The PVR potential of ARPE19 cells was enhanced by expression of PDGFRα. These studies suggest that preventing activation, signaling, or both by PDGFRα has the potential to prevent the most sight-threatening component of PVR.
Abstract
Purpose.
Previous studies indicate that the expression of platelet-derived growth factor (PDGF) receptor α (PDGFRα) dramatically increases the ability of fibroblasts to induce experimental proliferative vitreoretinopathy (PVR). The purpose of this study was to determine whether PDGFRα contributed to the PVR potential of retinal pigment epithelial (RPE) cells, one of the most abundant cell types in PVR membranes.
Methods.
PDGFRα expression in human ARPE19 cells was increased or decreased by stably expressing the PDGFRα cDNA or short hairpin (sh) RNA directed at PDGFRα, respectively. The level of PDGFRα expression in the resulting panel of cell lines was either barely detectable (KD), standard (similar to the level of primary RPE cells), or overexpressed approximately 80-fold. Western blot analysis was used to assess the level of p53 and the activation state of PDGFRα and Akt. The following cellular responses were monitored: proliferation, apoptosis, and contraction. The PVR potential of cells was tested in a rabbit model of PVR in which cells were coinjected with platelet-rich plasma into the vitreous.
Results.
Comparison of KD and overexpressing cells indicated that high-level expression of PDGFRα dramatically augmented signaling events, cellular responses, and the PVR potential of ARPE19 cells. However, all these outcomes were also significantly increased, albeit not as robustly, by PDGFRα expression to the level typically present in RPE cells.
Conclusions.
Even though RPE cells express substantially less PDGFRα than fibroblasts, it significantly boosts PVR-related signaling events, cellular responses, and the PVR potential of ARPE19 cells. These studies suggest that inhibiting activation, signaling, or both by PDGFRα has the potential to prevent the development of PVR.
Proliferative vitreoretinopathy (PVR) is a disorder characterized by the formation of membranes on both surfaces of the retina that contract and thereby induce retinal detachment. PVR results from various causes, but it is most often encountered after retinal tears and retinal detachment and after therapeutic interventions for these conditions. It occurs in approximately 8% to 10% of patients undergoing primary retinal detachment surgery and is the principal cause of failure of this procedure.1–5
The PVR membrane consists of extracellular matrix proteins (collagen I and II and fibronectin), and cells (retinal pigment epithelial cells [RPE], retinal glial cells, fibroblasts, and macrophages).6–9 RPE cells are among the most abundant cell type in PVR membranes, and this probably relates to the retinal break and dispersion of viable RPE cells into the vitreous during cryopexy treatment of retinal tears.10–12 Although there has been increased success in reattaching the retina, understanding the molecular mechanism of PVR is likely to enable the development of effective pharmacologic approaches to protect patients undergoing surgery to correct a retinal detachment from succumbing to PVR.
Results of recent studies support the growth factor hypothesis regarding the pathogenesis of PVR.13 For instance, growth factors are present in the vitreous and promote many of the cellular events that are intrinsic to PVR. Furthermore, the expression of platelet-derived growth factor (PDGF) receptor α (PDGFRα) increases the ability of fibroblasts to induce experimental PVR.14 Moreover, blocking growth factor-dependent activation of receptors or the downstream signaling events protects animals from developing experimental PVR.15–18 These studies begin to elucidate key events in the development of PVR and thereby identify potential therapeutic targets.
A potential shortcoming of the information obtained using a fibroblast-driven model of PVR is that fibroblasts are not the major cell type within clinical PVR membranes. Consequently, the goal of this study was to identify an Achilles heel in RPE cells, one of the most abundant cell types within clinical PVR membranes. In light of the fact that cultured RPE cell lines express PDGFRα19 and that this receptor is expressed and activated in PVR membranes isolated from patients,20,21 we focused on evaluating the importance of PDGFRα for RPE cells to induce experimental PVR.
Materials and Methods
Major Reagents and Cell Culture
Antibodies against PDGFRα, phospho-Akt (S473), Akt, p53, and keratin 17 were purchased from Cell Signaling Technology (Beverly, MA); anti-CRALBP (cellular retinaldehyde-binding protein) was from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho-Y742 PDGFRα antibody was raised against the phospho-peptide (KQADTTQpYVPMLDMK).18 Secondary antibodies (horseradish peroxidase [HRP]–conjugated goat anti-rabbit IgG, and goat anti-mouse IgG) were purchased from Santa Cruz Biotechnology. Enhanced chemiluminescent substrate for detection of horseradish peroxidase was from Pierce Protein Research Products (Rockford, IL).
Primary human RPE cells were from Lonza (Walkersville, MD); ARPE19 cells were purchased from American Type Culture Collection; ARPE19α cells are ARPE19 cells overexpressing human PDGFRα.22 RPE cells derived from a human epiretinal membrane (called RPEM) were described previously18,23 and generously provided by Jing Cui and Joanne Matsubara (University of British Columbia, Vancouver, BC, Canada). RPEM cells express typical markers of RPE cells that are observed in primary RPE cells (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental). Primary rabbit conjunctival fibroblasts (RCFs) and Fα cells (mouse embryo fibroblasts that were modified to express human PDGFRα) have been described previously.14,24
Suppression of PDGFRα Expression
An oligo (CCTGGAGAAGTGAGAAACAAA) corresponding to NM_011058.1–937 in a hairpin-pLKO.1 retroviral vector, the packaging plasmid (pCMV-dR8.91), the envelope plasmid (VSV-G/pMD2.G), and 293T packaging cells were from Dana Farber Cancer Institute/Harvard Medical School (Boston, MA).
To prepare PDGFRα shRNA lentivirus, a mixture of packaging plasmid (0.9 μg), envelope plasmid (0.1 μg), hairpin-pLKO.1 vector (1 μg) (or a hairpin-pLKO.1 containing the PDGFRα shRNA oligo), and TransIT-LT1 were mixed and incubated at room temperature for 30 minutes. The transfection mix was transferred to 293T cells that were approximately 70% confluent. After 18 hours, the medium was replaced with growth medium modified to contain 30% serum, and virus was harvested 40 hours after transfection. The viral harvest was repeated three times at 24-hour intervals. Virus-containing media were pooled and centrifuged at 800g for 5 minutes, and the supernatant was used to infect ARPE19 cells. Successfully infected cells were selected on the basis of their ability to proliferate in media containing puromycin (1 μg/mL). Resultant cells were characterized by Western blot analysis using an antibody against PDGFRα.
Western Blot Analysis
Cells were grown to 90% confluence and then incubated for 24 hours in the medium without serum. Cells were treated with or without vitreous (diluted 1:2 in DMEM) for 2 hours. After washing twice with ice-cold phosphate-buffered saline (PBS), cells were lysed in extraction buffer (10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 50 mM NaCl, 50 mM NaF, 1% Triton X-100, 20 μg/mL aprotinin, 2 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride). Lysates were centrifuged for 15 minutes at 13,000g and 4°C.25 Equal amounts of protein were separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and subjected to Western blot analysis using the indicated antibodies. Signal intensity was determined by densitometry (Quantity One; Bio-Rad, Hercules, CA) and normalized for the amount of PDGFRα in each sample.25
Cell Proliferation Assay
Cells were seeded into a 24-well plate at a density of 3 × 104 cells/well in DMEM + 10% FBS. After 8 hours, the cells had attached, the medium was aspirated, cells were rinsed twice with PBS, and 0.5 mL serum-free DMEM or vitreous (diluted 1:2 in DMEM) was added. The media were replaced every day to avoid depletion of the vitreous, which was being tested for its ability to promote proliferation. Cells were detached from the plates with trypsin and counted in a hemocytometer on day 3. Each experimental condition was assayed in duplicate, and at least three independent experiments were performed.25
Apoptosis Assay
Cells were seeded into 6-cm dishes at a density of 2 × 105 cells per dish in DMEM + 10% FBS. After 8 hours, the cells had attached, the medium was aspirated, cells were rinsed twice with PBS, and serum-free DMEM with or without vitreous (diluted 1:2 in DMEM) was added. The media were replaced every day. After 3 days cells were stained with FITC-conjugated Annexin V and propidium iodide according to instructions provided with the apoptosis kit (BD Biosciences, Palo Alto, CA). Cells were analyzed by flow cytometry (Epics XL Flow Cytometer; Beckman Coulter, Brea, CA). At least three independent experiments were performed.25
Collagen I Contraction Assay
Cells were suspended in 1.5 mg/mL neutralized collagen I (Inamed, Fremont, CA) (pH 7.2) at a density of 106 cells/mL.21,26 The mixture was transferred to a 24-well plate that had been preincubated overnight with 5 mg/mL bovine serum albumin in PBS. The collagen was solidified by incubation at 37°C for 90 minutes, and then 0.5 mL DMEM or rabbit vitreous (RV; diluted 1:2 in DMEM) was added. The media were replaced every day. The gel diameter was measured on day 3. The area was calculated using the formula πr2, where r is the radius of the gel. Each experimental condition was assayed in duplicate, and at least three independent experiments were performed.18
Rabbit Model for PVR
PVR was induced in the right eye of pigmented rabbits purchased from Covance (Denver, PA), as previously described.14,18 Briefly, a gas vitrectomy was performed by injecting 0.1 mL perfluoropropane (C3F8; Alcon, Fort Worth, TX) into the vitreous cavity 4 mm posterior to the corneal limbus. One week later, all rabbits received two injections: 0.1 mL platelet-rich plasma (PRP) and 0.1 mL Dulbecco's modified Eagle's medium (DMEM) containing 2.5 × 105 cells of ARPE19, ARPE19α, or ARPE19KD. Retinal status was evaluated with an indirect ophthalmoscope fitted with a +30 D fundus lens at days 1, 3, 5, 7, 14, 21, 28, 35, and 42. PVR was graded according to the Fastenberg classification from 0 through 5. On day 42, animals were killed, and the eyes were enucleated and frozen at −80°C. All surgeries were performed under aseptic conditions and pursuant to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol for the use of animals was approved by the Schepens Animal Care and Use Committee.
Preparation of Rabbit Vitreous
RV was prepared from frozen rabbit eyeballs as described previously.18,25 The eyes were not manipulated in any way and were normal and free of obvious abnormalities.
Statistical Analysis
Experimental data were analyzed using an unpaired t-test, survival curve log-rank test, and one-way ANOVA or posttest (or both). P < 0.05 was considered statistically significant.
Results
Expression of PDGFRα in ARPE19 Cells Enhanced Vitreous-Dependent Signaling Events Intrinsic to PVR
Although RPE cells abound in PVR membranes isolated from patients10–12 and are capable of inducing experimental PVR,23,27 the cell surface receptors that promote the pathologic behavior of RPE cells are unknown. Such information would be valuable because it would identify therapeutic targets, a stumbling block in ongoing efforts to develop effective pharmacologic approaches to protect patients from PVR.
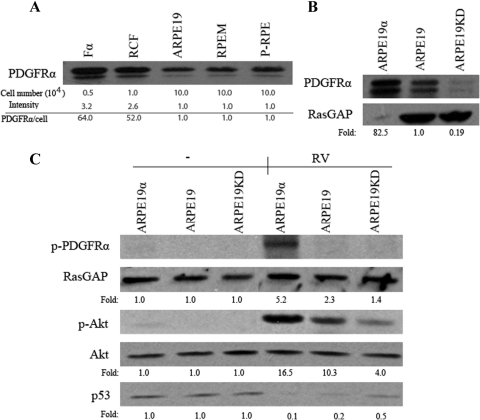
We considered PDGFRα a mediator of PVR because PDGFRα potently enhances the PVR potential of fibroblasts.14,28 Consistent with previous studies using RPE cell lines,19 we found that RPE cells isolated from an epiretinal membrane of a PVR patient (RPEM) also expressed PDGFRα (Fig. 1A). Furthermore, the level of PDGFRα in these cells was comparable to what primary RPE cells typically expressed (Fig. 1A). However, the level of PDGFRα in RPE cells was much lower than in fibroblasts (Fig. 1A). Consequently, we considered both whether the expression of PDGFRα contributed to the PVR potential of RPE cells, and whether the level of expression was an important factor. To this end, we established a panel of ARPE19 cell lines that either underexpressed, or overexpressed PDGFRα compared with the modest level present in RPEM cells (Fig. 1B). The overexpressing cells expressed slightly more PDGFRα than the level present in fibroblasts (Figs. 1A, 1B).
Figure 1.
The impact of PDGFRα expression on vitreous-dependent signaling events. (A) Fα (mouse embryo fibroblasts expressing PDGFRα), RCF (rabbit conjunctival fibroblasts), ARPE19, RPEM, and primary human RPE (P-RPE) cells were counted and lysed, and the indicated number of cells was subjected to anti-PDGFRα Western blot analysis. Values at the bottom of the panels are relative estimates of the expression of PDGFRα per cell. (B) Expression level of PDGFRα in ARPE19, ARPE19KD, and ARPE19α cells. Confluent dishes of the indicated cell lines were lysed and subjected to Western blot analysis using indicated antibodies. To compensate for the high level of PDGFRα expression in ARPE19α cells, a reduced amount of lysate was loaded. Values at the bottom of the panels indicate the relative amount of PDGFRα expression; these values were calculated by dividing the intensity of PDGFRα by the intensity of the loading control RasGAP (GTPase activating protein of Ras) and then correcting for the difference in the amount loaded. (C) ARPE19, ARPE19KD, and ARPE19α were grown to near confluence, serum-starved overnight, and left untreated or exposed to RV for 120 minutes. Cells were lysed, and the resultant lysates were subjected to Western blot analysis using indicated antibodies. “Fold” was calculated by first normalizing to the level of Akt and then calculating the ratio of the basal (i.e., unstimulated) over stimulated for each cell type. The experiment shown in this panel is representative of three independent trials.
Our previous studies with fibroblasts identified prolonged activation of Akt and suppression of p53 as PVR-associated signaling events that were induced by exposing cells to vitreous.29 RV triggered prolonged activation of Akt and a 50% reduction in the level of p53 in ARPE19KD cells (Fig. 1C), indicating that these signaling events occurred even when the level of PDGFRα was very low and, hence, probably driven by one or more of the many growth factors and cytokines present in vitreous. These signaling events were profoundly more robust in ARPE19 cells (Fig. 1C). Further increasing the level of PDGFRα boosted both the activation of Akt and the suppression of p53, but the magnitude of the increase in receptor level (approximately 80-fold) did not correspond to the increase in signaling events (approximately 2-fold) (Fig. 1C). These results revealed that even the relatively low (compared with fibroblasts) level of PDGFRα expression substantially improved the responsiveness of ARPE19 cells to RV and that only a relatively small increase in the response was obtained by overexpressing PDGFRα.
The enhanced ability of vitreous to promote signaling events in PDGFRα-expressing cells was not because of PDGF present in the vitreous. In these experiments we used vitreous from control rabbits, in which the concentration of PDGF was below the level required to induce the types of biochemical changes shown in Figure 1.22 The vitreal agents that activate PDGFRα are growth factors outside the PDGF family (non-PDGFs), which activate PDGFRα indirectly through an intracellular sequence of events that depends on both reactive oxygen species and Src family kinases.13,25,28
Expression of PDGFRα to the Level Typical of RPE Cells Significantly Enhanced RV-Induced Cellular Responses Intrinsic to PVR
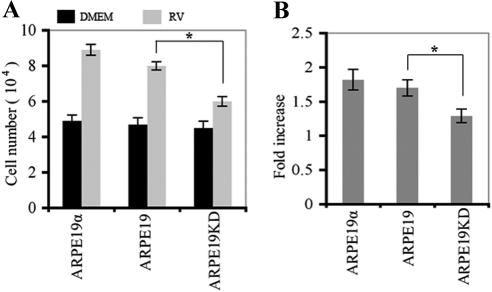
Exposure of ARPE19KD cells to vitreous promoted a small increase in proliferation (Fig. 2). The proliferative response to vitreous was significantly greater in ARPE19 cells, indicating that expressing PDGFRα to a physiologically relevant level increased the responsiveness of RPE cells to vitreous. Overexpressing PDGFRα did not further enhance the ability of cells to proliferate.
Figure 2.
Expression of PDGFRα promoted RV-induced proliferation of ARPE19 cells. (A) Cells (ARPE19, ARPE19α, ARPE19KD) were seeded into a 24-well plate at a density of 3 × 104 cells/well in DMEM + 10% FBS. After 8 hours, the cells had attached, the medium was changed to either 0.5 mL DMEM (black bars) or RV (diluted 1:2 in DMEM) (gray bars). Media were replaced every day. Cells were counted with a hemocytometer on day 3. Mean ± SD of three independent experiments is shown. *P < 0.05, unpaired t-test. (B) Raw data shown in A are expressed as fold increase in vitreous-stimulated cells compared with DMEM-stimulated cells. Knocking down the expression of PDGFRα blunted the ability of ARPE19 cells to proliferate in response to vitreous, whereas overexpressing PDGFRα had no effect.
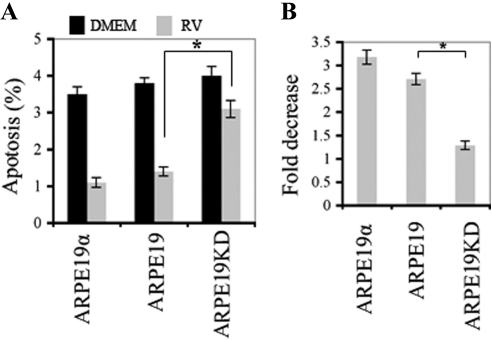
A similar phenomenon was observed when we monitored the ability of vitreous to protect ARPE19 cells from apoptosis. Survival was significantly improved by expression of PDGFRα to the level typically observed in RPE cells, and overexpression was not further advantageous (Fig. 3 and Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental).
Figure 3.
Expression of PDGFRα promoted vitreous-mediated survival of ARPE19 cells. (A) The indicated cells were seeded into 6 cm-dishes in DMEM + 10% FBS at a density of 2 × 105 cells per dish. After 8 hours, the medium was changed to either DMEM alone (black bars) or RV (diluted 1:2 in DMEM) (gray bars). Media were replaced every day. After 3 days, the cells were subjected to FACS analysis to determine the level of annexin V expression, a measure of apoptosis. Mean ± SD of three independent experiments is shown. *P < 0.05, unpaired t-test. An example of the raw FACS data is shown in Supplementary Figure S2 (http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental). (B) Data shown in A are expressed as a fold decrease in apoptosis. Knocking down the expression of PDGFRα resulted in a statistically significant decline in vitreous-mediated survival, whereas there was no further benefit of overexpressing PDGFRα.
Given that PDGFRα expression resulted in both a decline in apoptosis and an increase in proliferation, it is possible that the increased percentage of surviving cells led to an overestimation of the cell proliferation response. In the worst case scenario, the error would be minor because the magnitude of the change in percentage of surviving cells was much smaller than the percentage increase in proliferation. Vitreous induced 1.9%, 2.4%, and 1.5% increases in surviving cells, whereas the increases in cell proliferation for ARPE19α, ARPE19, and ARPE19KD cells were 169%, 157%, and 133%, respectively.
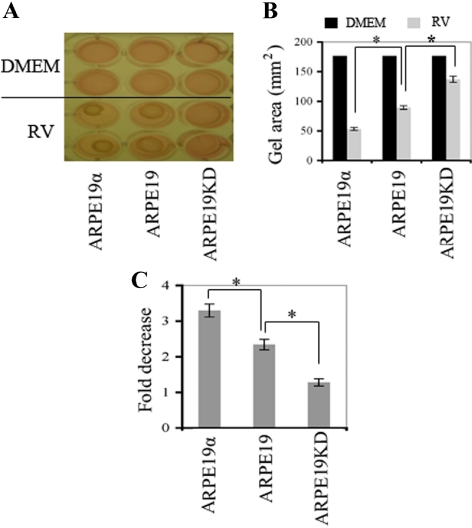
As with proliferation and survival, vitreous-induced contraction of ARPE19 cells was enhanced by the expression of PDGFRα to the standard level (Fig. 418,26). However, in contrast to the proliferative and survival responses, overexpressing PDGFRα improved the ability of cells to contract (Fig. 4). These studies show that the expression of PDGFRα in RPE cells to the standard level enhanced three PVR-related cellular responses when exposed to vitreous. Furthermore, contraction was the only vitreous-dependent response that was further enhanced by the overexpression of PDGFRα.
Figure 4.
Expression of PDGFRα to the standard level promoted RV-induced contraction of ARPE19 cells, and overexpressing PDGFRα further enhanced this response. (A) The indicated cell lines were suspended in 1.5 mg/mL neutralized collagen I (pH 7.2) at a density of 1 × 106 cells/mL18,26 and were seeded into wells of a 24-well plate that had been preincubated overnight with 5 mg/mL bovine serum albumin in PBS. The collagen was solidified by incubation at 37°C for 90 minutes. Solidified gels were overlaid with either DMEM alone (DMEM) or RV diluted 1:2 in DMEM. Media were replaced every day; each experimental condition was assayed in duplicate. The gel diameter was measured, and the gel was photographed on day 3; a photograph of a representative experiment is shown. (B) The area of the gel in each well was calculated using the formula πr2, where r is the radius of the gel. Mean ± SD of three independent experiments is shown. *P < 0.05, unpaired t-test. (C) Raw data shown in B are expressed as fold decrease of the gel diameter (which indicated increased contraction). Although ARPE19KD cells contracted in response to vitreous, a statistically greater response was observed in cells expressing a physiological level of PDGFRα, and overexpressing PDGFRα resulted in a further enhancement of vitreous-mediated contraction.
Expression of PDGFRα in ARPE19 Cells Contributed to Experimental PVR
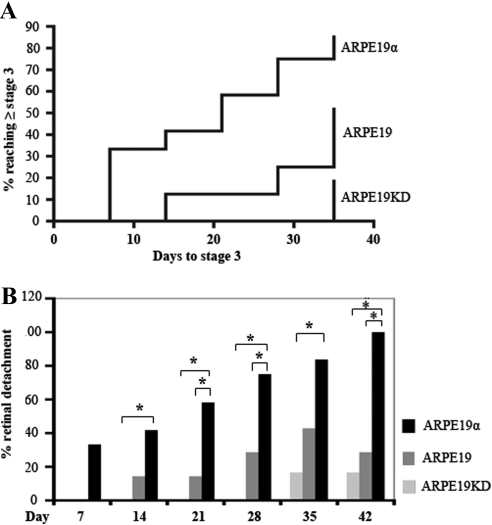
We assessed the importance of PDGFRα expression for the PVR potential of RPE cells by comparing the ability of our panel of ARPE19 cells to induce PVR when intravitreally coinjected with platelet-rich plasma into rabbits. In the context of this model, PDGFRα starkly augments the PVR potential of fibroblasts.14,28 Rabbits injected with ARPE19KD cells formed membranes that produced traction, but only 2 of 12 rabbits progressed to partial retinal detachment (Fig. 5A, Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental). The disease progressed faster and further in the group of rabbits injected with cells that expressed PDGFRα to the level typical of RPE (Fig. 5A, Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental). The PVR potential of ARPE19 cells was further increased by the overexpression of PDGFRα (Fig. 5A, Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental; the onset of retinal detachment was decreased, and penetrance was increased in the group injected with overexpressing cells (Fig. 5, Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental). This observation was consistent with the fact that PDGFRα-overexpressing cells were best at contracting collagen gels (Fig. 4). We conclude that the PVR potential of RPE cells is augmented by PDGFRα expression, as it is in fibroblasts. Furthermore, the level to which PDGFRα is naturally expressed in RPE cells was sufficient to substantially potentiate the PVR potential of RPE cells, and overexpression further enhanced this ability.
Figure 5.
PDGFRα in ARPE19 cells played a central role in experimental PVR. PVR was induced in the right eyes of pigmented rabbits, as described in Materials and Methods. One week after gas vitrectomy, each rabbit received two injections: 0.1 mL PRP and 2.5 × 105 ARPE19 cells, ARPE19α, or ARPE19KD in 0.1 mL DMEM. Retinal status was evaluated at days 1, 3, 5, 7, 14, 21, 28, 35, and 42. PVR was graded according to the Fastenberg classification from 0 through 5. Raw data are shown in Supplementary Figure S3 (http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7442/-/DCSupplemental). (A) Kaplan-Meier curves comparing the onset and frequency of retinal detachment (stage 3 or higher). As the level of PDGFRα expression increased, the onset of retinal detachment decreased and the number of rabbits affected increased. Differences between the three groups were statistically significant (P < 0.0001). (B) The percentage of rabbits that developed retinal detachment (stage 3 or higher) was calculated for each time point. Differences between groups that were statistically significant (as determined by one-way ANOVA and posttests) are indicated by asterisks.
Discussion
By characterizing a panel of ARPE19 cells that harbor various levels of PDGFRα, we learned that the level of PDGFRα that RPE cells normally express was sufficient to boost their responsiveness to vitreous and enhance their ability to promote PVR. Because RPE cells are among the most abundant cell types present in an epiretinal membrane,7–9 our studies suggest that approaches to block vitreous-driven activation of PDGFRα have the potential to provide protection from PVR.
Although the PVR potential of RPEs and fibroblasts is dependent on the expression of PDGFRα, the level of expression in fibroblasts is typically much higher than in ARPE19 cells (Fig. 1). This raises the possibility that fibroblasts need more PDGFRα to achieve their full PVR potential. Although we have not evaluated the impact of PDGFRα expression level on PVR potential with a panel of cell lines expressing different levels of PDGFRα, our work with a dominant negative PDGFRα suggests that modest expression of PDGFRα is sufficient to boost the PVR potential of fibroblasts, just as in RPEs. The dominant negative PDGFRα mutant is a truncation that ends at the beginning of the kinase domain, and it effectively prevents PVR, provided that it is expressed roughly 50-fold above the level of the full-length receptor.16 At lower levels of expression, the dominant negative is no longer capable of blocking PVR (H.L. and A.K., unpublished observations, 2009). These studies suggest that not all PDGFRα expressed in fibroblasts is necessary to enhance the PVR potential of this cell type.
Overexpressing PDGFRα did not increase all vitreous-driven cellular responses above the level observed in cells expressing the standard level of PDGFRα. This observation suggests that different thresholds of signaling are required for the three cellular responses we monitored. Since the greater decline in p53 (achieved in overexpressing cells) was associated with enhanced contraction, we speculate that p53 controls the expression of genes that prevent contraction. Identification of such genes may lead to approaches to prevent this most sight-threatening component of PVR.
It is important to point out that this model of PVR may underestimate the pathogenic potential of PDGFRα overexpression. Overexpressing cells may be substantially more responsive in traumatic settings that involve the release of large amounts of PDGF from platelets. Additional studies to evaluate the level of PDGFRα expression and how it correlates with the severity of PVR will provide the information needed to address this question.
Although our studies indicate that PDGFRα enhances the PVR potential of RPE cells, it is important to note that even when PDGFRα expression is very low (or null, as can be achieved using knockout fibroblasts14), the cells are still capable of inducing PVR. These observations indicate that PDGFRα is not the only driver of pathogenesis. Identification of other contributors will further increase our appreciation of how PVR develops and will enable us to prevent it.
In summary, our findings identify PDGFRα as an essential mediator of the PVR potential of RPE cells, one of the major cell types in PVR membranes. As a result, efforts directed at inhibiting vitreous-dependent activation of PDGFRα, signaling events, or cellular responses intrinsic to PVR have the potential to lead to therapies that effectively protect patients from PVR.
Acknowledgments
The authors thank Marie Ortega and Jessica Lanzim for help with the animal studies and Jing Cui and Joanne Matsubara for providing RPEM cells.
Footnotes
Supported by National Institutes of Health Grant EY012509 (AK).
Disclosure: H. Lei, None; M.-A. Rhéaume, None; G. Velez, None; S. Mukai, None; A. Kazlauskas, None
References
- 1. Ophir A, Blumenkranz MS, Claflin AJ. Experimental intraocular proliferation and neovascularization. Am J Ophthalmol. 1982;94:450–457 [DOI] [PubMed] [Google Scholar]
- 2. Sundaram V, Barsam A, Virgili G. Intravitreal low molecular weight heparin and 5-fluorouracil for the prevention of proliferative vitreoretinopathy following retinal reattachment surgery. Cochrane Database Syst Rev CD006421. [DOI] [PubMed] [Google Scholar]
- 3. Leaver PK. Proliferative vitreoretinopathy. Br J Ophthalmol. 1995;79:871–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han D. Proliferative Vitreoretinopathy. Philadelphia: WB Saunders; 2008 [Google Scholar]
- 5. Charteris DG. Growth factors in proliferative vitreoretinopathy. Br J Ophthalmol. 1998;82:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jerdan JA, Pepose JS, Michels RG, et al. Proliferative vitreoretinopathy membranes. Ophthalmology. 1989;9:801–810 [DOI] [PubMed] [Google Scholar]
- 7. Baudouin C, Fredj-Reygrobellet D, Brignole F, Negre F, Lapalus P, Gastaud P. Growth factors in vitreous and subretinal fluid cells from patients with proliferative vitreoretinopathy. Ophthalmic Res. 1993;25:52–59 [DOI] [PubMed] [Google Scholar]
- 8. Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 1990;31:14–28 [PubMed] [Google Scholar]
- 9. Cui JZ, Chiu A, Maberley D, Ma P, Samad A, Matsubara JA. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye. 2007;21:200–208 [DOI] [PubMed] [Google Scholar]
- 10. Laqua H, Machemer R. Glial cell proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol. 1975;80:602–618 [DOI] [PubMed] [Google Scholar]
- 11. Campochiaro PA. Mechanisms in ophthalmic disease: pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237–241 [DOI] [PubMed] [Google Scholar]
- 12. Ryan SJ. Traction retinal detachment: XLIX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1993;115:1–20 [DOI] [PubMed] [Google Scholar]
- 13. Lei H, Rheaume MA, Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res. 2010;90:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrews A, Balciunaite E, Leong FL, et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999;40:2683–2689 [PubMed] [Google Scholar]
- 15. Ikuno Y, Kazlauskas A. An in vivo gene therapy approach for experimental proliferative vitreoretinopathy using the truncated platelet-derived growth factor alpha receptor. Invest Ophthalmol Vis Sci. 2002;43:2406–2411 [PubMed] [Google Scholar]
- 16. Ikuno Y, Leong FL, Kazlauskas A. Attenuation of experimental proliferative vitreoretinopathy by inhibiting the platelet-derived growth factor receptor. Invest Ophthalmol Vis Sci. 2000;41:3107–3116 [PubMed] [Google Scholar]
- 17. Zheng Y, Ikuno Y, Ohj M, et al. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn J Ophthalmol. 2003;47:158–165 [DOI] [PubMed] [Google Scholar]
- 18. Lei H, Velez G, Cui J, et al. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am J Pathol. 2010;177:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campochiaro PA, Hackett SF, Vinores SA, et al. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci. 1994;107:2459–2469 [DOI] [PubMed] [Google Scholar]
- 20. Robbins SG, Mixon RN, Wilson DJ, et al. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest Ophthalmol Vis Sci. 1994;35:3649–3663 [PubMed] [Google Scholar]
- 21. Cui J, Lei H, Samad A, et al. PDGF receptors are activated in human epiretinal membranes. Exp Eye Res. 2009;88:438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lei H, Hovland P, Velez G, et al. A potential role for PDGF-C in experimental and clinical proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007;48:2335–2342 [DOI] [PubMed] [Google Scholar]
- 23. Wong CA, Potter MJ, Cui JZ, et al. Induction of proliferative vitreoretinopathy by a unique line of human retinal pigment epithelial cells. Can J Ophthalmol. 2002;37:211–220 [DOI] [PubMed] [Google Scholar]
- 24. Nakagawa M, Refojo MF, Marin JF, Doi M, Tolentino FI. Retinoic acid in silicone and silicone-fluorosilicone copolymer oils in a rabbit model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1995;36:2388–2395 [PubMed] [Google Scholar]
- 25. Lei H, Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem. 2009;284:6329–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ikuno Y, Kazlauskas A. TGFβ1-dependent contraction of fibroblasts is mediated by the PDGFα receptor. Invest Ophthalmol Vis Sci. 2002;43:41–46 [PubMed] [Google Scholar]
- 27. Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR. In vivo models of proliferative vitreoretinopathy. Nat Protoc. 2007;2:67–77 [DOI] [PubMed] [Google Scholar]
- 28. Lei H, Velez G, Hovland P, Hirose T, Gilbertson D, Kazlauskas A. Growth factors outside the PDGF family drive experimental PVR. Invest Ophthalmol Vis Sci. 2009;50:3394–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lei H, Velez G, Kazlauskas A. Pathological signaling via PDGFRa involves chronic activation of Akt and suppression of p53. Mol Cell Biol. 2011;31:1788–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]