Kappa opioid receptor (KOR) agonists lower IOP, but the specific cellular mechanisms involved have not been fully elucidated. This study demonstrates the presence of KORs in cells of the anterior chamber and also that nitric oxide may play a role in KOR-mediated IOP reduction.
Abstract
Purpose.
The present study was designed to determine whether kappa opioid receptors (KORs) are localized to cells of the inflow and outflow pathways of the eye and if activation of these receptors has an effect on nitric oxide (NO) production, because these effects could play a role in KOR agonist–mediated reduction of IOP.
Methods.
Human nonpigmented ciliary epithelial (NPCE) and trabecular meshwork (HTM-3) cells were treated with spiradoline (SPR), a selective KOR agonist, or estradiol, for 24 hours. Some cells were pretreated with the selective KOR antagonist norbinaltorphimine (norBNI) or the nonselective NO synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) for 30 minutes, followed by the addition of SPR. Immunofluorescent localization of KORs was determined in isolated rabbit iris–ciliary bodies (ICBs) and NPCE and HTM-3 cells.
Results.
Immunohistochemical data show the localization of KORs to the rabbit ICB and more specifically to the ciliary epithelial layer. KORs were also found on cell membranes of NPCE and HTM-3 cells. Treatment of both these cell types with spiradoline caused concentration-dependent increases in the release of NO. Spiradoline-induced release of NO from both cell types was inhibited by pretreatment with norBNI and L-NAME.
Conclusions.
Results from this study show the presence of KORs on rabbit ICBs and also on NPCE and HTM cells. Activation of these KORs on both cell types resulted in KOR-mediated increases in NO production. These findings provide evidence that previously demonstrated KOR-mediated reduction in IOP could be caused, in part, by NO production in both the ciliary body and the trabecular meshwork.
Elevated IOP is one of the major risk factors responsible for optic nerve damage in primary open-angle glaucoma (POAG). Therefore, achieving good control of IOP is very important in preventing the progression of optic neuropathy, which eventually leads to irreversible vision loss. IOP is determined primarily by the dynamic equilibrium between the production of aqueous humor in the ciliary body and its efflux, mainly through the trabecular meshwork and Schlemm's canal.1 Aqueous humor is produced by the ciliary epithelial cells located in the ciliary body.2 We3–5 and others6 have shown that opioid receptor activation modulates aqueous humor dynamics and, therefore, IOP. Specifically, we have shown that kappa opioid receptor (KOR) agonists reduce IOP by reducing aqueous humor formation and increasing aqueous humor outflow, thereby suggesting their possible usefulness in glaucoma therapy.
We have also provided evidence that the effect of selective KOR agonists on aqueous humor dynamics involves increased natriuretic peptide release in the anterior segment,5,7 but much remains to be understood about the specific cellular and molecular mechanisms underlying these events. Most of the actions of natriuretic peptides occur via activation of the guanylyl cyclase (GC) pathway, which induces the elevation of intracellular cGMP. cGMP, however, is synthesized by two different isoforms of GC: particulate or membrane GC (pGC), to which ANP binds, and soluble GC (sGC), which is activated by NO. Compounds that affect the NO/cGMP pathway lower IOP either by enhancing aqueous humor outflow facility, reducing aqueous humor formation, or a combination of these mechanisms.8 Because KOR agonists have effects on both the inflow and the outflow pathways, we propose that NO may also be involved in KOR-mediated effects in the anterior segment.
We conducted the present study using well established human nonpigmented ciliary epithelial cells (NPCEs) and human trabecular meshwork cells (HTM-3). Because the ciliary epithelium is a primary site for aqueous humor production, NPCE cells are often used to determine mechanisms of action of drugs that affect aqueous humor formation.9 These transformed NPCE cells were also shown to contain NO-activated heterodimeric soluble GC.10 HTM-3 cells are often used to determine the effect of drugs on the signal transduction pathways in the conventional outflow tract of the eye.11 Based on the reported use of these cultured cells in the determination of signaling pathways in the anterior chamber, we used these cellular models to determine the possible involvement of NO in KOR agonist–induced ocular hypotension.
Materials and Methods
Cell Culture
NPCE (gift from Miguel Coca-Prados, Yale University School of Medicine) and HTM-3 (gift from Iok-Hou Pang; Alcon Research Laboratories, Fort Worth, TX) cells were maintained and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 4 mM l-glutamine, and 50 μg/mL gentamicin, and kept at 37°C in 95% O2, 5% CO2 environment, per Pang et al.11 Cells were grown to approximately 90% confluence before being treated as described below.
Immunocytochemistry
NPCE and HTM-3 cells were grown on Laboratory-Tek II chamber slides and identification of KOR protein was carried out using standard immunocytochemical techniques with a polyclonal antibody directed against the human KOR (R&D Antibodies, Las Vegas, NV). The antibody was produced in rabbits and was specific for the COOH-terminal of the KOR. Cells were fixed in ice cold (-20°C) methanol for 5 to 10 minutes. After fixation, cells were washed with PBS and incubated at room temperature for 1 hour in blocking buffer (5% goat serum with 0.2% Triton X-100). The cells were exposed to the KOR primary antibody (1:200) overnight at 4°C. In experimental negative controls, the cells were incubated in blocking buffer alone. Primary antibody labeling was visualized using a fluorescent secondary antibody (Oregon Green 488 goat anti-rabbit IgG, Alexa Fluor 594 goat anti-rabbit IgG; Invitogen, Carlsbad, CA). Cells were then mounted in aqueous mount and staining was viewed using a confocal microscope.
Immunohistochemistry
Adult male New Zealand albino rabbits were killed with an overdose of Beuthanasia-D (pentobarbital sodium) and their ICBs were surgically removed and immediately fixed in 4% paraformaldehyde (in PBS [pH 7.4]). Fixed ICBs were cryoprotected in graded sucrose in PBS (10% to 30%) at 4°C. Tissues were embedded in ornithine carbamoyltransferase (OCT), sectioned at 10 μm on a cryostat, and mounted onto slides. Sections were treated with sodium borohydride (NaBH4; 0.5%) and were washed in PBS before blocking in buffer containing normal chicken serum (10%) and Triton X-100 (0.3%). Anti-peptide polyclonal goat anti-KOR (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse monoclonal anti-synaptic vesicle protein (gift from Peter MacLeish, Morehouse School of Medicine) were used in immunohistochemical studies to determine the distribution and localization of KORs in rabbit ICBs. Experimental negative controls did not include the primary antibody in the incubation medium or were coincubated with the control peptide. Tissue samples were washed with PBS and exposed for 2 hours (room temperature) to fluorescent secondary antibodies (Alexa Fluor 488 chicken anti-goat and Alexa Fluor 594 donkey anti-mouse IgG, 1:200 dilution; Invitrogen, Carlsbad, CA). The slides were examined on a confocal laser scanning microscope. All studies were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the Institutional Animal Care and Use Committee of Morehouse School of Medicine approved all protocols involving animals.
Drug Treatments for NO Determination
NPCE or HTM-3 cells seeded in 6-well plates were grown to approximately 90% confluence before drug treatment. Experimental protocols were carried out on cells incubated at 37°C in dye-free DMEM growth media containing protease inhibitor cocktail and L-arginine. L-arginine was included in the media of control and treated cells to stimulate NO production in vitro. Cells were treated with either spiradoline (SPR; 10, 100, 500, and 1000 μM; Sigma-Aldrich, Saint Louis, MO) or estradiol (100 μM; Sigma-Aldrich) for 24 hours. Some cells were pretreated with the selective KOR antagonist norbinaltorphimine (norBNI; Tocris Bioscience, Ellisville, MO) or the nonselective NO synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; Sigma-Aldrich) for 30 minutes, followed by treatment with SPR (100 or 1000 μM) for 24 hours. After the 24-hour drug treatments, media samples were assayed for NO and cell lysates analyzed for protein using the Bio-Rad protein assay.
Nitrite Measurements
NO is a very unstable molecule in solution, with a half-life of only a few seconds. Therefore, in these studies, NO was measured as its stable metabolites nitrate and nitrite. In the cell, NO undergoes a series of reactions with several molecules present in biologic fluids, and is eventually metabolized to nitrite (NO2–) and nitrate (NO3–). The incubation medium surrounding the cells was assayed for NO (nitrate plus nitrite) levels using a microplate assay (Active Motif, Carlsbad, CA). The principle of the NO quantitation kit is that nitrate in the sample is converted to nitrite in the presence of nitrate reductase and cofactors. Then, nitrate and nitrite levels are assayed using Griess reagent. Each experiment was performed in triplicate and repeated at least four times.
Statistical Analysis
Data were analyzed for differences using one-way ANOVA followed by the Holm–Sidak method for multiple comparisons. Results are expressed as mean values ± SEM and were considered significant when P < 0.05.
Results
KOR Expression
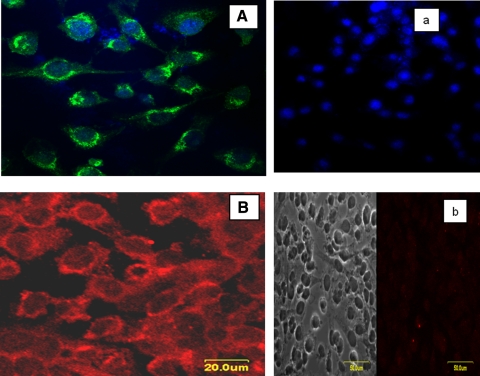
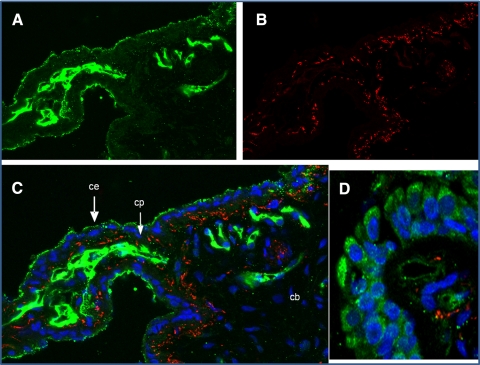
Figures 1A and 1B show confocal images of immunocytochemical staining of NPCE and HTM-3 cells, respectively, with an antibody against KOR protein. Dense receptor labeling is seen on the membrane surface of both cell types. To further confirm that these receptors are present in the tissue involved in aqueous humor production, and because KOR agonists were shown to reduce aqueous formation in rabbits, KOR immunoreactivity was assessed in ICBs of New Zealand white rabbits. We also assessed whether KOR immunoreactivity colocalized with labeling for synaptic vesicle protein, a marker for synaptic vesicles and nerve terminals. Our data confirms KOR immunoreactivity throughout the ICB (Fig. 2A, green). Staining in the ciliary processes (CPs) appears more intense than in the stroma of the ciliary body (Fig. 2C; CB). Specifically, in the CPs, the most intense staining appeared in the nonpigmented ciliary epithelium (CE), which consists of aqueous humor–producing cells. The bright green labeling in the center of the CPs appears to be capillaries located in the connective tissue core of the processes. KOR immunoreactivity was also found to be associated with cells within the stroma and epithelial cells (Fig. 2D) of the ICB. KORs are expected to be localized to synaptic vesicles and nerve terminals, but this was not a consistent observation in the ICB and requires ultrastructural investigation.
Figure 1.
Immunofluorescence (IF) localization of kappa opioid receptors to NPCE (A) and HTM-3 (B) cell membranes. Experimental negative controls (a and b) did not include the primary antibody in the incubation medium. The blue color indicates DAPI-stained nuclei.
Figure 2.
Immunolocalization of kappa opioid receptors (A; green) and synaptic vesicles/nerve endings (B; red) in rabbit ICB (40×). (C) Merged image with DAPI nuclear staining in blue. (D) Ciliary epithelial cells. CB, ciliary body; CE, ciliary epithelium; CP, ciliary process.
KOR Activation: Effects on NO Production in NPCE and HTM-3 Cells
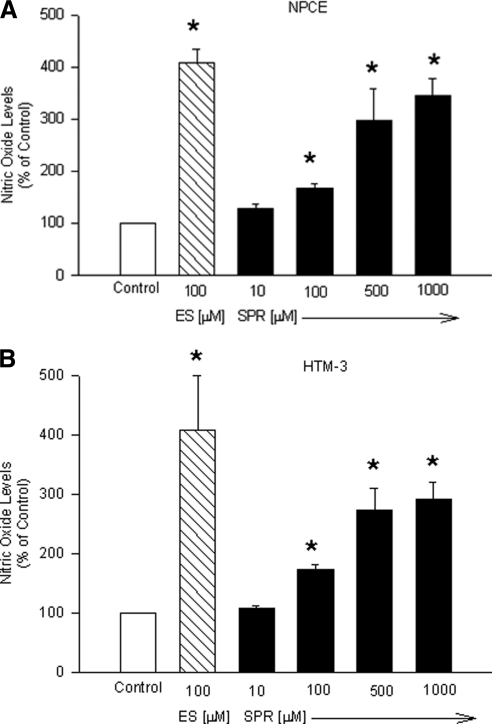
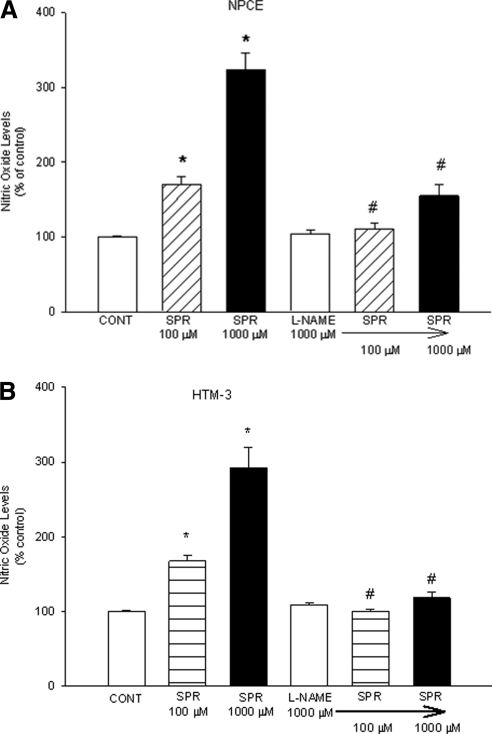
Of all the opioid receptors, the KOR has been shown to exhibit the strongest dependency on NO/cGMP to induce its effects in tissues, such as the pial artery.12,13 To determine whether NO plays a role in KOR-mediated effects in the anterior chamber, we used the relatively selective KOR agonist SPR to determine the effect of KOR activation (SPR treatment) on NO formation in NPCE and HTM-3 cells. SPR treatment (for 24 hours) caused a concentration-dependent increase in NO release in both human NPCE (Fig. 3A) and HTM-3 cells (Fig. 3B).
Figure 3.
Concentration response of spiradoline (SPR) on NO production in NPCE (A) or HTM-3 (B) cells. Cells were treated with the indicated concentrations of SPR or estradiol (ES) for 24 hours. At the end of drug treatments, NO in the incubation medium was analyzed as [nitrates + nitrates] using a microplate assay kit. Data are mean ± SEM of 4 to 10 experiments in triplicate. *P < 0.05 compared to control.
In NPCE cells, KOR activation caused significant increases in nitrite concentrations (the stable form of NO in solution) from 128 ± 9.69% of control to 347 ± 31.30% of control. In HTM-3 cells, nitrite concentrations increased from 108 ± 4.31% of control to 292 ± 28.02% of control. In these experiments, estradiol (ES) was used as a positive control for NO release.
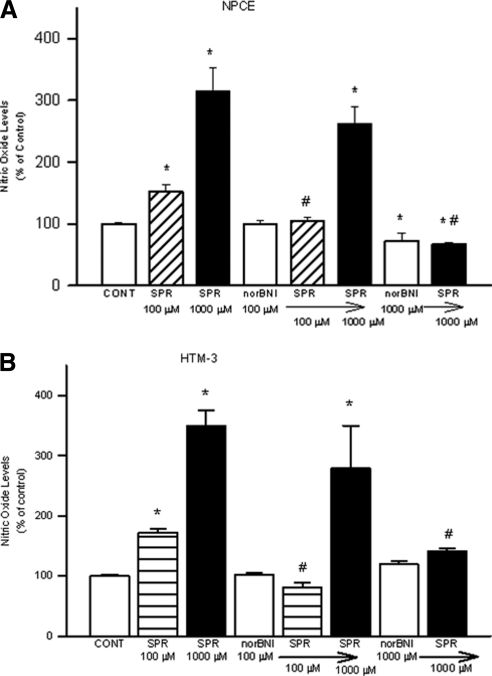
To confirm that SPR-stimulated NO release in NPCE and HTM-3 cells was mediated by activation of KORs, the selective KOR antagonist norBNI was used. NorBNI has a very high affinity for KOR binding sites. NorBNI (100 μM) alone did not significantly alter NO levels in media compared with control (Figs. 4A and 4B). The 1000 μM dose, however, did significantly reduce basal levels of NO in NPCE but not in HTM-3 cells. Pretreatment with norBNI (100 μM, 30 minutes) resulted in the inhibition of SPR (100 μM)-induced increases in NO levels in the media of both cell types, but did not significantly affect that generated by 1000 μM SPR, although there was a trend toward inhibition. Pretreatment with the 1000 μM dose of norBNI, however, did significantly inhibit the increases in NO levels generated by 1000 μM SPR. These results show that the elevation in NO levels produced by SPR is mediated by activation of KORs.
Figure 4.
Effect of norBNI on SPR-stimulated NO production in NPCE (A) or HTM-3 (B) cells. Cells were pretreated with norBNI for 30 minutes, followed by treatment with SPR for 24 hours. At the end of drug treatments, NO in the incubation medium was analyzed as [nitrates + nitrites] using a microplate assay kit. Data are mean ± SEM of four to nine experiments in triplicate. *P < 0.05 compared to control. #P < 0.05 compared to SPR alone.
To test whether the KOR-activated increases in NO levels in NPCE and HTM-3 cells is associated with activation of receptors linked to NO release, experiments using the nonselective nitric oxide synthase (NOS) inhibitor L-NAME, were performed. When administered alone, L-NAME did not cause any significant changes in NO levels when compared to basal levels, but pretreatment with L-NAME resulted in complete inhibition of SPR (100 and 1000 μM)-induced NO production (Figs. 5A and 5B). In NPCE cells, the NO-producing effects of high concentrations of SPR (1000 μM) were almost completely abolished in the presence of L-NAME. Also, in HTM-3 cells, SPR (1000 μM)-induced increases in NO levels (291.89 ± 28.02%) was significantly reduced to almost that of control levels in the presence of L-NAME (118.12 ± 8.61%). These data indicate that SPR-stimulated NO formation in both NPCE and HTM-3 cells results specifically from the activation of NOS enzymes and is not related to either physical or chemical phenomena.
Figure 5.
Effect of L-NAME on SPR-stimulated NO production in NPCE (A) or HTM-3 (B) cells. Cells were pretreated with L-NAME for 30 minutes, followed by treatment with SPR for 24 hours. At the end of drug treatments, NO in the incubation medium was analyzed as [nitrates + nitrites] using a microplate assay kit. Data are mean ± SEM of 4 to 11 experiments in triplicate. *P < 0.05 compared to control. #P < 0.05 compared to SPR alone.
Discussion
We previously reported that KOR agonists reduce IOP, in part, by reducing aqueous humor formation3,4 and increasing outflow facility,5 but the precise site and cellular mechanism of action of these agonists—and the localization and distribution of KORs that modulate aqueous humor dynamics—has not been clearly established. In this study, KORs were found localized to cell membranes of both NPCE and HTM-3 cells (Fig. 1). The presence of KORs on cultured cells of the inflow and outflow pathways has not been previously reported, and these data suggest that these NPCE and HTM-3 cells may be used to elucidate the mechanisms by which KOR agonists modulate aqueous humor dynamics. KOR proteins were also found localized to the ICB of the New Zealand white rabbit. Specifically, the immunoreactive staining on ciliary processes (CPs) suggests that these receptors could play a role in the regulatory mechanisms that modulate aqueous humor dynamics because CPs are responsible for the active production of aqueous humor. Overall, these immunofluorescence data provide evidence that the nonpigmented ciliary epithelium of the ciliary body, and the trabecular meshwork, could be direct targets for KOR agonists, and supports our previous work that these agonists influence the rate of aqueous humor formation and egress from the anterior chamber in the regulation of IOP.
NO appears to be an important physiologic regulator of IOP, and it might also be directly involved in the increase in IOP observed in POAG as a result of low NO production.14,15 The underproduction of NO could be corrected by providing NOS substrates or NO donors to lower IOP, increase ocular blood flow, and relax ciliary muscle.16 The control of NO levels in the eye may therefore be a therapeutic target in glaucoma. The effects of various drugs acting through NO in aqueous humor modulation suggest a role for this cellular mediator in the regulation of IOP. Endothelial NOS (eNOS) and neuronal NOS (nNOS) were found to be present in most ocular tissues, including those responsible for aqueous dynamics—that is, the CPs, ciliary muscle, and trabecular meshwork17–19—further confirming the involvement of these NO producing enzymes in IOP regulation.
A number of inconsistencies currently exist in the literature regarding the effects of NO on IOP. A study performed by Kiel et al.20 indicated that the inhibition of NOS with L-NAME caused a large rapid decrease in IOP in rabbits. They concluded that this reduction could be related to ciliary vasoconstriction and a reduction in aqueous humor production. Other investigators also indicated the inhibition of IOP by L-NAME in laser irradiated21 and α-chymotrypsin22 ocular hypertensive rabbits. No effect of this agent was seen in normotensive rabbits. Another group revealed that the topical application of various NOS inhibitors, including L-NAME, did not prevent the IOP increase induced by water intake in rabbits.23 Several studies however, have shown IOP-lowering effects of NO. For example, it was shown that by increasing the L-arginine/NO/cGMP pathway, it was possible to lower IOP in rabbits equally to antiglaucoma agents currently being used.24 A NO donor was also found to enhance the IOP-lowering effects of latanoprost, a commonly used ocular hypotensive agent.25 In a clinical study, L-arginine, applied intravenously to human subjects, was shown to lower IOP.26 In addition, a number of other studies indicate that the mechanisms involved in the reduction of aqueous humor flow27–30 and increased outflow facility31,32 are likely to involve the NO/cGMP pathway. Others have shown that the release of NO is implicated in avoiding ocular hypertension in a rabbit glaucoma model.33 Taken together, these results suggest that the NO/GC system plays a pivotal role in aqueous humor dynamics and, therefore, in the regulation of IOP.
Previous studies in our laboratory have shown that the adenylyl cyclase/cAMP and natriuretic peptide (NP) pathways are involved in KOR-mediated regulation of aqueous humor dynamics in the reduction of IOP. The present study supports our hypothesis that NO could also play a role in KOR-mediated changes in aqueous humor dynamics. Whether or not the NP and NO pathways interact with each other in the reduction of IOP remains to be determined. Here, we focused on NO released by NPCE cells that secrete aqueous humor, and HTM-3 cells that are involved in aqueous humor egress from the anterior chamber. IOP is maintained as a result of a balance between the secretion of aqueous humor by the CPs and its outflow through the trabecular and uveoscleral outflow pathways. We showed that activation of KORs in these cells of the anterior chamber, which participate in aqueous humor regulation, caused a dose-dependent increase in NO levels. The selectivity of SPR for KORs is 84 times that for mu opioid receptors and 100 times that for delta opioid receptors,34 so the relatively selective, competitive KOR antagonist norBNI was used to confirm that the response to SPR was KOR mediated. The observed increases in NO generated by 100 μM SPR were abolished in the presence of norBNI (100 μM), whereas that produced by the 1000-μM dose of SPR was not significantly affected. At the equivalent molar concentration however, norBNI (1000 μM) significantly inhibited the observed KOR-induced (SPR 1000 μM) increases in NO. These effects of norBNI suggest that in these cells, high levels of KOR activation require an equivalent high molar concentration of antagonist for inhibition.
We have shown in previous studies5 that activation of KORs in the anterior chamber increases outflow facility, and that this increase, is caused in part, by KOR-activated paracrine effects of natriuretic peptides on tissues within ocular outflow tracts. Based on in vitro evidence presented here, this increase in outflow facility may also be related to the release of NO from trabecular meshwork cells. This NO release could cause in vivo relaxation of trabecular meshwork cells, thereby increasing trabecular outflow facility and lowering IOP. Others have shown that NO induces relaxation of the trabecular meshwork and ciliary muscle cells and therefore may be involved in the regulation of aqueous humor dynamics.35 Their experiments on relaxation/contraction in trabecular meshwork and ciliary muscle of the bovine eye indicated that cGMP is the final common effector molecule of vasodilators, which activate the NO system, thereby influencing the outflow of aqueous humor. Studies by Ellis et al.36,37 have revealed that NO plays a direct role in aqueous humor outflow facility in bovine trabecular meshwork. They provided evidence that NO decreases trabecular meshwork cell volume by the activation of the sGC/cGMP/PKG pathway,36 and that observed changes in trabecular meshwork cell volume are correlated with changes in outflow facility. This group's anterior segment perfusion studies also revealed that the activation of sGC is necessary for NO-induced increases in outflow facility.37 Taken together, these data suggest that the NO/cGMP pathway plays a role in increasing outflow facility, thereby reducing IOP.
The CPs, which contain the cells responsible for aqueous humor formation, are enriched with nitric oxide synthases, the enzymes involved in the synthesis of NO. Several studies indicate that the NO/cGMP pathway is involved in the secretion of aqueous humor. Sodium azide, a vasodilator drug that acts through the generation of NO, was shown to lower IOP by reducing aqueous humor formation.38 In a bovine model, this effect of NO donors on aqueous humor secretion was shown to involve cGMP.28 NO donors were also shown to elevate cGMP in bovine CPs.39,40 Based on these studies and the data obtained here, we propose that KOR-mediated NO release from NPCE and trabecular meshwork cells could be associated with the mechanistic changes in aqueous humor inflow and outflow that result in reduced IOP.
To our knowledge, we are the first to show that KORs are expressed in CPs and on NPCE and HTM-3 cells, and that these receptors are coupled to NO production in the anterior segment of the eye. However, whether or not the receptor is linked to a specific NOS isoform is yet to be determined. KOR agonists have been shown to elicit their nociceptive and other actions, in part, by activating the L-arginine/NO/cGMP pathway, and there is evidence to suggest that some of these actions may be related to effects on neuronal NOS41,42 (nNOS; NOS1). In conclusion, results from this study show that KOR receptors are present in the ICB and on HTM-3 and NPCEs. Also, activation of KORs in human NPCE and trabecular meshwork cells increase NO production. These findings provide evidence that KOR-mediated reduction in IOP could be caused, in part, by NO production in both the ciliary body and the trabecular meshwork.
Acknowledgments
We thank Dr. Peter MacLeish for the SV2 antibody and for allowing use of the facilities in the Neuroscience Institute, Morehouse School of Medicine, Mr. Sidney Pitts of the Department of Neurobiology for his invaluable assistance with sectioning and microscopy, and Dr. Jeffrey Boatright for helpful comments on the manuscript.
Footnotes
Supported by the National Centers for Research Resources at the National Institutes of Health, Research Centers in Minority Institutions Grant G12-RR03034.
Disclosure: K. Russell-Randall, None; J. Dortch-Carnes, None.
References
- 1. Wiederholt M, Bielka S, Schweig F, Lütjen-Drecoll E, Lepple-Wienhues A. Regulation of outflow rate and resistance in the perfused anterior segment of the bovine eye. Exp Eye Res. 1995;61:223–234 [DOI] [PubMed] [Google Scholar]
- 2. Krupin T, Wax M, Moolchandani J. Aqueous production. Trans Ophthalmol Soc U K. 1986;105:156–161 [PubMed] [Google Scholar]
- 3. Russell KR, Wang DR, Potter DE. Modulation of ocular hydrodynamics and iris function by bremazocine, a kappa opioid receptor agonist. Exp Eye Res. 2000;70:675–682 [DOI] [PubMed] [Google Scholar]
- 4. Russell KR, Potter DE. Dynorphin modulates ocular hydrodynamics and releases atrial natriuretic peptide via activation of kappa-Opioid receptors. Exp Eye Res. 2002;75:259–270 [PubMed] [Google Scholar]
- 5. Potter DE, Russell KR, Manhiani M. Bremazocine increases C-type natriuretic peptide levels in aqueous humor and enhances outflow facility. J Pharmacol Exp Ther. 2004;309:548–553 [DOI] [PubMed] [Google Scholar]
- 6. Green K. Ocular effects of diacetyl morphine and lysergic acid diethylamide in rabbit. Invest Ophthalmol. 1975;14:325–329 [PubMed] [Google Scholar]
- 7. Russell KR, Moore TT, Potter DE. Elevation of atrial natriuretic peptide levels in aqueous humor of the rabbit by kappa opioid receptor agonists. Neuropeptides. 2001;35:232–237 [DOI] [PubMed] [Google Scholar]
- 8. Kotikoski H, Kankuri E, Vapaatalo H. Incubation of porcine iris-ciliary bodies to study the mechanisms by which nitric oxide donors lower intraocular pressure. Med Sci Monit. 2003;9:BR1–BR7 [PubMed] [Google Scholar]
- 9. Coca-Prados M, Wax MB. Transformation of human ciliary epithelial cells by simian virus 40: induction of cell proliferation and retention of beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1986;83:8754–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danziger RS, Star RA, Matsumoto S, Coca-Prados M, DeSantis L, Pang IH. Characterization of soluble guanylyl cyclase in transformed human non-pigmented epithelial cells. Biochem Biophys Res Commun. 1993;195:958–962 [DOI] [PubMed] [Google Scholar]
- 11. Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13:51–63 [DOI] [PubMed] [Google Scholar]
- 12. Devine JO, Armstead WM. The role of nitric oxide in opioid-induced pial artery vasodilation. Brain Res. 1995;675:257–263 [DOI] [PubMed] [Google Scholar]
- 13. Armstead WM. Opioids and nitric oxide contribute to hypoxia-induced pial arterial vasodilation in newborn pigs. Am J Physiol. 1995;268:H226–H232 [DOI] [PubMed] [Google Scholar]
- 14. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1774–1784 [PubMed] [Google Scholar]
- 15. Doganay S, Evereklioglu C, Turkoz Y, Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12:44–48 [DOI] [PubMed] [Google Scholar]
- 16. Chiou GCY. Review: effects of nitric oxide on eye diseases and their treatment. J Ocul Pharmacol Ther. 2001;17:189–198 [DOI] [PubMed] [Google Scholar]
- 17. Osborne NN, Barnett NL, Herrera AJ. NADPH diaphorase localization and nitric oxide synthetase activity in the retina and anterior uvea of the rabbit eye. Brain Res. 1993;610:194–198 [DOI] [PubMed] [Google Scholar]
- 18. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995;36:1765–1773 [PubMed] [Google Scholar]
- 19. Meyer P, Champion C, Schlotzer-Schrehardt U, Flammer J, Haefliger IO. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999;18:375–380 [DOI] [PubMed] [Google Scholar]
- 20. Kiel JW, Reitsamer HA, Walker JS, Kiel FW. Effects of nitric oxide synthase inhibition on ciliary blood flow, aqueous production and intraocular pressure. Exp Eye Res. 2001;73:355–364 [DOI] [PubMed] [Google Scholar]
- 21. Taniguchi T, Kawakami H, Sawada A, et al. Effects of nitric oxide synthase inhibitor on intraocular pressure and ocular inflammation following laser irradiation in rabbits. Curr Eye Res. 1998; 17:308–315 [DOI] [PubMed] [Google Scholar]
- 22. Giuffrida S, Bucolo C, Drago F. Topical application of a nitric oxide synthase inhibitor reduces intraocular pressure in rabbits with experimental glaucoma. J Ocul Pharmacol Ther. 2003;19:527–534 [DOI] [PubMed] [Google Scholar]
- 23. Fleischhauer JC, Liu R, Elena PP, Flammer J, Haefliger IO. Topical ocular instillation of nitric oxide synthase inhibitors and intraocular pressure in rabbits. Klin Monatsbl Augenheilkd. 2001;218:351–353 [DOI] [PubMed] [Google Scholar]
- 24. Kotikoski H, Alajuuma P, Moilanen E, et al. Comparison of nitric oxide donors in lowering intraocular pressure in rabbits: role of cyclic GMP. J Ocul Pharmacol Ther. 2002;18:11–23 [DOI] [PubMed] [Google Scholar]
- 25. Orihashi M, Shima Y, Tsuneki H, Kimura I. Potent reduction of intraocular pressure by nipradilol plus latanoprost in ocular hypertensive rabbits. Biol Pharm Bull. 2005;28:65–68 [DOI] [PubMed] [Google Scholar]
- 26. Chuman H, Chuman T, Nao-i N, Sawada A. The effect of L-arginine on intraocular pressure in the human eye. Curr Eye Res. 2000;20:511–516 [PubMed] [Google Scholar]
- 27. Millar JC, Shahidullah M, Wilson WS. Atriopeptin lowers aqueous humor formation and intraocular pressure and elevates ciliary cyclic GMP but lacks uveal vascular effects in the bovine perfused eye. J Ocul Pharmacol Ther. 1997;13:1–11 [DOI] [PubMed] [Google Scholar]
- 28. Shahidullah M, Yap M, To CH. Cyclic GMP, sodium nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br J Pharmacol. 2005;145:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneemann A, Dijkstra BG, van den Berg TJ, Kamphuis W, Hoyng PF. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:936–941 [DOI] [PubMed] [Google Scholar]
- 30. Schneemann A, Leusink-Muis A, van den Berg T, Hoyng PF, Kamphuis W. Elevation of nitric oxide production in human trabecular meshwork by increased pressure. Graefes Arch Clin Exp Ophthalmol. 2003;241:321–326 [DOI] [PubMed] [Google Scholar]
- 31. Kee C, Kaufman PL, Gabelt BT. Effect of 8-Br cGMP on aqueous humor dynamics in monkeys. Invest Ophthalmol Vis Sci. 1994;35:2769–2773 [PubMed] [Google Scholar]
- 32. Kotikoski H, Vapaatalo H, Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003;26:119–123 [DOI] [PubMed] [Google Scholar]
- 33. Galassi F, Masini E, Giambene B, et al. A topical nitric oxide-releasing dexamethasone derivative: effects on intraocular pressure and ocular haemodynamics in a rabbit glaucoma model. Br J Ophthalmol. 2006;90:1414–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lahti RA, Mickelson MM, McCall JM, Von Voigtlander PF. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985;109:281–284 [DOI] [PubMed] [Google Scholar]
- 35. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994;35:2515–2520 [PubMed] [Google Scholar]
- 36. Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008;294:C1378–C1386 [DOI] [PubMed] [Google Scholar]
- 37. Ellis DZ, Dismuke WM, Chokshi BM. Characterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50:1808–1813 [DOI] [PubMed] [Google Scholar]
- 38. Millar JC, Shahidullah M, Wilson WS. Intraocular pressure and vascular effects of sodium azide in bovine perfused eye. J Ocul Pharmacol Ther. 2001;17:225–234 [DOI] [PubMed] [Google Scholar]
- 39. Ellis DZ, Nathanson JA, Rabe J, Sweadner KJ. Carbachol and nitric oxide inhibition of Na, K-ATPase activity in bovine ciliary processes. Invest Ophthalmol Vis Sci. 2001;42:2625–2631 [PubMed] [Google Scholar]
- 40. Shahidullah M, Delamere NA. NO donors inhibit Na, K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Picolo G, Cury Y. Peripheral neuronal nitric oxide synthase activity mediates the antinociceptive effect of Crotalus durissus terrificus snake venom, a delta- and kappa-opioid receptor agonist. Life Sci. 2004;75:559–573 [DOI] [PubMed] [Google Scholar]
- 42. Zeynalov E, Nemoto M, Hurn PD, Koehler RC, Bhardwaj A. Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide. J Cereb Blood Flow Metab. 2006;26:414–420 [DOI] [PubMed] [Google Scholar]