The present study describes the role of the sodium-dependent monocarboxylate transporter SLC5A8 (SMCT1) in the transport of the cysteine prodrug 2-oxothiazolidine-4-carboxylate (OTC), and the resultant augmentation of glutathione production in RPE cells. Given the key causative role oxidative damage to RPE plays in the pathogenesis of AMD, the present study is critically important and highly clinically relevant.
Abstract
Purpose.
To evaluate the role of SLC5A8 in the transport of 2-oxothiazolidine-4-carboxylate (OTC) and to determine whether OTC augments glutathione production in RPE cells, thereby providing protection against oxidative stress.
Methods.
SLC5A8-mediated transport of OTC was monitored in Xenopus laevis oocytes by electrophysiological means. Saturation kinetics, Na+-activation kinetics, and inhibition by ibuprofen were analyzed by monitoring OTC-induced currents as a measure of transport activity. Oxidative stress was induced in ARPE-19 cells and primary RPE cells isolated from wild type and Slc5a8-/- mouse retinas using H2O2, and the effects of OTC on cell death and intracellular glutathione concentration were examined.
Results.
Heterologous expression of human SLC5A8 in X. laevis oocytes induced Na+-dependent inward currents in the presence of OTC under voltage-clamp conditions. The transport of OTC via SLC5A8 was saturable, with a Kt of 104 ± 3 μM. The Na+-activation kinetics was sigmoidal with a Hill coefficient of 1.9 ± 0.1, suggesting involvement of two Na+ in the activation process. Ibuprofen, a blocker of SLC5A8, inhibited SLC5A8-mediated OTC transport; the concentration necessary for half-maximal inhibition was 17 ± 1 μM. OTC increased glutathione levels and protected ARPE-19 and primary RPE cells isolated from wild type mouse retinas from H2O2-induced cell death. These effects were abolished in primary RPE isolated from Slc5a8-/- mouse retinas.
Conclusions.
OTC is a transportable substrate for SLC5A8. OTC augments glutathione production in RPE cells, thereby protecting them from oxidative damage. Transport via SLC5A8 is obligatory for this process.
Age-related macular degeneration (AMD) is the leading cause of blindness in elderly Americans.1 The vision loss in AMD ultimately results from damage to photoreceptor cells in the central retina, but the initial pathogenesis involves degeneration of RPE cells.1,2 The exact mechanisms responsible for RPE degeneration in AMD are not known; however, there is a burgeoning literature supporting a key causative role for oxidant-induced damage/death of RPE cells in the pathogenesis/progression of the disease.3,4 Glutathione is the most abundant endogenous antioxidant in retina, and is essential for protection of cells against oxidative stress.5–7 Interestingly, plasma levels of glutathione and glutathione-related enzymes are decreased in association with aging and AMD.8,9 As such, discovery of novel therapeutic strategies to enhance the antioxidant capacity of the RPE has immense clinical significance.
A number of mechanisms for modulating the availability of glutathione in cells have been described.10 Exogenous glutathione can be provided either directly or in the form of glutathione esters; however, previous studies suggest that administering glutathione may be of limited benefit to RPE cells.5,7,11–13 A very effective alternative to providing intact glutathione is provision of its amino acid constituents, especially cysteine, as it is the availability of cysteine that largely determines glutathione synthesis.10,14 The ability of RPE cells to rapidly synthesize glutathione from exogenously supplied cysteine has been documented.11 Therefore, promoting endogenous glutathione synthesis via administration of cysteine may be a very effective therapeutic strategy for protecting RPE cells against oxidative insult.
2-Oxothiazolidine-4-carboxylate (OTC) is a prodrug of cysteine. On entering cells, OTC interacts with the ubiquitous intracellular enzyme 5-oxoprolinase, readily generating cysteine.15 Providing cysteine in the form of OTC is ideal because the direct administration of cysteine is associated with high toxicity, specifically in the brain and retina.16 The ability of OTC to significantly augment intracellular glutathione synthesis is well documented in animals and humans.17–24 In fact, OTC is more effective than other cysteine precursors in repleting intracellular glutathione stores.18,19,24 Although the beneficial effects of OTC administration on intracellular glutathione levels have been shown in a number of different cell types, there have been no studies of the effect of OTC on glutathione synthesis in RPE. In addition, the mechanism by which OTC crosses the cell membrane to gain entry into cells is not known.
SLC5A8 (SMCT1) is a Na+-coupled transporter for a variety of monocarboxylates such as lactate, pyruvate, short-chain fatty acids, ketone bodies, and nicotinate.25 In retina, the transporter is thought to play a critical role in the transcellular transfer of lactate and ketone bodies, thereby serving a key role in the maintenance of energy status in the retina.26 We recently discovered that pyroglutamate (5-oxoproline) is a transportable substrate for SLC5A8.27 Given that OTC is a pyroglutamate analog, we asked whether OTC might also be recognized as a transportable substrate for SLC5A8. This question is relevant to RPE because the transporter is expressed in the basolateral membrane of this cell and therefore may be able to mediate the entry of OTC from choroidal circulation into RPE. The present studies show that SLC5A8 does indeed transport OTC. We also show for the first time that OTC augments endogenous glutathione production in RPE cells and that transport via SLC5A8 is obligatory for this process.
Methods
Materials
[14C]-Nicotinate was purchased from American Radiolabeled Chemicals (Saint Louis, MO). L-2-Oxo-4-thiazolidine carboxylate (OTC), ibuprofen, and γ-glutamyl p-nitroanilide were purchased from Sigma-Aldrich (Saint Louis, MO). Cell culture media and related reagents were from Invitrogen (Grand Island, NY). Glutathione (GSH) Glo assay kit was from Promega (Madison, WI), and Annexin V-FITC apoptosis kit from eBioscience (San Diego, CA). SLC5A8 cDNA was originally cloned from human intestine.28
Animals and Cell Culture
Slc5a8-/- mice have been described previously.29,30 The care and use of the animals adhered to institutional guidelines for the humane treatment of animals and to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Human RPE cells (ARPE-19) were obtained from American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM:F12), supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. Primary RPE cells (mRPE) were isolated from wild type and Slc5a8-/- mouse eyes as described previously.31 mRPE cells were cultured in DMEM:F12 medium supplemented with 25% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Passage 2 mRPE cells were used for all experiments.
Transport of OTC via Human SLC5A8 in the X. laevis Oocyte Heterologous Expression System
The preparation of capped cRNA from human SLC5A8 cDNA has been described.28 Mature oocytes from X. laevis were injected with 50 ng cRNA. Water-injected oocytes served as controls. The oocytes were used for electrophysiological studies 3 to 6 days after cRNA injection. Electrophysiological studies were performed by the two-microelectrode/voltage-clamp method.28,32 Oocytes were perifused with a NaCl-containing buffer (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES/Tris, pH 7.5) followed by the same buffer containing OTC or nicotinate. The membrane potential was clamped at −50 mV. The differences between the steady state currents measured in the presence or absence of substrates were considered as the substrate-induced currents. In the analysis of the saturation kinetics of OTC-induced currents, the Km Kt was calculated by fitting the values of the OTC-induced currents at different OTC concentrations to the Michaelis–Menten equation. The Na+-activation kinetics of OTC-induced currents was analyzed by measuring the OTC-specific currents in the presence of increasing concentrations of Na+, and the data were analyzed according to the Hill equation to determine the Hill coefficient (the number of Na+ ions involved in the activation process) and Kt for Na+. Because the expression levels varied significantly from oocyte to oocyte, data were normalized by taking the maximally induced SLC5A8-specific current in each oocyte as 1. The kinetic parameters were determined using SigmaPlot software (version 10; Systat Software, Chicago, IL).
The transport of OTC via SLC5A8 was also investigated by an alternative approach in which the ability of OTC to compete with nicotinate for transport via SLC5A8 was monitored. Uptake of [14]-nicotinate (30 μM) in water-injected and SLC5A8 cRNA-injected oocytes was measured for 1 hour in the presence and absence of 1 mM OTC. The effect of ibuprofen, an inhibitor of SLC5A8,33 on SLC5A8-mediated OTC transport was also studied. The dose–response relationship for ibuprofen-induced inhibition of OTC-induced currents in these oocytes was investigated by fitting the data to the Michaelis–Menten equation, which enabled us to determine the value for K0.5 (i.e., the concentration of ibuprofen necessary to cause 50% maximal inhibition of OTC-induced currents). Each uptake experiment was performed individually in 10 different oocytes, and the data are presented as mean ± SEM.
Induction of Oxidative Stress and Fluorescence-Activated Cell Sorting Analysis
ARPE-19 cells were seeded in 6-well plates (0.2 × 106 cells/well) and grown to ∼80% confluency. Various concentrations of hydrogen peroxide (H2O2) were then tested for induction of oxidative stress in these cells. Protective effects of OTC were studied by first preincubating cells with various concentrations of OTC for 2 hours, followed by treatment with H2O2 for 4 hours. Cell viability was determined via trypan blue exclusion assay and also by flow cytometric analysis of apoptosis using the Annexin V-FITC kit. Similar studies were performed in mRPE cells isolated from wild type or Slc5a8-/- mouse retinas.
Measurement of Cellular Levels of Glutathione and γ-Glutamyl Transpeptidase Activity
ARPE-19 cells and wild type and Slc5a8-/- mRPE cells were cultured in the presence or absence of OTC for 1 hour. Cellular glutathione concentrations were measured using the GSH Glo assay kit following both the manufacturer's instructions and our previously published protocol.34 In brief, the assay involves a series of two reactions. Luciferin is generated first from a luminogenic substrate, catalyzed by glutathione-S-transferase (GST) in the presence of glutathione. This luciferin is then detected as a luminescent signal that is directly proportional to the amount of luciferin formed and therefore to the amount of GSH present in the sample. The effect of OTC on γ-glutamyltranspeptidase (γ-GTPase) was measured using mouse kidney homogenates as the source of the enzyme.35 In addition, RT-PCR was performed to analyze the effects of OTC treatment in mRPE cells on the expression of enzymes responsible for regulating glutathione homeostasis: glutathione synthetase (GS), γ-glutamatecysteine ligase modulatory (GCLM) and catalytic (GCLC) subunits and γ-GTPase. The RT-PCR primers and corresponding annealing temperatures are listed in Table 1.
Table 1.
Sequence of PCR Primers
| Gene | Primer Sequences | Annealing Temperature (°C) | Expected Product Size (bp) |
|---|---|---|---|
| γ-GTPase | FWD: 5′-GATTCGGCACCACCATACAG-3′ | 56 | 160 |
| REV: 5′-TGGCTGCGGGTCAATCT-3′ | |||
| GS | FWD: 5′-CTAATGCGGTGGTGCTACTG-3′ | 55.2 | 278 |
| REV: 5′-ACACTTGGCAGCACGAGA-3′ | |||
| GCLM | FWD: 5′-GGCATGCTCCGTCCTT-3′ | 54.4 | 327 |
| REV: 5′-AGCAGTTCTTTGGGTCATT-3′ | |||
| GCLC | FWD: 5′-ATGGGGCTGCTGTCCCAAGG-3′ | 56 | 219 |
| REV: 5′-CCTTGGGACAGCAGCCCCAT-3 |
GCLM, γ-glutamatecysteine ligase modulatory subunit; GCLC, γ-glutamatecysteine ligase catalytic subunit; GS, glutathione synthetase; γ-GTPase; γ-glutamyltranspeptidase.
Data Analysis
Electrophysiological measurements of OTC-induced currents were repeated at least three times with separate oocytes. For studies with mRPE cells, two independent preparations of mRPE cells were made from wild type and Slc5a8-/- mouse eyes. All cell treatments (mRPE and ARPE-19) were performed in duplicate and measurements performed in triplicate. For all experiments, data are presented as mean ± SEM.
Results
Structural Relationship between Nicotinate, Pyroglutamate, and OTC
OTC is a monocarboxylate and is structurally similar to nicotinate and to pyroglutamate, two of the transportable substrates of SLC5A8.27 The chemical structures of OTC, pyroglutamate, and nicotinate are given in Figure 1. This suggested that OTC is likely to be transported by SLC5A8.
Figure 1.
Structures of pyroglutamate, OTC, and nicotinate.
Transport of OTC via Human SLC5A8 in a Heterologous Expression System Using X. laevis Oocytes
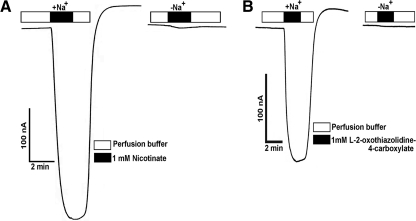
To determine whether OTC is a transportable substrate for SLC5A8, we used the X. laevis expression system. SLC5A8 is an electrogenic transporter, and transport of monocarboxylate substrates via this transporter occurs with a Na+:substrate stoichiometry of 2:1.27,28 This makes the transport process electrogenic, with a net positive charge entering the cells during the cotransport of Na+ and the monocarboxylate substrate via the transporter. This results in membrane depolarization that can be detected as inward currents under voltage-clamp conditions. With this rationale, we expressed human SLC5A8 in X. laevis oocytes and monitored its transport function by electrophysiological means. Water-injected oocytes served as negative controls. Nicotinate was used as a positive control, because we have shown previously that exposure of SLC5A8-expressing oocytes to this monocarboxylate induces Na+-dependent inward currents under voltage-clamp conditions.36 Exposure of human SLC5A8-expressing oocytes to 1 mM nicotinate induced marked inward currents in the presence of Na+ (Fig. 2A). Such currents were not detectable in SLC5A8-expressing oocytes in the absence of Na+, nor in water-injected oocytes (data not shown). We then examined whether OTC was recognized as a substrate by human SLC5A8 by monitoring the inward currents in human SLC5A8-expressing oocytes on exposure to this compound in the presence of Na+ (Fig. 2B). At a concentration of 1 mM, OTC induced inward currents in SLC5A8-expressing oocytes. These currents were not detectable in water-injected oocytes (data not shown). As seen with nicotinate, the currents induced by OTC were obligatorily dependent on the presence of Na+. Similar results were obtained with three different oocytes. These data show that OTC is indeed a transportable substrate for SLC5A8 and that the transport process is Na+-coupled and electrogenic.
Figure 2.
Demonstration of human SLC5A8-mediated OTC transport in the Xenopus laevis oocyte expression system. (A) SLC5A8 cRNA-injected oocytes were perifused with 1 mM nicotinate in the presence of NaCl (+Na+) or NMDG chloride (-Na+). Currents were monitored by the two-microelectrode voltage-clamp technique. (B) SLC5A8 cRNA-injected oocytes were perifused with 1 mM OTC in the presence of NaCl (+Na+) or NMDG chloride (-Na+).
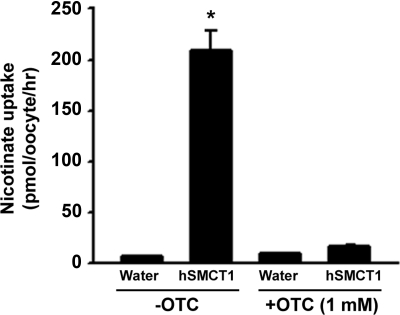
As an additional means of determining whether OTC is a transportable substrate of SLC5A8, we studied the effect of OTC on SLC5A8-specific [14C]-nicotinate uptake in human SLC5A8-expressing oocytes (Fig. 3). If OTC is a substrate of SLC5A8, it should be able to compete with nicotinate for the uptake process. The uptake of nicotinate was ∼40-fold higher in SLC5A8-expressing oocytes than in water-injected control oocytes. When present at 1 mM, OTC caused 80% to 90% inhibition of SLC5A8-specific nicotinate uptake. These data show that OTC interacts with human SMCT1 and competes with nicotinate for the uptake process.
Figure 3.
Inhibition of human SLC5A8 (SMCT1)-mediated nicotinate transport by OTC in Xenopus laevis oocytes. Na+-dependent uptake of [14C]-nicotinate (30 μM) was measured in water-injected (control) oocytes and in SLC5A8 (SMCT1)-expressing oocytes in the presence or absence of OTC (1 mM).
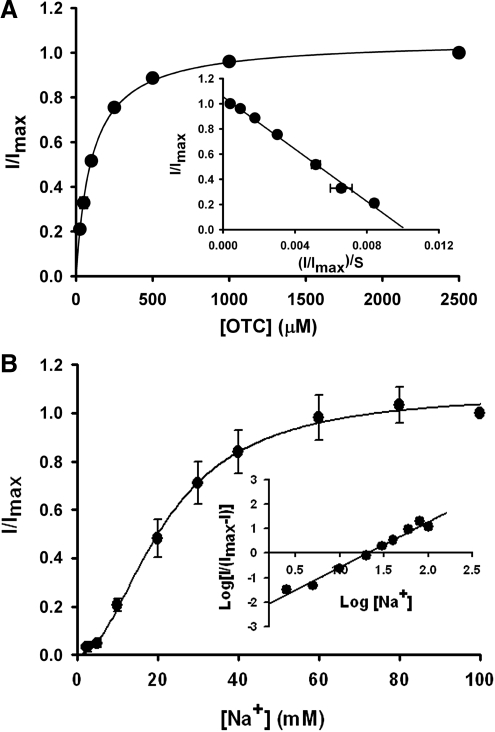
We then investigated the kinetic features of OTC transport via human SLC5A8 using the same electrophysiological approach. Here we used OTC-induced currents as the measure of transport activity. The transport of OTC via SLC5A8 was saturable, with a Km (Kt) of 104 ± 3 μM (Fig. 4A). Na+-activation kinetics indicated that the relationship between SLC5A8-mediated OTC transport and Na+ concentration was sigmoidal (Fig. 4B), suggesting involvement of more than one Na+ in the activation process. The Hill coefficient was 1.9 ± 0.1, which indicates that for every molecule of OTC transported, 2 Na+ ions are also cotransported. The concentration of Na+ necessary for half-maximal activation of OTC-induced currents was 22 mM. The observed Na+:OTC stoichiometry of 2:1 provides the molecular basis for the electrogenic nature of the transport process.
Figure 4.
Saturation kinetics (A) and Na+-activation kinetics (B) of human SLC5A8-mediated OTC transport in the Xenopus laevis oocyte expression system. (A) Inward currents were monitored in SLC5A8 cRNA-injected oocytes in the presence of increasing concentrations of OTC in perfusion buffer. The experiment was performed in four different oocytes. Because the expression levels of SLC5A8 varied to some extent in different oocytes, the data were normalized by taking the maximal current induced by the highest concentration of OTC (2.5 mM) as 1 in each oocyte and then calculating the currents induced by OTC at other concentrations as a fraction of this maximal current. Inset: Eadie–Hofstee plot. (B) OTC (1 mM)-induced inward currents were monitored in SLC5A8 cRNA-injected oocytes in the presence of increasing concentrations of Na+ (2.5–100 mM). The concentration of Cl− was maintained at 100 mM by appropriately substituting NaCl with NMDG chloride. The experiment was performed with three different oocytes, and the currents were normalized as described above to adjust for variations in the expression levels in different oocytes. Inset: Hill plot.
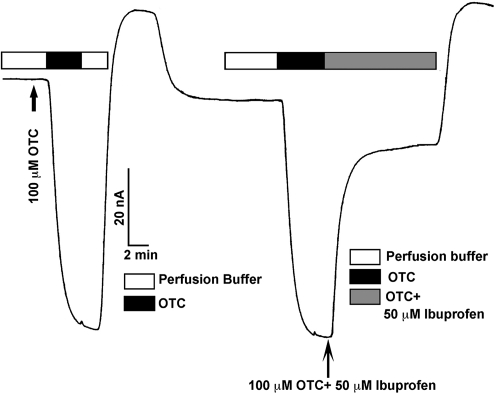
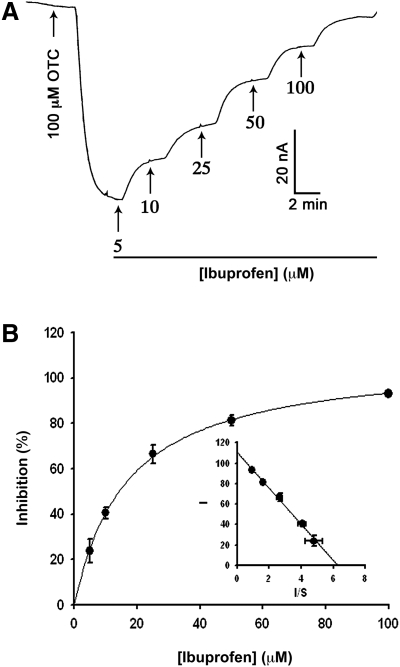
Our previous studies have shown that ibuprofen is a potent blocker of human SLC5A8.33 Therefore, if OTC is a transportable substrate for SLC5A8, the addition of ibuprofen to the perifusion medium should interfere with OTC-induced inward currents in SLC5A8-expressing oocytes. This was indeed the case. In the absence of ibuprofen, OTC (100 μM) induced marked inward currents in human SLC5A8-expressing oocytes (Fig. 5). However, when ibuprofen (50 μM) was included along with OTC in the perifusion medium, there was ∼80% inhibition of currents induced by OTC. We investigated the dose–response relationship for blockade of OTC-induced currents by ibuprofen (Fig. 6). We monitored OTC transport with perifusion of SLC5A8-expressing oocytes with OTC (100 μM) in the presence of Na+ and increasing concentrations of ibuprofen. Ibuprofen decreased the magnitude of OTC-induced currents in a dose-dependent manner. The concentration of ibuprofen needed for half-maximal blockade of the OTC-induced currents was 17 ± 1 μM.
Figure 5.
Blockade of human SLC5A8-mediated OTC transport by ibuprofen. OTC (100 μM)-induced currents were monitored in SLC5A8 cRNA-injected oocytes in the absence and presence of 50 μM ibuprofen.
Figure 6.
Dose-dependent blockade of human SLC5A8-mediated OTC transport by ibuprofen. (A) OTC (100 μM)-induced currents were monitored in SLC5A8 cRNA-injected oocytes in the presence of NaCl and increasing concentrations of ibuprofen. (B) The percent inhibition of OTC-induced currents by each concentration of ibuprofen was calculated from the above experiment, and the data were used to determine the concentration of ibuprofen needed to cause 50% maximal inhibition by fitting the Michaelis–Menten equation to the data. Inset: Eadie–Hofstee plot. I, percent inhibition; S, ibuprofen concentration in μM.
Cell Viability and Apoptosis in ARPE-19 Cells Treated with H2O2 in the Presence or Absence of OTC
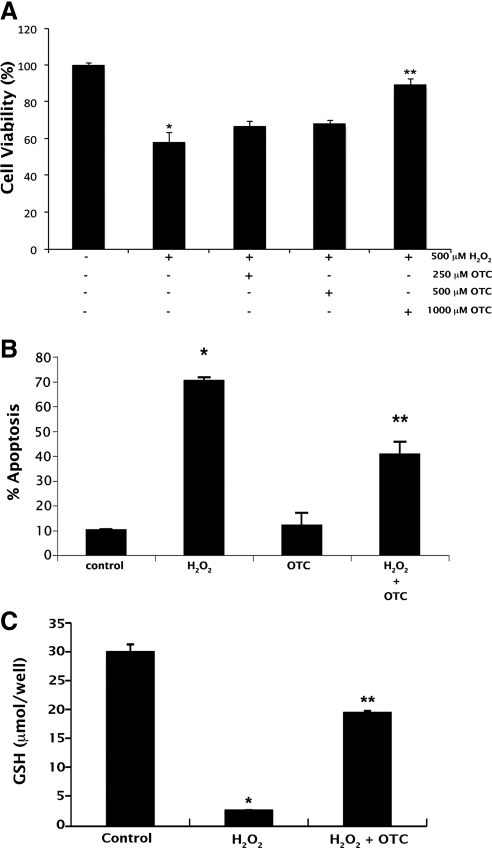
We then asked whether OTC could protect RPE cells from oxidative stress. Oxidative stress was induced in ARPE-19 cells using 500 μM H2O2. The viability of H2O2-treated cells cultured in the presence or absence of varying concentrations of OTC was then analyzed by trypan blue exclusion assay and Annexin V-FITC apoptosis assay (Fig. 7). Treatment of cells with H2O2 decreased the percentage of viable cells significantly. However, inclusion of OTC (1 mM) in the culture media reduced this effect on cell viability (Fig. 7A). The effectiveness of OTC in protecting ARPE-19 cells from oxidant-induced cell death was confirmed by Annexin V-FITC apoptosis assay (Fig. 7B). The protection of cells conferred by OTC treatment was associated with an increase in intracellular glutathione levels (Fig. 7C).
Figure 7.
OTC protects ARPE-19 cells against H2O2-induced cell death. (A) Oxidative stress was induced in ARPE-19 cells using H2O2 (500 μM) in the presence or absence of OTC (0.25, 0.5, and 1 mM). Cell viability was analyzed by trypan blue exclusion assay (*P < 0.01 compared to control; **P < 0.01 compared to H2O2-treated cells). (B, C) Oxidative stress was induced in ARPE-19 cells using H2O2 (500 μM) in the presence or absence of OTC (1 mM). Cells were then used for Annexin V-FITC apoptosis assay (*P < 0.001 compared to control; **P < 0.001 compared to H2O2-treated cells) and glutathione measurements (*P < 0.001 compared to control; **P < 0.001 compared to H2O2-treated cells).
Measurement of Glutathione Levels in Wild Type and Slc5a8-/- mRPE Cells and Protection against Oxidative Stress
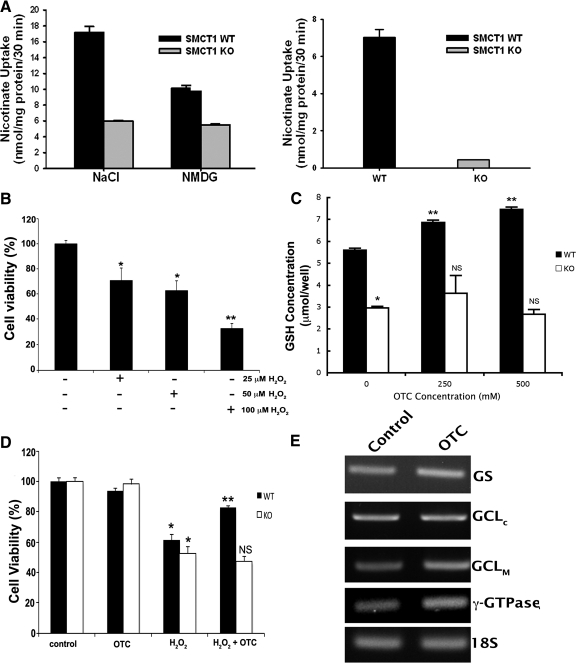
If SLC5A8 is required for OTC entry into cells, then culturing cells in the presence of OTC should only have an effect on cell viability and intracellular glutathione status in cells in which SLC5A8 is present. To determine whether this is the case, we isolated primary RPE cells (mRPE) from wild type and Slc5a8-/- mouse retinas. Uptake measurements showed Na+-dependent nicotinate uptake in wild type mRPE but not in knockout mRPE (Fig. 8A). We also studied the effect of OTC on Slc5a8-specific [14C]-nicotinate uptake in mRPE cells. When present at 1 mM, OTC inhibited uptake of nicotinate in wild type mRPE almost 100%. Na+-dependent nicotinate uptake in Slc5a8-/- mRPE was negligible, and OTC did not affect nicotinate uptake in the presence or absence of Na+ (data not shown). These studies demonstrate the obligatory requirement of Slc5a8 for the entry of OTC into RPE cells.
Figure 8.
Functional analysis of Slc5a8 in primary RPE (mRPE) cells isolated from wild type (WT) and Slc5a8-/- (KO) mouse retinas, and OTC-mediated protection of wild type mRPE against H2O2-induced cell death. (A) Wild type and Slc5a8-/- mRPE cells were used for analysis of [14C]-nicotinate (30 μM) uptake. Uptake measurements were made in the presence (NaCl) or absence of Na+ (NMDG). Data in the right panel represent the values for Na+-dependent nicotinate uptake in WT and KO mRPE cells. (B) Dose–response for H2O2-induced oxidative stress/cell death in wild type mRPE. (C) Wild type and Slc5a8-/- mRPE cells were incubated in the presence or absence of two different concentrations of OTC (250 and 500 μM) for 60 minutes, followed by determination of intracellular glutathione concentration (*P < 0.001 compared to WT cells; **P < 0.001 compared to WT cells cultured in the absence of OTC; NS, not significant compared to KO cells cultured in the absence of OTC). (D) Oxidative stress was induced in WT and KO mRPE using H2O2 (25 μM) in the presence or absence of 500 μM OTC. Cell viability was analyzed by trypan blue exclusion assay (*P < 0.01 compared to control cells cultured in the absence of H2O2 and of OTC; **P < 0.01 compared to WT cells cultured in the presence of H2O2; NS, not significant compared to KO cells cultured in the presence of H2O2). (E) RT-PCR analysis of mRNA expression of genes involved in glutathione homeostasis.
We then examined the role of OTC in protection against oxidative stress in wild type and Slc5a8-/- mRPE cells. Because mRPE proved to be more sensitive to H2O2 than ARPE-19 cells, we first performed a dose–response experiment to determine the optimal concentration of H2O2 for use in these cells (Fig. 8B). Treatment of cells with 25 μM H2O2 reduced the percentage of viable cells significantly compared with untreated control cells. Wild type and Slc5a8-/- mRPE cells were then treated with 25 μM H2O2 in the presence or absence of OTC (250 and 500 μM), followed by analysis of intracellular glutathione concentrations and cell viability. OTC increased glutathione levels in a dose-dependent manner in wild type mRPE cells, but had no effect on glutathione levels in Slc5a8-/- cells (Fig. 8C). In addition, OTC (500 μM) protected wild type mRPE from H2O2-induced cell death, but had no effect on the viability of Slc5a8-/- mRPE cells (Fig. 8D). These data suggest that the ability of OTC to enter RPE cells and increase intracellular glutathione levels as a means to protect against oxidative insult is obligatorily dependent on Slc5a8. The effect of OTC on the activity of γ-GTPase—a plasma membrane enzyme that plays a critical role in regulating glutathione homeostasis—was also examined. Given the extremely low γ-GTPase activity in RPE cells in culture, we used mouse kidney homogenates as the source of the enzyme. OTC had no effect on γ-GTPase activity (activity of the enzyme in the presence of 1 mM OTC was 94 ± 2% of control activity; P > 0.05). In addition, RNA samples from wild type mRPE cultured in the presence or absence of OTC (1 mM) were used for RT-PCR to examine the effects of OTC on steady state levels of mRNAs for γ-GTPase and other enzymes involved in glutathione synthesis. OTC had no effect on the expression of any these enzymes (Fig. 8E).
Discussion
OTC is a substrate for 5-oxoprolinase and is rapidly converted into cysteine, the rate-limiting amino acid in glutathione synthesis, upon entering cells. The administration of OTC is a therefore a very effective means of enhancing endogenous glutathione synthesis.15–24 The majority of previous studies of OTC have focused on its use in augmenting glutathione synthesis in a variety of disease conditions in which oxidative stress and resultant glutathione depletion are implicated, such as HIV infection, asthma, and liver disease.22,37–40 Although the beneficial effects of OTC as an antioxidant drug are well established, before the present study, whether OTC might also be beneficial in protecting RPE cells against oxidative stress had not been studied. The mechanism by which OTC crosses the plasma membrane thereby gaining entry into the cell was also not known. Here we show for the first time that OTC protects RPE cells against oxidative damage. We show also the obligatory involvement of the Na+-coupled monocarboxylate transporter SLC5A8 (SMCT1) in the transport of OTC.
RPE is exposed to considerable amounts of oxidative stress on a continuous basis, even in the absence of disease. This stems from its high oxygen consumption and the fact that a number of routine functions performed by RPE cells by themselves generate a significant amount of oxidative stress.4,41,42 As such, healthy RPE cells are equipped with very effective endogenous defenses against oxidative stress.4 Considerable evidence indicates that aging is associated with an increase in production of oxidative stress but a decrease in the antioxidant capacity of RPE cells.4,43 Indeed, severe oxidative stress is known to cause degeneration of RPE cells and is a critical factor in the pathogenesis of AMD. The development of novel strategies for enhancing the ability of RPE cells to combat oxidative stress is therefore critically important and highly relevant clinically. A compound like OTC that is capable of augmenting endogenous glutathione synthesis may prove beneficial in protecting RPE cells from oxidative stress. OTC is similar in structure to pyroglutamate and nicotinate, both of which are substrates for SLC5A8. In retina, SLC5A8 is expressed in retinal neurons and in the basolateral membrane of RPE,26 a location conducive to interaction of the transporter with substrates present in the choroidal circulation. Therefore, if OTC is a substrate for SLC5A8, then the transporter expressed on the basolateral membrane of RPE cells can mediate Na+-dependent active entry of OTC from the choroidal circulation into the RPE, resulting in increased glutathione production inside the cells. This could represent a novel strategy to enhance glutathione levels and increase the antioxidant capacity in RPE cells. This rationale led us to the present study, where we evaluated the ability of OTC to protect RPE cells against oxidative stress and the transport of OTC by SLC5A8.
We sought first to determine whether OTC is a substrate for SLC5A8. Indeed this was the case, as our data show that SLC5A8 transports OTC very effectively. Expression of the human SLC5A8 in X. laevis oocytes induced Na+-dependent inward currents in the presence of OTC under voltage-clamp conditions, suggesting that OTC is a transportable substrate for SLC5A8. SLC5A8-mediated transport of OTC was saturable with a Km of 104 ± 3 μM. The Na+-activation kinetics was sigmoidal with a Hill coefficient of 1.9 ± 0.1. Ibuprofen, a specific blocker of SLC5A8, suppressed OTC-induced currents in SLC5A8-expressing oocytes; the concentration of the blocker necessary for causing half-maximal inhibition was 17 ± 1 μM. The ability of OTC to increase intracellular glutathione levels and protect cells against oxidant-induced cell death has been documented in a number of differing cell types but never examined in RPE. Here we revealed that OTC protects ARPE-19 cells against cell death caused by oxidative damage. In the presence of H2O2, a known inducer of oxidative cell damage, there was ∼60% cell death. However, in the presence of OTC, the H2O2-induced death of ARPE-19 cells was reduced significantly. The protective effects of OTC were associated with increased levels of glutathione in these cells. Collectively, these data suggest that RPE cells are indeed amenable to antioxidant treatment using OTC. To determine definitively whether SLC5A8 is required in this process, we studied the effects of OTC on nicotinate uptake, cell viability, and glutathione levels in mRPE cells isolated from wild type and Slc5a8-/- mouse retinas. OTC competed with nicotinate for uptake in wild type mRPE cells but had no effect in Slc5a8-/- mRPE cells. When added to the culture medium of wild type mRPE cells, OTC induced a significant increase in intracellular glutathione levels and protected these cells from oxidant-induced cell death. However in Slc5a8-/- mRPE cells, OTC treatment had no significant effect, confirming the obligatory requirement of Slc5a8 for OTC entry into RPE cells. Interestingly, we found that glutathione levels were significantly lower in knockout mRPE cells compared to wild type cells under normal culture conditions in the absence of OTC treatment. This suggests that the absence of Slc5a8 expression in these cells may have an effect on other, hitherto unidentified, factor(s) associated with the glutathione homeostasis in mRPE cells. Additional studies are needed to evaluate this possibility.
Oxidative stress is associated with a number of changes at the cellular and molecular level, including low antioxidant levels, protein modification, mitochondrial DNA damage, and lipid peroxidation. Among these, a decline in endogenous synthesis of glutathione has been the most consistently observed phenomenon in in vitro studies, animal studies, and human patients.44,45 As such, strategies for increasing endogenous glutathione synthesis in RPE cells may be of tremendous benefit in preserving the viability of these cells under conditions of increased oxidative stress such as occurs with aging, exposure to cigarette smoking, and AMD. Given that cysteine is regarded as the limiting precursor in glutathione synthesis, provision of cysteine prodrugs as a means of increasing endogenous glutathione levels has received a great deal of attention. OTC is a cysteine prodrug. In comparison to other cysteine prodrugs that have been used clinically with success, OTC appears to be superior in its ability to raise intracellular glutathione levels and protect cells from oxidative stress.18,19,24 In addition, OTC treatment is associated with low toxicity and is well tolerated in patients.10,15,22,38–40 Our present study showing transport of OTC by the Na+-coupled monocarboxylate transporter SLC5A8 and the resultant increase in intracellular glutathione levels in RPE cells suggests that administration of OTC may be beneficial as a therapeutic strategy for the treatment of AMD and other retinal diseases in which oxidative damage to RPE plays a significant role. SLC5A8 is expressed in the basolateral membrane of the RPE, a location ideal for mediating the active entry of OTC from the choroidal blood into RPE cells. Therefore, based on the results of the present study, we predict that OTC, when present in systemic circulation, will be transported actively into RPE cells via SLC5A8 at the basolateral membrane and will enhance glutathione synthesis and antioxidant capacity in these cells as a means to protect the cells from oxidative damage.
Footnotes
Supported by the National Institutes of Health Grant K99/R00 EY018053 (PMM).
Disclosure: E. Babu, None; S. Ananth, None; R. Veeranan-Karmegan, None; V. Coothankandaswamy, None; S.B. Smith, None; T. Boettger, None; V. Ganapathy, None; P.M. Martin, None
References
- 1. Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293 [DOI] [PubMed] [Google Scholar]
- 2. Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Ret Eye Res. 2009;28:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134 [DOI] [PubMed] [Google Scholar]
- 4. Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Ret Eye Res. 2000;19:205–221 [DOI] [PubMed] [Google Scholar]
- 5. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 6. Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31:1–11 [DOI] [PubMed] [Google Scholar]
- 7. Kannan R, Tang D, Hu J, Bok D. Glutathione transport in human retinal pigment epithelial (HRPE) cells: apical localization of sodium-dependent GSH transport. Exp Eye Res. 2001;72:661–666 [DOI] [PubMed] [Google Scholar]
- 8. Cohen SM, Olin KL, Feuer WJ, Hjelmeland L, Keen CL, Morse LS. Low glutathione reductase and peroxidase activity in age-related macular degeneration. Br J Ophthalmol. 1994;78:791–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Samiec PS, Drews-Botsch C, Flagg EW, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Rad Biol Med. 1998;24:699–704 [DOI] [PubMed] [Google Scholar]
- 10. Wernerman J, Hammarqvist F. Modulation of endogenous glutathione availability. Curr Opin Clin Nutr Metab Care. 1999;2:1363–1950 [DOI] [PubMed] [Google Scholar]
- 11. Davidson PC, Sternberg P, Jr, Jones DP, Reed RL. Synthesis and transport of glutathione by cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:2843–2849 [PubMed] [Google Scholar]
- 12. Sternberg P, Jr, Davidson PC, Jones DP, Hagen TM, Reed RL, Drews-Botsch C. Protection of retinal pigment epithelium from oxidative injury by glutathione and precursors. Invest Ophthalmol Vis Sci. 1993;34:3661–3668 [PubMed] [Google Scholar]
- 13. Lu SC, Sun W, Nagineni CN, Hooks JJ, Kannan R. Bidirectional glutathione transport by cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36:2523–2530 [PubMed] [Google Scholar]
- 14. Bridges CC, Kekuda R, Wang H, et al. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:47–54 [PubMed] [Google Scholar]
- 15. Porta P, Aebi S, Summer K, Lauterburg BH. L-2-Oxothiazolidine-4-carboxylic acid, a cysteine prodrug: pharmacokinetics and effects on thiols in plasma and lymphocytes in human. J Pharmacol Exp Ther. 1991;257:331–334 [PubMed] [Google Scholar]
- 16. Olney JW, Ho O-L. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature. 1970;227:609–611 [DOI] [PubMed] [Google Scholar]
- 17. Anderson ME, Meister A. Marked increase of cysteine levels in many regions of the brain after administration of 2-oxothiazolidine-4-carboxylate. FASEB J. 1989;3:1632–1636 [DOI] [PubMed] [Google Scholar]
- 18. Williamson JM, Boettcher B, Meister A. Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis. Proc Natl Acad Sci U S A. 1982;79:6246–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williamson J, Meister A. Stimulation of hepatic glutathione formation by administration of L-2-oxothiazolidine-4-carboxylate, a 5-oxo-L-prolinase substrate. Proc Natl Acad Sci U S A. 1981;78:936–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moslen MT, Whitehead RF, Ferguson AE, Kanz MF. Protection by L-2-oxothiazolidine-4-carboxylate, a cysteine produg, against 1,1-dichloroethylene hepatotoxicity in rats is associated with decreases in toxin metabolism and cytochrome P-450. J Pharmacol Exp Ther. 1989;248:157–163 [PubMed] [Google Scholar]
- 21. Brodeur J, Goyal R. Effect of a cysteine prodrug, L-2-oxothiazolidine-4-carboxylic acid, on the metabolism and toxicity of bromobenzene: an acute study. Can J Physiol Pharmacol. 1987;65:816–822 [DOI] [PubMed] [Google Scholar]
- 22. Vita JA, Frei B, Holbrook M, Gokce N, Leaf C, Keaney JF., Jr L-2-Oxothiazolidine-4-carboxylic acid reverses endothelial dysfunction in patients with coronary artery disease. J Clin Invest. 1998;101:1408–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee KS, Park HS, Park SJ, et al. A prodrug of cysteine, L-2-oxothiazolidine-4-carboxylic acid, regulates vascular permeability by reducing vascular endothelial growth factor expression in asthma. Mol Pharmacol. 2005;68:1281–1290 [DOI] [PubMed] [Google Scholar]
- 24. Mesina JE, Page RH, Hetzel FW, Chopp M. Administration of L-2-oxothiazolidine-4-carboxylate increases glutathione levels in rat brain. Brain Res. 1989;478:181–183 [DOI] [PubMed] [Google Scholar]
- 25. Ganapathy V, Thangaraju M, Gopal E, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin PM, Dun Y, Mysona B, et al. Expression of the sodium-coupled monocarboxylate transproters SMCT1 (SLC5A8) and SMCT2 (SLC5A12) in retina. Invest Ophthalmol Vis Sci. 2007;48:3356–3363 [DOI] [PubMed] [Google Scholar]
- 27. Miyauchi S, Gopal E, Ellapan B, et al. Sodium-coupled electrogenic transport of pyroglutamate (5-oxoproline) via SLC5A8 a monocarboxylate transporter. Biochim Biophys Acta. 2010;1798:1164–1171 [DOI] [PubMed] [Google Scholar]
- 28. Miayuchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor downregulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279:13293–13296 [DOI] [PubMed] [Google Scholar]
- 29. Frank H, Gröger N, Diener M, Becker C, Braun T, Boettger T. Lactaturia and loss of sodium-dependent lactate uptake in the colon of SLC5A8-deficient mice. J Biol Chem. 2008;283:24729–24737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh N, Thangaraju M, Prasad PD, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–27608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin PM, Ananth S, Cresci G, Roon P, Smith S, Ganapathy V. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol Vis. 2009;15:362–372 [PMC free article] [PubMed] [Google Scholar]
- 32. Martin PM, Gopal E, Ananth S, et al. Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of l-lactate and ketone bodies in the brain. J Neurochem. 2006;98:279–288 [DOI] [PubMed] [Google Scholar]
- 33. Itagaki S, Gopal E, Zhuang L, et al. Interaction of ibuprofen and other structurally related NSAIDs with the sodium-coupled monocarboxylate transporter SMCT1 (SLC5A8). Pharm Res. 2006;23:1209–1216 [DOI] [PubMed] [Google Scholar]
- 34. Gnana-Prakasam JP, Thangaraju M, Liu K, et al. Absence of the iron-regulatory protein Hfe results in hyperproliferation of retinal pigment epithelium: role of cystine/glutamate exchanger. Biochem J. 2009;424:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ganapathy V, Mendicino J, Leibach FH. Transport of glycyl-L-proline into intestinal and renal brush border vesicles from rabbit. J Biol Chem. 1981;256:118–124 [PubMed] [Google Scholar]
- 36. Gopal E, Miyauchi S, Martin PM, et al. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res. 2007;24:575–584 [DOI] [PubMed] [Google Scholar]
- 37. Ho WZ, Starr SE, Sison A, Douglas SD. L-2-Oxothiazolidine-4-carboxylic acid inhibits immunodefeicinecy virus type 1 replication in mononuclear phagocytes and lymphocytes. Clin Diag Lab Immunol. 1997;4:352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barditch-Crovo P, Noe D, Skowron G, et al. A phase I/II evaluation of oral L-2-oxothiazolidine-4-carboxylic acid in asymptomatic patients infected with human immunodeficiency virus. J Clin Pharmacol. 1998;38:357–363 [DOI] [PubMed] [Google Scholar]
- 39. Homes RP, Assismos DG, Leaf CD, Whalen JJ. The effects of (L)-2-oxothiazolidine-carboxylate on urinary oxalate excretion. J Urol. 1997;158:34–37 [DOI] [PubMed] [Google Scholar]
- 40. Moberly JB, Logan J, Borum PR, et al. Elevation of whole-blood glutathione in peritoneal dialysis patients by L-2-oxothiazolidine-4-carboyxlate, a cysteine prodrug (Procysteine). J Am Soc Nephrol. 1998;9:1093–1099 [DOI] [PubMed] [Google Scholar]
- 41. Miceli MV, Newsome DA, Schriver GW. Glucose uptake, hexose monophosphate shunt activity, and oxygen consumption in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1990;31:277–283 [PubMed] [Google Scholar]
- 42. Futterman S, Kinoshita HJ. Metabolism of the retina. I. Respiration of cattle retina. J Biol Chem. 1959;234:723–726 [PubMed] [Google Scholar]
- 43. Frank RN, Amin RH, Puklin JE. Antioxidant enzymes in the macular retinal pigment of eyes with neovascular age-related macular degeneration. Am J Ophthalmol. 1999;127:694–709 [DOI] [PubMed] [Google Scholar]
- 44. Moriarty Siobhan E, Shah JH, Lynn M, et al. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Rad Biol Med. 2003;35:1582–1588 [DOI] [PubMed] [Google Scholar]
- 45. Sies H. Oxidative stress. London: Academic Press; 1985 [Google Scholar]