This is the first study to demonstrate that all lysyl oxidase (LOX) genes are expressed in human TM cells. This study also illustrates that transforming growth factor–β employs both Smad and non-Smad signaling to induce the LOX genes.
Abstract
Purpose.
The profibrotic cytokine TGFβ is associated with glaucoma and plays an important role in the regulation of extracellular matrix metabolism in the trabecular meshwork (TM). The purpose of this study was to determine whether expression of ECM cross-linking LOX genes is regulated by TGFβ in TM cells.
Methods.
Expression of the five LOX genes (LOX, LOXL1, LOXL2, LOXL3, and LOXL4) was examined in cultured human TM cells by using RT-PCR, quantitative RT-PCR, and Western immunoblot analysis. TM cells were treated with recombinant TGFβ1, -2, and -3, to determine the effects on LOX and LOXL1 to -4 expression. The TM cells were pretreated with TGFBR inhibitors (LY364947, SB431542), canonical Smad signaling pathway (SIS3 or Smad2, -3, and -4 siRNAs) inhibitors, or inhibitors of the non-Smad signaling pathways (SP600125, SR11302), to identify the signaling pathway(s) involved in TGFβ induction of LOX and LOXL gene and protein expression. A novel LOX activity assay was used to determine the effects of the LOX inhibitor BAPN on tropoelastin cross-linking.
Results.
All five LOX genes (LOX, LOXL1 to -4) were expressed in cultured human TM cells and were induced by all three isoforms of TGFβ. This TGFβ induction of LOX and LOXL expression was blocked by TGFβ inhibitors as well as by inhibitors of the canonical Smad2, -3, and -4 signaling and non-Smad JNK/AP-1 signaling pathways (P < 0.05).
Conclusions.
Both Smad and non-Smad signaling pathways are involved in TGFβ-mediated LOX induction, suggesting complex regulation of these important extracellular matrix cross-linking enzymes. Increased LOX activity may be at least partially responsible for TGFβ-mediated IOP elevation and increased aqueous humor outflow resistance.
Glaucoma is a leading cause of irreversible visual impairment and blindness in the world, with primary open-angle glaucoma (POAG) being the major form.1,2 Elevated intraocular pressure (IOP) is a major risk factor for the development and progression of glaucoma,3,4 and this ocular hypertension is due to increased aqueous humor outflow resistance in the trabecular meshwork (TM) and is associated with increased deposition of extracellular matrix (ECM) material within the TM. The profibrotic cytokine TGFβ2 has been implicated in the pathogenesis of POAG.5 Levels of TGFβ2 are elevated in the aqueous humor6–8 and TM (Tovar-Vidalez T et al., manuscript submitted) of POAG patients. TM cells express TGFβ receptors, and TGFβ2 has direct effects on the TM.9 TGFβ2 has been shown to increase aqueous outflow resistance and to elevate IOP in perfusion cultured human and porcine eyes10–12 and in rodent eyes.13 TGFβ1 is elevated in the aqueous humor of patients with exfoliation glaucoma,14 suggesting that this TGFβ isoform is associated with the accumulation of exfoliation material, including ECM proteins, in the anterior segments of patients with this syndrome.
TGFβ2 regulates ECM metabolism in TM cells and tissues. This cytokine increases expression of a variety of ECM proteins, including fibronectin, collagen, elastin, and proteoglycans, as well as PAI-1 and TIMP-1, inhibitors that suppress proteolytic degradation of the ECM.15 In addition, TGFβ2 increases expression of the ECM cross-linking enzyme transglutaminase (TGM)-2 in TM cells.16 The combination of increased ECM synthesis, increased cross-linking, and decreased degradation causes increased ECM deposition in the TM, which may be responsible for the TGFβ2-mediated increased resistance to aqueous humor outflow. Similar changes occur in the TM of POAG patients, with increased levels of fibronectin,17 collagen,18 PAI-1,19 and TGM2.20
In addition to TGM2, there is a second important class of ECM cross-linking enzymes. The lysyl oxidase (LOX) family contains five genes (LOX and LOXL1 to -4) encoding enzymes that covalently cross-link elastin and collagens via generation of aldehydes on lysine residues.21,22 This cross-linking reaction provides additional mechanical strength to the ECM and makes it more resistant to degradation. LOX enzymes play a role in a variety of fibrotic diseases.21–25 Single-nucleotide polymorphisms (SNPs) in LOXL1 are associated with a significantly increased risk of exfoliation glaucoma,26,27 further suggesting potential roles for LOXs in glaucoma pathogenesis. The purpose of the present study was to determine (1) whether the LOX and LOXL genes and proteins are expressed in human TM cells, (2) whether TGFβ induces LOX gene expression and activity in the TM, and (3) which TGFβ signaling pathway(s) regulate LOX expression in the TM.
Methods
TM Cell Culture
Human TM cells were isolated from carefully dissected human TM tissue explants derived from patients with glaucoma or from normal donors and characterized as previously described.9 All donor tissues were obtained from regional eye banks and managed according to the guidelines in the Declaration of Helsinki for research involving human tissue. Isolated TM cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen-Gibco, Grand Island, NY) containing l-glutamine (0.292 mg/mL; Invitrogen-Gibco), penicillin (100 U/mL)/streptomycin (0.1 mg/mL; Invitrogen-Gibco), and 10% fetal bovine serum (Invitrogen-Gibco).
TM Cell Treatments
TM cells were grown to 100% confluence, and the medium was then replaced with serum-free medium for 24 hours before the treatments, to avoid the effects of serum proteins on the treatments. The cells were incubated with fresh medium containing specific signaling inhibitors for 1 to 12 hours before the addition of various concentrations of recombinant human TGFβ1, -2, or -3 proteins (R&D System, Minneapolis, MN). The small-molecule inhibitors LY364947 (5 μM; Tocris Biosciences, Ellisville, MO) and SB431542 (5 μM; Sigma-Aldrich, St. Louis, MO) were used to examine the effects of inhibition of TGFβR-1/2. The Smad-3 phosphorylation inhibitor SIS3 (10 μM; Sigma-Aldrich), the JNK inhibitor SP600125 (10 μM; Sigma-Aldrich), and the AP-1 inhibitor SR11302 (5 μM; Tocris Biosciences) were used to examine the effects of inhibition on canonical Smad, JNK, and AP-1 signaling. Various concentrations of the LOX inhibitor β-aminopropionitrile (BAPN; Sigma-Aldrich) were used for the LOX activity assay.
Small Interfering RNA and Transfection
siRNAs (SMARTpool) for Smad2, -3, and -4; TGFBR1; and nontargeting control siRNAs were purchased from Dharmacon (Lafayette, CO). Transfection of siRNA was performed as described previously.28,29 Three TM cell strains were grown in 12-well plates containing DMEM with 10% FBS. In one tube, 4 μL of transfection reagent (DharmaFECT 1; Dharmacon) was mixed gently with 200 μL of reduced-serum medium (Opti-MEM; Invitrogen) and incubated for 5 minutes at room temperature. In separate tubes, various concentrations of siRNA were mixed gently with 200 μL of the medium. These two tubes were combined, gently mixed, and incubated for 20 minutes at room temperature. After incubation, DMEM without FBS and antibiotics was added to obtain a final volume of 2 mL in each well (10 nM of test siRNA and 10 nM of control siRNA). The cells were washed with sterile PBS and incubated with siRNA transfection solution for 24 hours at 37°C. They were washed with sterile PBS and incubated with 10% FBS containing DMEM for 24 hours at 37°C and then were washed with serum-free DMEM for 24 hours and treated with TGFβ2 in serum-free DMEM for 48 hours. The cell lysates were analyzed for various proteins by Western immunoblot analysis (Table 1).
Table 1.
Antibodies Used in Western Immunoblot Analyses
| Antibody | Dilution | Source |
|---|---|---|
| Rabbit anti-LOX | 1:10,000 | Novus Biologicals, Littleton, CO |
| Rabbit anti-LOXL1 | 1:500 | Abnova, Walnut, CA |
| Mouse anti-LOXL2 | 1:2,000 | R&D Systems, Minneapolis, MN |
| Mouse anti-LOXL4 | 1:250 | Abcam, Cambridge, MA |
| Mouse anti-ACTB | 1:1,000 | Millipore, Billerica, MA |
| Rabbit anti-GAPDH | 1:1,000 | Cell Signaling, Danvers, MA |
| Rabbit anti-TGFBR1 | 1:250 | Abcam |
| Rabbit anti-SMAD2 | 1:1,000 | Cell Signaling |
| Rabbit anti-phos-SMAD2 | 1:1,000 | Cell Signaling |
| Rabbit anti-SMAD3 | 1:1,000 | Cell Signaling |
| Rabbit anti-phos-SMAD3 | 1:1,000 | Cell Signaling |
| Rabbit anti-SMAD4 | 1:1,000 | Cell Signaling |
| Rabbit anti-JNK1/2 | 1:1,000 | Cell Signaling |
| Rabbit anti-phos-JNK1/2 | 1:1,000 | Cell Signaling |
| Mouse anti-ELN | 1:500 | Millipore |
| Donkey anti-mouse IgG | 1:10,000 | Santa Cruz Biotechnology, Santa Cruz, CA |
| Goat anti-rabbit IgG | 1:10,000 | Santa Cruz Biotechnology |
RNA Isolation, RT-PCR, and Agarose Gel Electrophoresis
Total cellular RNA was prepared from cultured TM cells (TRI Reagent RT extraction; MRC Inc., Cincinnati, OH), and a cDNA synthesis kit (SuperScript VILO; Invitrogen) was used for first-strand cDNA synthesis with 1 μg of total RNA. Primers for the various LOX proteins were designed with Primer3 software (http://frodo.wi.mit.edu/primer3/; Whitehead Institute, Massachusetts Institute of Technology, Cambridge, MA). The primer pairs are listed in Table 2. The PCR products were loaded and electrophoresis performed on a 1.5% agarose gel.
Table 2.
Primers Used for PCR Studies
| Gene | Primer (5′ → 3′) |
|---|---|
| LOX | |
| Left | CGACCCTTACAACCCCTACA |
| Right | AAGTAGCCAGTGCCGTATCC |
| LOXL1 | |
| Left | AGAGCCTCTCTGTCCACCAG |
| Right | GTACACCTGCCCGTTGTTGTTCT |
| LOXL2 | |
| Left | CCTGGGGAGAGGACATACAA |
| Right | CTCGCAGGTGACATTCTTCA |
| LOXL3 | |
| Left | CAACGCGGCCTTCTACAG |
| Right | GGTGTCATTGGCACGATAGA |
| LOXL4 | |
| Left | CGACAGCCACTACTACAGGAAA |
| Right | CTGGTGGATCCAGAAGGAGTT |
| ACTB | |
| Left | GTCCACCTTCCAGCAGATGT |
| Right | AAAGCCATGCCAATCTCATC |
Quantitative Real-Time RT-PCR
Real-time RT-PCR was performed as described previously.30 Briefly, 2.5 μL (∼100 ng) of cDNA was used in a reaction consisting of 1.5 units per reaction of antibody-bound Taq enzyme (Jump Start; Sigma-Aldrich), 10× PCR buffer, 1.5 mM MgCl2, 200 nM dNTP mix, 100 nM respective primers, 2.5 μL green nucleic acid dye (EvaGreen; Biotium, Hayward, CA), and 30 nM passive reference dye (Rox; USB, Cleveland, OH) per 50-μL reaction. PCR was performed on a real-time thermal cycler (model Mx3000p; Stratagene, La Jolla, CA), with cycling parameters of initial denaturation at 95°C; 40 cycles of 95°C, 30 seconds; 60°C, 30 seconds; and 72°C, 60 seconds; and a denaturation cycle for creation of dissociation curves. Reactions for each sample and gene of interest were run in duplicate, cycle thresholds (Ct) were normalized to β-actin expression as a housekeeping gene, and comparative quantitation was performed (MxPro ver. 4.0 software; Stratagene). Only individual PCR samples with single-peak dissociation curves were selected for data analysis.
Protein Extraction and Western Immunoblot Analysis
Total cellular protein was extracted from the TM cells using mammalian protein extraction buffer (MPER; Pierce Biotech, Rockford, IL) containing protease inhibitor and phosphatase inhibitor cocktails (both from Pierce Biotech). Protein concentration was determined with a protein assay system (Dc assay; Bio-Rad Laboratories, Hercules, CA). The cellular proteins were separated on denaturing polyacrylamide gels and then transferred to PVDF membranes by electrophoresis. Blots were blocked with 5% fat-free dry milk in tris-buffered saline-Tween (TBST) buffer for 1 hour and then incubated overnight with primary antibodies (Table 1). The membranes were washed with TBST and processed with corresponding horseradish peroxidase-conjugated secondary antibodies (Table 1). The proteins were then visualized in an imager (Fluor Chem 8900; Alpha Innotech, San Leandro, CA) using ECL detection reagent substrate (SuperSignal West Femto Maximum Sensitivity Substrate; Pierce Biotechnology). To ensure equal protein loading, the same blot was subsequently developed for β-actin or GAPDH expression.
Statistical Analysis
For comparing results between two groups, Student's t-test was performed. For comparison of results between more than two groups, one-way ANOVA was used. Data reported are the mean ± SD.
Results
Expression of LOX Family Members in TM Cells
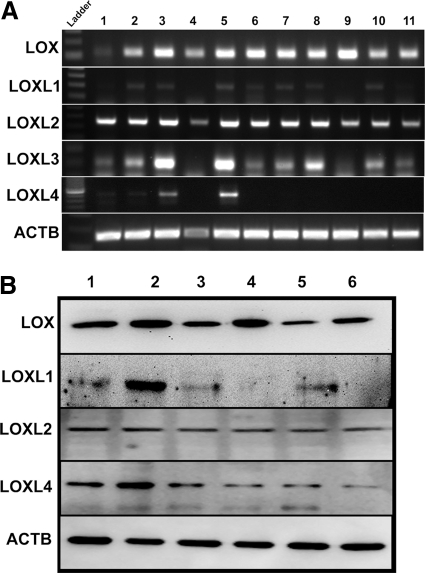
The expression of all five members of the LOX gene family in the TM has not been studied. Therefore, we sought to determine whether LOX and LOXL mRNA and proteins are expressed in cultured human TM cells. Using RT-PCR, we profiled the cDNA samples obtained from 11 TM cell strains (Fig. 1A), and the protein expression was studied in 6 TM cell strains (Fig. 1B). LOX and LOXL1 to -4 mRNA (Fig. 1A) and LOX, LOXL1, -2, and -4 proteins were expressed in multiple TM cell strains, although there appeared to be differences in basal LOX protein expression among the TM cell strains. We could not study LOXL3 protein expression because of the lack of a commercially available antibody.
Figure 1.
Expression of LOX and other LOX-like genes in TM. (A) RT-PCR on RNA samples from 11 TM cell strains for the five LOX genes and β-actin as the housekeeping gene. The agarose gel image represents data generated in three independent experiments. (B) Western immunoblots of protein samples from six TM cell strains. Antibodies specific to LOX, LOXL1, LOXL2, and LOXL4 were used with β-actin (ACTB) as the loading control. The blot represents data from three independent experiments.
TGFβ1, -2, and -3 Induce LOX Genes in TM Cells
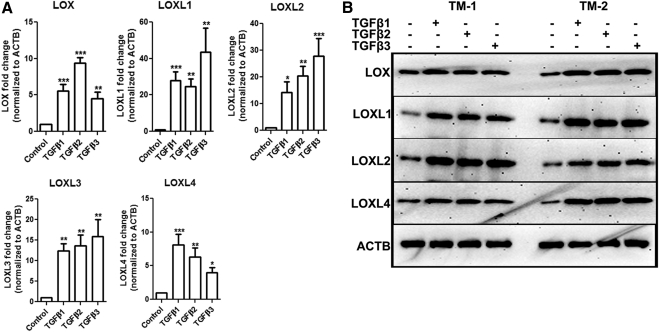
TM cells were treated with TGFβ1, -2, or -3 (5 ng/mL) for 12 hours followed by RNA extraction and qRT-PCR. Each TGFβ isoform significantly induced LOX and LOXL1 to -4 mRNA (n = 3, P < 0.05; Fig. 2A). We also treated six TM cell strains with TGFβ1, -2, or -3 (5 ng/mL) for 48 hours, and Western immunoblot analysis of TM cell lysates was used to study the effects on LOX and LOXL protein expression. TGFβ1, -2, and -3 induced LOX and LOXL1, -2, and -4 protein expression in all the TM cell strains tested. The blot represents data generated in two of the six cell strains used for the experiment (Fig. 2B).
Figure 2.
TGFβ1, -2, and -3 each induced LOXs in TM cells. (A) Induction of LOX/LOXL mRNA in three TM cell strains treated with TGFβ1, -2, or -3 (5 ng/mL) for 12 hours. Data represent the ratio of induction of LOXs normalized to ACTB. Three replicates of each sample were used. All three TGFβ isoforms induced LOXs in all the three cell lines. Student's t-test was used for statistical analyses. *0.01 < P < 0.05; **0.0001 < P < 0.01; ***P < 0.0001. (B) Western immunoblots of LOX/LOXL in two TM cell strains treated with TGFβ1, -2, or -3 (5 ng/mL) for 48 hours. The TGFβ isoforms induced LOX proteins compared with ACTB. Similar results were observed in four additional TM cell strains. The image is representative of three independent experiments.
TGFβ1, -2, and -3 Induce LOXs in a Concentration- and Time-Dependent Fashion
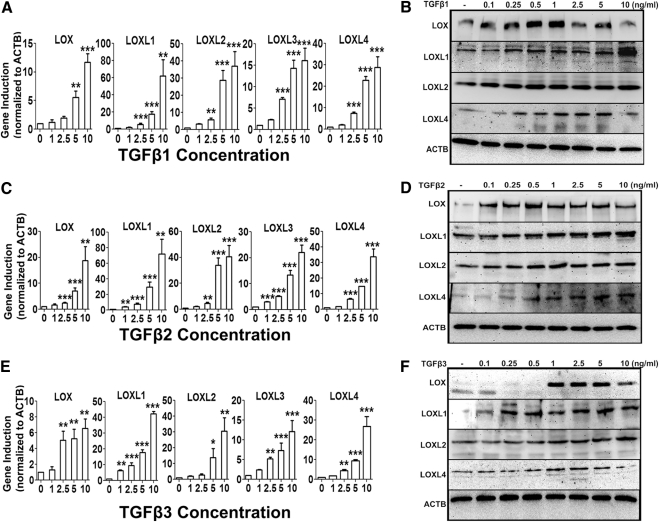
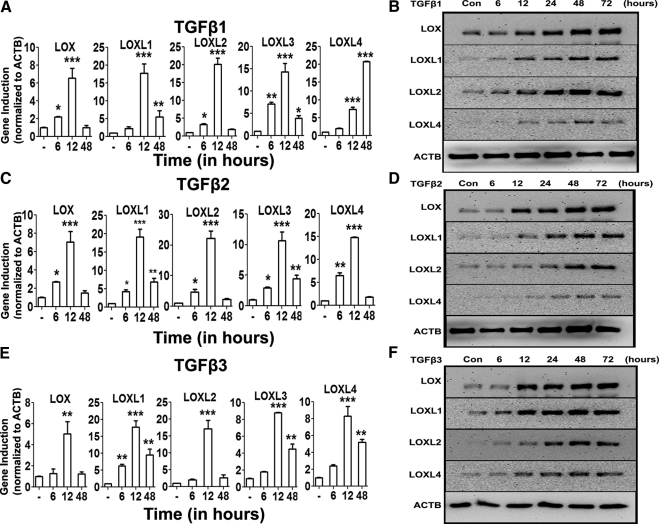
TM cell strains (n = 3) were treated with increasing concentrations of TGFβ1, -2, or -3 (0–10 ng/mL) for 48 hours. The mRNA and protein expression of LOX and LOXL genes were determined with qRT-PCR and Western immunoblot analysis, respectively. TGFβ1 (Figs. 3A, 3B), TGFβ2 (Figs. 3C, 3D), and TGFβ3 (Figs. 3E, 3F) each induced LOX and LOXL1- to -4 mRNA and LOX and LOXL1, -2, and -4 protein expression in a concentration-dependent manner. The maximum induction for mRNA appeared to be ∼10 ng/mL, whereas the maximum induction of proteins was seen at ∼1 ng/mL. TM cells were treated with TGFβ1, -2, or -3 for 6, 12, and 48 hours, to examine whether TGFβ induces LOXs mRNA in a time-dependent manner. The greatest mRNA induction of LOXs was observed at 12 hours (P < 0.05), and by 48 hours, there was little or no difference in expression between untreated and TGFβ1, -2, or -3–treated groups (Figs. 4A, 4C, 4E). Similarly, TM cell strains (n = 2) were treated with TGFβ1, -2, or -3 (5 ng/mL) for 6, 12, 24, 48, and 72 hours, to evaluate effects on LOX and LOXL protein expression. TGFβ ligands induced LOX and LOXL proteins as early as 24 hours and maintained the induction up to 72 hours (Figs. 4B, 4D, 4F). Therefore, TGFβ induction of LOX and LOXL gene and protein expression was both time and dose dependent.
Figure 3.
Concentration-dependent TGFβ induction of the LOXs. Dose-dependent induction of LOX and LOXL mRNA (A, C, E) and protein (B, D, F) by 0 to 10 ng/mL TGFβ1 (A, B), -2 (C, D), and -3 (E, F) in cultured TM cell strains (n = 3). qRT-PCR values (A, C, E) represent TGFβ induction compared with the controls and normalized to ACTB as housekeeping gene. Three replicates of each sample were used. One-way ANOVA was used for statistical analyses. **0.0001 < P < 0.01; ***P < 0.0001. Western immunoblots (B, D, F) are representative of data obtained in the three TM cell strains; 5 ng/mL of TGFβ1, -2, and -3 induced maximum LOX/LOXL expression.
Figure 4.
Time-dependent TGFβ induction of the LOXs. Time course induction (0–72 hours) of LOX and LOXL mRNA (A, C, E) and protein (B, D, F) by 5 ng/mL TGFβ1 (A, B), -2 (C, D), and -3 (E, F) in cultured TM cell strains (n = 2). qRT-PCR values (A, C, E) represent TGFβ ratio of induction compared with controls and normalized to ACTB as the housekeeping gene. Three replicates of each sample were used. One-way ANOVA was used for statistical analyses: *0.01 < P < 0.05; **0.0001 < P < 0.01; ***P < 0.0001. Western immunoblots (B, D, F) are representative of data obtained in the three TM cell strains.
TGFβ Signaling in LOX and LOXL Induction
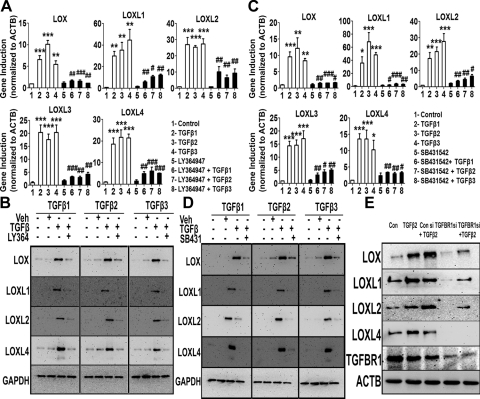
We used various small-molecule inhibitors to determine the TGFβ signaling pathway(s) involved in LOX and LOXL induction. SB431542 is a widely used, selective TGFBR1 and -2 receptor inhibitor.31 LY364947 is a relatively selective inhibitor for the TGFBR2 receptor.32 We treated TM cell strains (n = 3) with recombinant TGFβ1, -2, or -3 (5 ng/mL) for 12 hours, with or without a 1-hour pretreatment with 5 μM SB431542 or LY364947. Total RNA was isolated for qRT-PCR analysis. Each TGFβ isoform elevated LOX and LOXL1 to -4 expression compared with untreated or inhibitor-only–treated samples (P < 0.01). Pretreatment with either of the two inhibitors LY364947 (Fig. 5A) or SB431542 (Fig. 5C) blocked the TGFβ-mediated induction in all the cell strains (P < 0.05).
Figure 5.
TGFβ receptor inhibition blocked TGFβ induction of the LOXs. Effect of the TGFBR inhibitors LY364947 (A, B) and SB431542 (C, D) on TGFβ1, -2, and -3 induction of LOX/LOXL mRNA (A, C) and protein (B, D) expression. qRT-PCR values (A, C) represent the ratio of gene induction normalized to ACTB as the housekeeping gene in treated samples compared with the controls (triplicates of three TM strains). One-way ANOVA was used for statistical analyses. *,#0.01<P < 0.05; **,##0.0001<P < 0.01; ***,###P < 0.0001. #Differences between TGFβ samples versus TGFβ+inhibitor samples; *differences between TGFβ-treated and the untreated cells. (B, D) Western immunoblots of TM cells treated with 5 ng/mL of TGFβ1, -2, and -3 for 48 hours along with 5 μM of LY364947 (B) or SB431542 (D). Untreated and DMSO-treated cells served as negative controls. GAPDH was used as the loading control. Blots shown are representative of data from four TM cell strains. (E) Western immunoblots of LOX and LOXL proteins after siRNA-mediated TGFBR1 knockdown followed by TGFβ2 treatment. TM cells were treated with TGFBR1 or control siRNA, followed by treatment with 5 ng/mL of TGFβ2 for 48 hours. ACTB was used as a loading control. Blots are representative data from two TM cell strains.
We also treated three TM cell strains (n = 4) with or without TGFβ1, -2, or -3 (5 ng/mL) for 48 hours, with or without a 1-hour pretreatment with 5 μM SB431542 or LY364947. The LOX and LOXL proteins were analyzed by Western immunoblot. TGFβ1, -2, or -3 each elevated LOX and LOXL1, -3, and -4 compared with untreated or vehicle-treated samples. Each of the two inhibitors, LY364947 (Fig. 5B) and SB431542 (Fig. 5D), inhibited the TGFβ-mediated induction of LOX proteins. Treatment with the inhibitors alone did not have any effect on LOX and LOXL expression (data not shown).
In addition to the TGFBR1/2 inhibitors, we also used siRNA-mediated TGFB receptor 1 (TGFBR1) knockdown to confirm the role of TGFβ receptor signaling in LOX and LOXL induction. TGFβ2-treated TM cells were untransfected or transfected with a nontargeting siRNA control or TGFBR1 siRNA. As previously shown, TGFβ2-induced LOX and LOXL protein expression. Control siRNAs did not affect endogenous TGFBR1 levels and did not affect TGFβ2-induction of LOX and LOXL expression. Consistent with the data with small-molecule TGFBR1 or -2 inhibition, TGFBR1 knockdown inhibited TGFβ2-induction of LOX and LOXL proteins (Fig. 5E). These results strongly support TGFβ receptor–dependent regulation of LOX and LOXL protein expression.
TGFβ Induces LOX and LOXLs Using Both Smad and JNK Signaling Pathways
The profibrotic cytokine TGFβ has been shown to activate both canonical Smad and noncanonical signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway in various cells and tissues.33–35 TGFβ signaling is complex, because these different signal transduction pathways can interact with each other.36,37 We wanted to determine which of these TGFβ signaling mechanisms are involved in LOX and LOXL induction in TM cells.
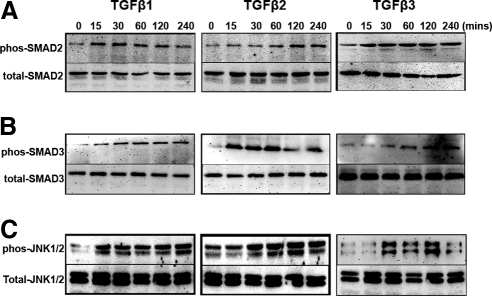
TGFβ2 activates canonical Smad and MAPK signaling in the TM cells.38 Three primary TM cell strains were treated with TGFβ1, -2, or -3 (5 ng/mL) for 15, 30, 60, 120, and 240 minutes, and total and phosphorylated Smad2, Smad3, and JNK1/2 proteins were evaluated by Western immunoblot. All three TGFβ ligands phosphorylated both Smad2 (Fig. 6A), Smad3 (Fig. 6B), and JNK1/2 (Fig. 6C) proteins during this time course. There were no changes in total Smad2, Smad3, or JNK1/2 levels.
Figure 6.
TGFβ activates both canonical and noncanonical signaling pathways in TM cells. Western immunoblots of Smad2/pSmad2 (A), Smad3/pSmad3 (B), and JNK1/2/pJNK1/2 (C) in four TM cell strains treated for 0 to 240 minutes with TGFβ1, -2, and -3. TGFβ1, -2, and -3 treatment caused a time-dependent increase in pSmad2, pSmad3, and pJNK1/2 expression.
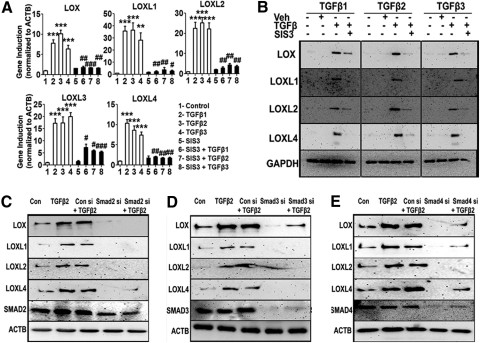
Phosphorylated Smad2 and -3 form a complex with co-Smad4 to regulate transcription of their target genes. To determine whether the Smad2/3 complex transcriptionally regulates the LOXs, we used SIS3, a selective small-molecule inhibitor of Smad3. Three TM cell strains were treated with SIS3 (10 μM) 6 hours before treatment with recombinant human TGFβ1, -2, or -3 for 12 or 48 hours to study mRNA and protein expression of LOXs, respectively. Untreated cells, DMSO-treated cells, and SIS3-alone–treated cells were the negative controls. TGFβ-induction of LOX and LOXL mRNA and protein expression were inhibited by SIS3 pretreatment (P < 0.01; Figs. 7A, 7B). Therefore, TGFβ-mediated Smad2/3 signaling in TM cells induced all members of the LOX gene family.
Figure 7.
Smad2, -3, and -4 inhibition blocked TGFβ-induction of the LOXs. Treatment of TM cells with the Smad3 inhibitor SIS3 blocked TGFβ1, -2, and -3 induction of LOX/LOXL mRNA (A) and protein (B) expression. (A) qRT-PCR analysis of the TGFβ1, -2, and -3 induction of the LOXs in the presence of a specific inhibitor of Smad3 (SIS3). qRT-PCR results represent the ratio of induction of LOX/LOXL genes normalized to ACTB, the housekeeping gene, in TGFβ-treated samples compared with the controls (triplicates of three TM cell strains). One-way ANOVA was used for statistical analyses. **,##0.0001 < P < 0.01; ***,###P < 0.0001. #Differences between TGFβ1, -2, and -3 samples versus TGFβ+inhibitor samples; *differences between TGFβ1, -2, and -3–treated versus untreated cells. (B) Western immunoblots of LOX/LOXL proteins after pretreatment with SIS3 followed by TGFβ treatment. Immunoblots are representative of three different TM cell strains treated with 5 ng/mL of TGFβ1, -2, and -3 for 48 hours along with 10 μM of SIS3. GAPDH was used as loading control. Untreated and DMSO-treated cells served as negative controls. (C, D, E) Western immunoblots of LOX/LOXL proteins in TM cells pretreated with Smad2 (C), -3 (D), or -4 (E) siRNAs followed by TGFβ2 treatment. Control cells were transfected with nontargeting siRNA. Immunoblots are representative of results from two TM cell lines. Each Smad siRNA not only knocked down its target protein, but also suppressed the TGFβ2 induction of LOX/LOX proteins.
To confirm the role of Smad signaling in regulation of LOX expression, we used siRNA-mediated knockdown of Smad2, -3, and -4. Nontargeting siRNA served as the negative control. Cells transfected with Smad2 (Fig. 7C), Smad3 (Fig. 7D), or Smad4 (Fig. 7E) siRNAs were subsequently treated with or without TGFβ2. Untransfected and untreated cells served as negative controls, whereas untransfected cells treated with TGFβ2 served as the positive control. TGFβ2 induced expression of LOX and LOXL proteins. Control siRNAs neither affected TGFβ2-induction of LOXs nor did they affect the endogenous Smad2, -3, and -4 levels. As expected, knockdown of Smad2, -3, or -4 inhibited TGFβ2-induction of the LOX and LOXL proteins.
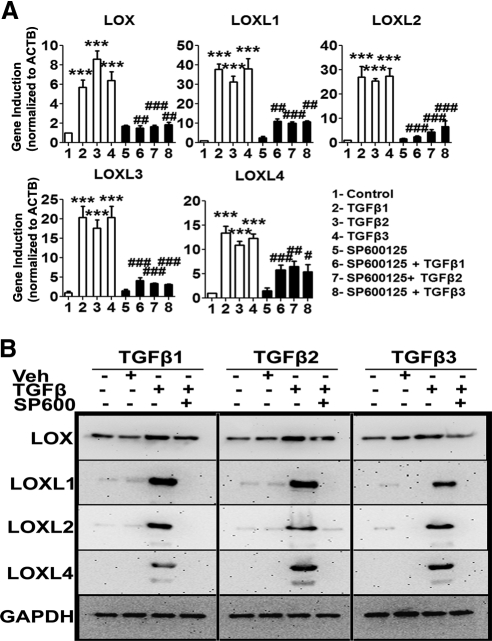
In addition to activating Smad signaling, TGFβ also activated the non–Smad JNK1/2 signaling pathway (Fig. 6C). We used the selective JNK inhibitor SP600124 to determine whether TGFβ-activated JNK signaling regulates LOX and LOXL induction. Three TM cell strains were pretreated for 6 hours with SP600125 (10 μM) before incubation with or without recombinant human TGFβ1, -2, and -3 (5 ng/mL) for 12 and 48 hours, to study LOX and LOXL mRNA and protein expression, respectively. Untreated, DMSO-treated, and SP600125-only–treated cells served as negative controls. SP600125 pretreatment inhibited TGFβ-induction of LOX and LOXL mRNA (P < 0.05; Fig. 8A) and protein (Fig. 8B) expression in TM cells. SP600125 alone did not alter endogenous LOX or LOXL mRNA (Fig. 8A) or protein (data not shown) expression. Taken together, these data strongly suggest that both Smad and JNK signaling regulate LOXs in TM cells.
Figure 8.
JNK1/2 inhibition blocked TGFβ induction of LOXs. Effect of JNK1/2 inhibitor SP600125 on TGFβ1, -2, and -3 induction of LOX/LOXL mRNA (A) and protein (B) expression in cultured TM cells. (A) qRT-PCR analysis results represent the induction ratio of LOX/LOXL genes normalized to ACTB in treated samples compared with controls. Cumulative data for experiments performed in triplicate in two TM strains. One-way ANOVA was used for statistical analyses. #0.01<P < 0.05; ##0.0001<P < 0.01; ***,###P < 0.0001. #Differences between TGFβ samples versus TGFβ+inhibitor samples; *differences in TGFβ-treated versus the untreated cells. (B) Western immunoblots of LOX/LOXL proteins in TM cells pretreated with SP600125 followed by TGFβ1, -2, and -3 treatment. Immunoblots are representative of three different TM cell strains. GAPDH was used as the loading control. Untreated and DMSO-treated cells served as negative controls. SP600125 suppressed TGFβ induction of LOX/LOXL mRNAs and proteins, suggesting involvement of the JNK1/2 pathway.
AP-1 Regulates TGFβ-Induction of LOXs
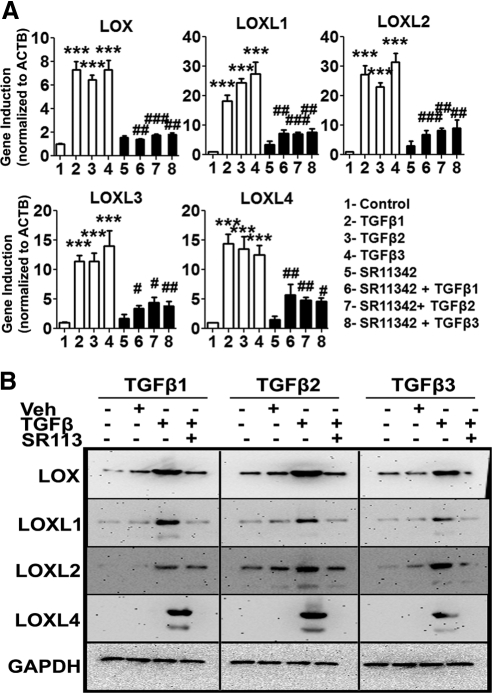
The transcription factor AP-1 comprises two units: c-Fos and c-Jun. The Jun subunit is phosphorylated by active JNK1/2, which subsequently activates transcription of AP-1 target genes.39 However, AP-1 also acts as a cotranscription factor of other gene regulators, such as the Smad2, -3, and -4 complex.40 Our data strongly indicate that both Smad (Fig. 7) and JNK1/2 signaling (Fig. 8) regulate LOX and LOXL expression, so we wanted to determine the role of AP-1 as the transcriptional regulator of the LOXs. We treated TM cell strains (n = 3) with SR11302 (5 μM), a small molecule AP-1 inhibitor,41 for 12 hours before treatment for 12 or 48 hours with 5 ng/mL of TGFβ1, -2, or -3. The 12-hour TGFβ treatment group was analyzed for effects on mRNA and the 48-hour treatment group for effects on LOX and LOXL protein expression. Pretreatment with the AP-1 inhibitor significantly reduced the TGFβ1, -2, and -3-induction of LOXs and LOXL mRNA (P < 0.01; Fig. 9A) and protein (Fig. 9B) expression. These data indicate that regulation of LOX genes by TGFβ involves both canonical and noncanonical signaling pathways, which may interact by sharing common transcription factors, such as AP-1.
Figure 9.
AP-1 inhibition blocked TGFβ-induction of the LOXs. Effect of AP1 inhibitor SR11302 on TGFβ1, -2, and -3 induction of LOX/LOXL mRNA (A) and protein (B) expression in cultured TM cells. (A) qRT-PCR analysis values represent the induction ratio of the of LOX/LOXL genes normalized to ACTB in treated samples compared with the controls. Cumulative data for experiments performed in triplicate in two TM strains. One-way ANOVA was used for statistical analyses. #0.01 < P < 0.05; ##0.0001 < P < 0.01; ***,###P < 0.0001. #Differences between TGFβ samples versus TGFβ+inhibitor samples; *differences for TGFβ-treated versus the untreated cells. (B) Western immunoblots of LOX/LOXL proteins in TM cells pretreated with SR11342 followed by TGFβ1, -2, and -3 treatment. Immunoblots are representative of three different TM cell strains studied. GAPDH was used as the loading control. Untreated and DMSO-treated cells served as negative controls. SP600125 suppressed TGFβ induction of LOX/LOXL mRNAs and proteins supporting involvement of the JNK1/2 and AP-1 signaling pathways.
TGFβ Regulates LOX Enzymatic Activity in the Cultured TM Cells
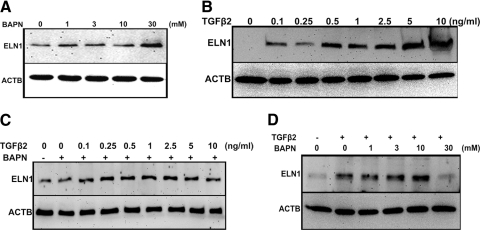
Finally, we wanted to determine whether TGFβ regulates the enzymatic activity of LOXs in TM cells. Since LOXs catalyze elastin and collagen cross-linking, we wanted to determine the potential role of LOXs in TGFβ cross-linking of elastin in the TM cells. We used the irreversible LOX inhibitor, β-aminopropionitrile (BAPN), to block elastin cross-linking.42,43 TM cells were treated for 48 hours with increasing concentrations of BAPN, and cell lysates were analyzed by Western immunoblot for tropoelastin, the soluble non–cross-linked form of elastin. BAPN should decrease the cross-linking of elastin leading to higher levels of the substrate, tropoelastin. Indeed, we observed that increasing concentrations of BAPN elevated tropoelastin levels in the TM cells (Fig. 10A; n = 3).
Figure 10.
LOX activity assay. (A) Representative Western immunoblot for tropoelastin (normalized to ACTB) in TM cells treated with increasing concentrations (1, 3, 10, and 30 mM) of the LOX inhibitor BAPN. There was a concentration-dependent increase in tropoelastin levels. (B) Representative Western immunoblot for tropoelastin (normalized to ACTB) in TM cells treated with increasing concentrations of TGFβ2 (0.1, 0.25, 0.5, 1, 2.5, 5, and 10 ng/mL). There was a concentration-dependent increase in tropoelastin levels. (C) Representative Western immunoblot for tropoelastin (normalized to ACTB) in TM cells treated with 1 mM of BAPN, along with increasing concentrations of TGFβ2 (0.1, 0.25, 0.5, 1, 2.5, 5, and 10 ng/mL). (D) TM cells were treated with 5 ng/mL of TGFβ2, along with increasing concentrations of BAPN (1, 3, 10, and 30 mM) and probed for tropoelastin levels. Each of these Western blots is representative of independent experiments performed in three different TM cell strains.
We next examined the role of TGFβ-induced LOXs in regulating elastin cross-linking. TM cells were treated with increasing concentrations of TGFβ2 for 48 hours, and levels of tropoelastin were determined by Western immunoblot. Increasing TGFβ2 concentrations increased tropoelastin in the TM cells (Fig. 10B). We followed these studies by treating TM cells with increasing concentrations of TGFβ2, along with BAPN (1 mM) for 48 hours. Cells treated with TGFβ2 and BAPN showed greater tropoelastin levels, even at lower TGFβ2 concentrations (Fig. 10C). We observed similar results with TGFβ1 and -β3 treatment (data not shown). In addition to inducing LOX and LOXL expression, TGFβ2 also increased tropoelastin expression. Since BAPN is an irreversible inhibitor of LOX, it blocked the LOX enzymatic activity and appeared to result in higher levels of the LOX substrate tropoelastin, both in the absence and presence of TGFβ2.
We also used the reverse strategy, treating TM cells with a single concentration of TGFβ2 (5 ng/mL) along with increasing concentrations of BAPN. TGFβ2 elevated tropoelastin levels. However, cells co-treated with various BAPN concentrations further increased tropoelastin levels (Fig. 10D). The effect of BAPN appeared to be concentration dependent at 1 to 10 mM BAPN concentrations, but not at 30 mM, perhaps owing to BAPN toxicity at this concentration. TGFβ2 induced both tropoelastin and LOXs, but inhibiting the LOXs decreased tropoelastin cross-linking thereby increasing levels of uncross-linked tropoelastin. Similar results were observed with TGFβ1 and -3 (data not shown), indicating that the three TGFβ ligands increase LOX activity in TM cells. Taken together, these data show that LOXs are enzymatically active in TM cells and that TGFβ regulates LOX activity.
Discussion
We have shown that all five LOX genes are expressed in multiple human TM cell strains and that all three TGFβ isoforms induce mRNA and protein expression of these LOX and LOXL genes. We developed a novel LOX activity assay and showed basal LOX enzyme activity in TM cells, which can be further induced by TGFβ. Finally, we demonstrated that, canonical Smad as well as non-Smad JNK1/2 and AP-1 signaling pathways are involved in the TGFβ induction of the LOX and LOXL genes. Figure 11 schematically summarizes these data. It was somewhat surprising to find that all five LOX genes were expressed in the TM and induced by TGFβ. Why would the TM require this redundancy in this class of enzyme? The LOX and LOXL enzymes may have subtle differences in enzyme activity, secondary regulation, and/or cellular or tissue localization, which warrants further investigation.
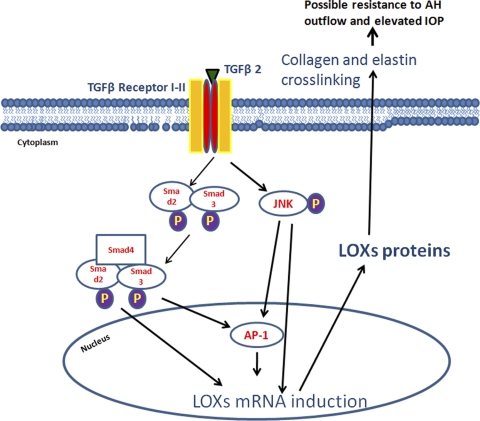
Figure 11.
Proposed mechanism of TGFβ regulation of LOXs in TM and implications in glaucoma. TGFβ ligands bind to the TGFBR1/2 receptor heterotetramer complex on the cell surface and activate the Smad2/Smad3 complex, which utilizes Smad4 to translocate to the nucleus. This complex may bind by itself or with AP-1 to the upstream promoter regions of LOX genes to regulate their transcription. TGFβ receptor activation also appears to phosphorylate and activate JNK1/2, which phosphorylates the Jun component of the transcription factor AP-1. AP-1 binds to the upstream promoter region of LOXs to regulate their gene transcription. LOX mRNA transcribes to proteins and cross-links collagen and elastin fibrils in the ECM of TM cells.
These LOX enzymes may play a role in the pathogenesis of glaucoma. TGFβ2 levels are higher in the aqueous humor5–8 and TM (Tovar-Vidales et al. manuscript submitted) of POAG patients, and TGFβ2 also regulates ECM metabolism in the TM.11,15,16 The TM of POAG patients increases elastic-sheath–derived material44,45 and increases type VI curly collagen.18 Elastin, fibrillins, and collagen are substrates for the LOX enzymes, and increased levels of these molecules in glaucoma TM tissues may be due to decreased turnover of these cross-linked molecules (Fig. 11). A recent report used atomic force microscopy to show that TM tissues from glaucomatous eyes are significantly stiffer than the TM from nonglaucomatous eyes.46 Greater cross-linking of the TM ECM could lead to greater tissue stiffness. TGFβ induction of the two major classes of ECM cross-linking enzymes, LOXs and TGM2, may be involved in enhanced ECM deposition and greater TM stiffness, which ultimately may be responsible for the increased outflow resistance and elevated IOP in POAG.
There is a confirmed genetic association of LOXL1 with exfoliation glaucoma.26,27 Allele frequency differences in LOXL1 SNPs significantly increase the risk for development of exfoliation glaucoma. Interestingly, TGFβ1 has been reported to be elevated in the aqueous humor of patients with exfoliation syndrome and exfoliation glaucoma,14 and we have shown that TGFβ1 enhances LOXL1 expression in the TM. Exfoliation syndrome and exfoliation glaucoma are associated with increased levels of exfoliation material in the anterior segment and elsewhere in the body.47 This exfoliation material comprises a variety of ECM proteins, including elastic fibrillins, among others. It is possible that the LOXL1 gene of exfoliation glaucoma alters the cross-linking activity, which may cause the formation of exfoliated material in the anterior segment leading to compromised aqueous outflow and elevated IOP. The morphologic changes in exfoliation glaucoma may be due to TGFβ1-induced LOX cross-linking of exfoliation material in the outflow pathway. In contrast, elevated TGFβ2 levels in POAG may activate LOXs to cross-link endogenous ECM molecules in the TM. Therefore, it is not surprising that the two forms of glaucoma have differing morphologies.
TGFβ1 and -2 are profibrotic cytokines that play a pathogenic role in other fibrotic diseases, such as sclerosis, fibrosclerosis, and kidney, lung, and liver fibroses. Similar to POAG, the fibrotic changes at the cellular and tissue levels arise from disordered and exaggerated deposition of the ECM, including collagens, elastin, and fibronectin. Fibrotic ECM remodeling also involves cross-linking of ECM molecules. LOX and LOXL1 enzymes are overexpressed in several types of tissue fibrosis.21,22,48,49 Therefore, understanding the roles of LOX and LOXL enzymes in glaucoma may have broader implications for other serious fibrotic diseases.
The potential relationship between the LOX and LOXL genes in regulating aqueous outflow in TGFβ2-induced ocular hypertension, POAG, and exfoliation glaucoma warrants further study. Are all five LOX genes functionally redundant or does each serve a specific role in TM ECM metabolism? Do any of these LOX genes play a direct role in TGFβ2-induced ocular hypertension? Which LOX genes are more important in normal TM homeostasis, and are any of them directly involved in glaucoma pathogenesis? Does increased expression of any of the LOX or LOXL genes cause glaucomalike morphologic changes in the TM and directly cause IOP elevation? Our current results provide a foundation for addressing these questions.
Acknowledgments
The authors thank Allan Shepard, Nasreen Jacobson, and Michael R. Waggoner for their insights and help with the project.
Footnotes
Supported by National Institutes of Health, National Eye Institute Grant EY-017374 (RJW).
Disclosure: A. Sethi, None; W. Mao, None; R.J. Wordinger, None; A.F. Clark, None
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720 [DOI] [PubMed] [Google Scholar]
- 3. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713, discussion 829–730 [DOI] [PubMed] [Google Scholar]
- 4. The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440 [DOI] [PubMed] [Google Scholar]
- 5. Lütjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4 [DOI] [PubMed] [Google Scholar]
- 6. Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727 [DOI] [PubMed] [Google Scholar]
- 7. Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113 [DOI] [PubMed] [Google Scholar]
- 8. Picht G, Welge-Luessen U, Grehn F, Lütjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207 [DOI] [PubMed] [Google Scholar]
- 9. Wordinger RJ, Clark AF, Agarwal R, et al. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest Ophthalmol Vis Sci. 1998;39:1575–1589 [PubMed] [Google Scholar]
- 10. Bachmann B, Birke M, Kook D, Eichhorn M, Lütjen-Drecoll E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–2020 [DOI] [PubMed] [Google Scholar]
- 11. Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234 [DOI] [PubMed] [Google Scholar]
- 12. Gottanka J, Chan D, Eichhorn M, Lütjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–158 [DOI] [PubMed] [Google Scholar]
- 13. Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076 [DOI] [PubMed] [Google Scholar]
- 14. Schlotzer-Schrehardt U, Zenkel M, Kuchle M, Sakai LY, Naumann GO. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001;73:765–780 [DOI] [PubMed] [Google Scholar]
- 15. Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res. 2009;88:683–688 [DOI] [PubMed] [Google Scholar]
- 16. Welge-Lussen U, May CA, Lütjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-β1 and TGF-β2. Invest Ophthalmol Vis Sci. 2000;41:2229–2238 [PubMed] [Google Scholar]
- 17. Babizhayev MA, Brodskaya MW. Immunohistochemical monitoring of the effect of a synthetic fibronectin-like peptide (Arg-Gly-Asp) on the age-related changes in the isolated human corneoscleral tissue of glaucomatous eyes. Mech Ageing Dev. 1993;72:1–12 [DOI] [PubMed] [Google Scholar]
- 18. Lütjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989;48:139–147 [DOI] [PubMed] [Google Scholar]
- 19. Dan J, Belyea D, Gertner G, Leshem I, Lusky M, Miskin R. Plasminogen activator inhibitor-1 in the aqueous humor of patients with and without glaucoma. Arch Ophthalmol. 2005;123:220–224 [DOI] [PubMed] [Google Scholar]
- 20. Tovar-Vidales T, Roque R, Clark AF, Wordinger RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2008;49:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660 [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez C, Rodriguez-Sinovas A, Martinez-Gonzalez J. Lysyl oxidase as a potential therapeutic target. Drug News Perspect. 2008;21:218–224 [DOI] [PubMed] [Google Scholar]
- 23. Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol. 2010; 299:H1–H9. [DOI] [PubMed] [Google Scholar]
- 24. Vadasz Z, Kessler O, Akiri G, et al. Abnormal deposition of collagen around hepatocytes in Wilson's disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol. 2005;43:499–507 [DOI] [PubMed] [Google Scholar]
- 25. Akiri G, Sabo E, Dafni H, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666 [PubMed] [Google Scholar]
- 26. Thorleifsson G, Magnusson KP, Sulem P, et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–1400 [DOI] [PubMed] [Google Scholar]
- 27. Schlotzer-Schrehardt U. Molecular pathology of pseudoexfoliation syndrome/glaucoma: new insights from LOXL1 gene associations. Exp Eye Res. 2009;88:776–785 [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi T, Liu X, Wen FQ, et al. Smad3 mediates TGF-beta1 induction of VEGF production in lung fibroblasts. Biochem Biophys Res Commun. 2005;327:393–398 [DOI] [PubMed] [Google Scholar]
- 29. Zode GS, Clark AF, Wordinger RJ. Bone morphogenetic protein 4 inhibits TGF-beta2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia. 2009;57:755–766 [DOI] [PubMed] [Google Scholar]
- 30. Wordinger RJ, Fleenor DL, Hellberg PE, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200 [DOI] [PubMed] [Google Scholar]
- 31. Laping NJ, Grygielko E, Mathur A, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64 [DOI] [PubMed] [Google Scholar]
- 32. Sawyer JS, Anderson BD, Beight DW, et al. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem. 2003;46:3953–3956 [DOI] [PubMed] [Google Scholar]
- 33. Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22(suppl 1):S79–S84 [DOI] [PubMed] [Google Scholar]
- 34. Omori S, Kitagawa H, Koike J, et al. Activated extracellular signal-regulated kinase correlates with cyst formation and transforming growth factor-beta expression in fetal obstructive uropathy. Kidney Int. 2008;73:1031–1037 [DOI] [PubMed] [Google Scholar]
- 35. Tsukada S, Westwick JK, Ikejima K, Sato N, Rippe RA. SMAD and p38 MAPK signaling pathways independently regulate alpha1(I) collagen gene expression in unstimulated and transforming growth factor-beta-stimulated hepatic stellate cells. J Biol Chem. 2005;280:10055–10064 [DOI] [PubMed] [Google Scholar]
- 36. Wrighton KH, Lin X, Feng XH. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009;19:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424 [DOI] [PubMed] [Google Scholar]
- 38. Liton PB, Li G, Luna C, Gonzalez P, Epstein DL. Cross-talk between TGF-beta1 and IL-6 in human trabecular meshwork cells. Mol Vis. 2009;15:326–334 [PMC free article] [PubMed] [Google Scholar]
- 39. O'Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–657 [PubMed] [Google Scholar]
- 40. Hall MC, Young DA, Waters JG, et al. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. J Biol Chem. 2003;278:10304–10313 [DOI] [PubMed] [Google Scholar]
- 41. Li JJ, Dong Z, Dawson MI, Colburn NH. Inhibition of tumor promoter-induced transformation by retinoids that transrepress AP-1 without transactivating retinoic acid response element. Cancer Res. 1996;56:483–489 [PubMed] [Google Scholar]
- 42. Narayanan AS, Siegel RC, Martin GR. On the inhibition of lysyl oxidase by aminopropionitrile. Biochem Biophys Res Commun. 1972;46:745–751 [DOI] [PubMed] [Google Scholar]
- 43. Saad FA, Torres M, Wang H, Graham L. Intracellular lysyl oxidase: effect of a specific inhibitor on nuclear mass in proliferating cells. Biochem Biophys Res Commun. 2010;396:944–949 [DOI] [PubMed] [Google Scholar]
- 44. Lütjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW. Quantitative analysis of ‘plaque material’ in the inner- and outer wall of Schlemm's canal in normal- and glaucomatous eyes. Exp Eye Res. 1986;42:443–455 [DOI] [PubMed] [Google Scholar]
- 45. Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775 [DOI] [PubMed] [Google Scholar]
- 46. Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ritch R, Schlotzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315 [DOI] [PubMed] [Google Scholar]
- 48. Thomassin L, Werneck CC, Broekelmann TJ, et al. The pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. J Biol Chem. 2005;280:42848–42855 [DOI] [PubMed] [Google Scholar]
- 49. Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182 [DOI] [PubMed] [Google Scholar]