Characteristics of corneal xenograft rejection in a discordant species combination. Invest Ophthalmol Vis Sci. 1993;34:2469–2476 [PubMed] [Google Scholar]
- 27. Holan V, Vitova A, Krulova M, et al. Susceptibility of corneal allografts and xenografts to antibody-mediated rejection. Immunol Lett. 2005;100:211–213 [DOI] [PubMed] [Google Scholar]
- 28. Cooper DK, Dorling A, Pierson RN, 3rd, et al. Alpha1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84:1–7 [DOI] [PubMed] [Google Scholar]
- 29. Tai HC, Ezzelarab M, Hara H, Ayares D, Cooper DK. Progress in xenotransplantation following the introduction of gene-knockout technology. Transpl Int. 2007;20:107–117 [DOI] [PubMed] [Google Scholar]
- 30. Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loveland BE, Milland J, Kyriakou P, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183 [DOI] [PubMed] [Google Scholar]
- 32. Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174 [DOI] [PubMed] [Google Scholar]
- 33. Ebato B, Friend J, Thoft RA. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1988;29:1533–1537 [PubMed] [Google Scholar]
- 34. Harvey SA, Guerriero E, Charukamnoetkanok N, Piluek J, Schuman JS, Sundarraj N. Responses of cultured human keratocytes and myofibroblasts to ethyl pyruvate: a microarray analysis of gene expression. Invest Ophthalmol Vis Sci. 2010;51:2917–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45:1743–1751 [DOI] [PubMed] [Google Scholar]
- 36. Wada M, Amae S, Sasaki H, et al. The functional roles of porcine CD80 molecule and its ability to stimulate and regulate human anti-pig cellular response. Transplantation. 2003;75:1887–1894 [DOI] [PubMed] [Google Scholar]
- 37. Hara H, Campanile N, Tai HC, et al. An in vitro model of pig liver xenotransplantation: pig complement is associated with reduced lysis of wild-type and genetically modified pig cells. Xenotransplantation. 2010;17:370–378 [DOI] [PubMed] [Google Scholar]
- 38. Lin YJ, Hara H, Tai HC, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ezzelarab M, Welchons D, Torres C, et al. Atorvastatin down-regulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008;86:733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889 [DOI] [PubMed] [Google Scholar]
- 41. Alexandre GP. From AB. O-incompatible human kidney transplantation to xenotransplantation. Xenotransplantation. 2004;11:233–236 [DOI] [PubMed] [Google Scholar]
- 42. Cooper DK, Human PA, Lexer G, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246 [PubMed] [Google Scholar]
- 43. Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762 [PubMed] [Google Scholar]
- 44. Good AH, Cooper DK, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562 [PubMed] [Google Scholar]
- 45. Cooper DK. Depletion of natural antibodies in non-human primates: a step towards successful discordant xenografting in humans. Clin Transplant. 1992;6:178–183 [PubMed] [Google Scholar]
- 46. Cooper DK, Good AH, Koren E, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205 [DOI] [PubMed] [Google Scholar]
- 47. Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56:1433–1442 [DOI] [PubMed] [Google Scholar]
- 48. Larkin DF, Takano T, Standfield SD, Williams KA. Experimental orthotopic corneal xenotransplantation in the rat: mechanisms of graft rejection. Transplantation. 1995;60:491–497 [DOI] [PubMed] [Google Scholar]
- 49. Amano S, Shimomura N, Kaji Y, Ishii K, Yamagami S, Araie M. Antigenicity of porcine cornea as xenograft. Curr Eye Res. 2003;26:313–318 [DOI] [PubMed] [Google Scholar]
- 50. Lee HI, Kim MK, Oh JY, et al. Gal alpha(1–3)Gal expression of the cornea in vitro, in vivo and in xenotransplantation. Xenotransplantation. 2007;14:612–618 [DOI] [PubMed] [Google Scholar]
- 51. Koizumi N, Sakamoto Y, Okumura N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48:4519–4526 [DOI] [PubMed] [Google Scholar]
- 52. Beauregard C, Huq SO, Barabino S, Zhang Q, Kazlauskas A, Dana MR. Keratocyte apoptosis and failure of corneal allografts. Transplantation. 2006;81:1577–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993;34:3579–3584 [PubMed] [Google Scholar]
- 54. Hargrave SL, Mayhew E, Hegde S, Niederkorn J. Are corneal cells susceptible to antibody-mediated killing in corneal allograft rejection? Transpl Immunol. 2003;11:79–89 [DOI] [PubMed] [Google Scholar]
- 55. Hegde S, Mellon JK, Hargrave SL, Niederkorn JY. Effect of alloantibodies on corneal allograft survival. Invest Ophthalmol Vis Sci. 2002;43:1012–1018 [PubMed] [Google Scholar]
- 56. Barcia RN, Dana MR, Kazlauskas A. Corneal graft rejection is accompanied by apoptosis of the endothelium and is prevented by gene therapy with bcl-xL. Am J Transplant. 2007;7:2082–2089 [DOI] [PubMed] [Google Scholar]
- 57. Gong N, Ecke I, Mergler S, et al. Gene transfer of cyto-protective molecules in corneal endothelial cells and cultured corneas: analysis of protective effects in vitro and in vivo. Biochem Biophys Res Commun. 2007;357:302–307 [DOI] [PubMed] [Google Scholar]
- 58. Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966 [DOI] [PubMed] [Google Scholar]
- 59. Oropeza M, Petersen B, Carnwath JW, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009;16:522–534 [DOI] [PubMed] [Google Scholar]
- 60. Petersen B, Lucas-Hahn A, Lemme E, et al. Generation and characterization of human hemeoxygenase-1 (hHO-1) transgenic pigs. Xenotransplantation. 2009;16:373–374 [Google Scholar]
- 61. Hara H, Ezzelarab M, Rood PP, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365 [DOI] [PubMed] [Google Scholar]
- 62. Ezzelarab M, Ayares D, Cooper DK. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005;83:396–404 [DOI] [PubMed] [Google Scholar]
- 63. Kim YG, Oh JY, Gil GC, et al. Identification of alpha-Gal and non-Gal epitopes in pig corneal endothelial cells and keratocytes by using mass spectrometry. Curr Eye Res. 2009;34:877–895 [DOI] [PubMed] [Google Scholar]
- 64. LeibundGut-Landmann S, Waldburger JM, Krawczyk M, et al. Mini-review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol. 2004;34:1513–1525 [DOI] [PubMed] [Google Scholar]
- 65. Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806 [DOI] [PubMed] [Google Scholar]
- 66. Arancibia-Carcamo CV, Osawa H, Arnett HA, et al. A CIITA-independent pathway that promotes expression of endogenous rather than exogenous peptides in immune-privileged sites. Eur J Immunol. 2004;34:471–480 [DOI] [PubMed] [Google Scholar]
- 67. Yun S, Gustafsson K, Fabre JW. Suppression of human anti-porcine T-cell immune responses by major histocompatibility complex class II transactivator constructs lacking the amino terminal domain. Transplantation. 1998;66:103–111 [DOI] [PubMed] [Google Scholar]
- 68. Hara H, Crossley T, Witt W, et al. Dominant-negative CIITA transgenic pigs - effect on the human anti-pig T cell immune response and immune status (abstract 503). Am J Transplant. 2010;10:187 [Google Scholar]
- 69. Jin R, Greenwald A, Peterson MD, Waddell TK. Human monocytes recognize porcine endothelium via the interaction of galectin 3 and alpha-GAL. J Immunol. 2006;177:1289–1295 [DOI] [PubMed] [Google Scholar]
- 70. Saethre M, Schneider MK, Lambris JD, et al. Cytokine secretion depends on Gal alpha(1,3)Gal expression in a pig-to-human whole blood model. J Immunol. 2008;180:6346–6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilhite T, Ezzelarab C, Hara H, et al. Absence of gal expression on pig cells is associated with a reduced primate cellular response (abstract 889). Am J Transplant. 2010;10:299 [Google Scholar]
- 72. Rogers NJ, Jackson IM, Jordan WJ, Hawadle MA, Dorling A, Lechler RI. Cross-species costimulation: relative contributions of CD80, CD86, and CD40. Transplantation. 2003;75:2068–2076 [DOI] [PubMed] [Google Scholar]
- 73. Phelps CJ, Ball SF, Vaught TD, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–485 [DOI] [PubMed] [Google Scholar]
- 74. Comer RM, King WJ, Ardjomand N, Theoharis S, George AJ, Larkin DF. Effect of administration of CTLA4-Ig as protein or cDNA on corneal allograft survival. Invest Ophthalmol Vis Sci. 2002;43:1095–1103 [PubMed] [Google Scholar]