Abstract
Trex2 is a 3′ → 5′ exonuclease that removes 3′-mismatched sequences in a biochemical assay; however, its biological function remains unclear. To address biology we previously generated trex2null mouse embryonic stem (ES) cells and expressed in these cells wild-type human TREX2 cDNA (Trex2hTX2) or cDNA with a single-amino-acid change in the catalytic domain (Trex2H188A) or in the DNA-binding domain (Trex2R167A). We found the trex2null and Trex2H188A cells exhibited spontaneous broken chromosomes and trex2null cells exhibited spontaneous chromosomal rearrangements. We also found ectopically expressed human TREX2 was active at the 3′ ends of I-SceI–induced chromosomal double-strand breaks (DSBs). Therefore, we hypothesized Trex2 participates in DNA DSB repair by modifying 3′ ends. This may be especially important for ends with damaged nucleotides. Here we present data that are unexpected and prompt a new model. We found Trex2-altered cells (null, H188A, and R167A) were not hypersensitive to camptothecin, a type-1 topoisomerase inhibitor that induces DSBs at replication forks. In addition, Trex2-altered cells were not hypersensitive to γ-radiation, an agent that causes DSBs throughout the cell cycle. This observation held true even in cells compromised for one of the two major DSB repair pathways: homology-directed repair (HDR) or nonhomologous end joining (NHEJ). Trex2 deletion also enhanced repair of an I-SceI–induced DSB by both HDR and NHEJ without affecting pathway choice. Interestingly, however, trex2null cells exhibited reduced spontaneous sister chromatid exchanges (SCEs) but this was not due to a defect in HDR-mediated crossing over. Therefore, reduced spontaneous SCE could be a manifestation of the same defect that caused spontaneous broken chromosomes and spontaneous chromosomal rearrangements. These unexpected data suggest Trex2 does not enable DSB repair and prompt a new model that posits Trex2 suppresses the formation of broken chromosomes.
DNA double-strand breaks (DSBs) are cytotoxic lesions generated by exogenous and endogenous agents (Friedberg et al. 1995). Breaks may also occur spontaneously at collapsed replication forks (Petermann and Helleday 2010). There are two major pathways that repair DNA DSBs: homology-directed repair (HDR) and nonhomologous end joining (NHEJ). Even though much is known about these processes, they are not fully understood. Both HDR and NHEJ are frequently used in mammalian cells (Jasin 2000) and disruption of either leads to gross chromosomal rearrangements that may cause cancer (Hoeijmakers 2001) or aging-like phenotypes (Vijg and Dolle 2002; Hasty et al. 2003; Lombard et al. 2005). HDR repairs general DSBs by annealing the broken strand to a homologous template usually provided by the sister chromatid during DNA replication (West 2003; San Filippo et al. 2008). Importantly, the generation of 3′ overhangs is required before strand annealing can take place. These ends may need modification if damaged nucleotides are present. NHEJ repairs general DSBs by joining the ends together without the use of a homologous template (Lieber et al. 2004) and like HDR, the ends may need modification if damaged nucleotides are present (Roberts et al. 2010). Thus a protein with nonprocessive 3′ → 5′ exonuclease activity could be useful for HDR and NHEJ to remove damaged nucleotides from 3′ ends.
Trex2 is a nonprocessive 3′ → 5′ exonuclease that removes 3′ mismatches in biochemical assays (Mazur and Perrino 2001). It is present from worms to mammals but is not found in lower life forms like bacteria and yeast. Trex2 functions as a homodimer and has a DNA-binding domain along with the catalytic domain. Single-amino-acid mutations can selectively disrupt these functions, including H188A that completely ablates exonuclease activity but also impairs DNA binding by ∼60% and R167A that impairs DNA binding by ∼85% without diminishing exonuclease activity (Chen et al. 2007b). Therefore, these activities are separable, at least in part.
To understand biological function, we generated trex2null mouse embryonic stem (ES) cells (Chen et al. 2007a). We introduced into these trex2null cells wild-type human TREX2 cDNA or human TREX2 cDNA with a single-amino-acid change in the catalytic domain (Trex2H188A) or DNA-binding domain (Trex2R167A); these cells were made by knock-in such that the human cDNA is expressed by the mouse Trex2 promoter in situ (Dumitrache et al. 2009). The trex2null and Trex2H188A cells, but not the Trex2hTX2 or Trex2R167A cells, exhibited high levels of spontaneous broken chromosomes. Broken chromosomes were especially apparent in the Trex2H188A cells, suggesting a dominant activity. These breaks were often located in or adjacent to the pericentromere, a highly repetitive region that is composed of 6–8 Mb of tandem major satellite repeats (MSRs) that undergoes dynamic regulation (Guenatri et al. 2004). This observation suggests Trex2 maintains highly repetitive regions. In addition, the trex2null cells exhibited spontaneous chromosomal rearrangements (Trex2H188A and Trex2R167A cells were not analyzed for rearrangements) (Chen et al. 2007a). Therefore, Trex2 suppresses spontaneous broken chromosomes and spontaneous chromosomal rearrangements.
It is possible that Trex2 participates in repairing DSBs by either HDR or NHEJ. Trex2’s potential role in HDR and NHEJ is especially attractive since it is a nonprocessive 3′ → 5′ exonuclease that would be useful for modifying 3′ ends with damaged nucleotides. To support this possibility ectopically expressed human TREX2 removed nucleotides from the 3′ ends of DNA DSBs generated by I-SceI, a site-specific endonuclease (Bennardo et al. 2009). Typically two to five nucleotides were removed such that the I-SceI site was destroyed. Presumably these ends did not have damaged nucleotides, presenting the possibility that Trex2 activity can modify 3′ DNA ends even without damage. However, these cells ectopically expressed human TREX2 at levels 10 times higher than endogenous mouse Trex2 as measured by mRNA. Therefore, we do not know whether these endogenous levels of mouse Trex2 are sufficient to modify I-SceI–induced 3′ ends. We also do not know whether Trex2 participates in the repair of DSBs induced by chemical agents or ionizing radiation. Thus, it is possible that Trex2-defective cells exhibit elevated levels of broken chromosomes and chromosomal rearrangements due to a defect in DSB repair.
Here, we test the possibility that Trex2 participates in the repair of DNA DSBs by either HDR or NHEJ. We report that Trex2-altered cells did not exhibit hypersensitivity to agents that generate DSBs (camptothecin or γ-radiation) even in cells compromised for either HDR or NHEJ. Trex2 deletion enhanced the repair of an I-SceI–induced DSB by both HDR and NHEJ without affecting pathway choice. In addition, Trex2 deletion did not diminish the formation of sister chromatid exchanges (SCEs) in cells elevated for HDR-induced crossing over. Instead, Trex2 deletion diminished spontaneous SCEs, suggesting a defect in a strand-exchange pathway that is independent of HDR and DSB repair. Our data suggest Trex2 does not participate in DSB repair by either HDR or NHEJ; therefore, Trex2 may instead suppress DSB formation.
Materials and Methods
Tissue culture conditions
ES cells were maintained in M15 [high-glucose DMEM supplemented with 15% fetal bovine serum, 100 μM β-mercaptoethanol, 2 mM L-Glutamine, 3 mg/ml penicillin, 5 mg/ml streptomycin, 1000 units/ml ESGRO (LIF)] and seeded on 0.1% gelatin-coated plastic plates in a 37° incubator at atmospheric O2.
Substrains and gene targeting
We performed an epistatic analysis for this study and used genetically modified cells derived from multiple investigators. These cells are called AB2.2, IB10, TC1, and J1. All cells were derived from the 129 mouse strain; however, 129 mice rapidly undergo genetic changes so cells derived from different substrains should not be considered isogenic (Simpson et al. 1997; Threadgill et al. 1997). Therefore, we mutated Trex2 in control and mutant cells of each parental cell to make a valid comparison. All these cells are XY; therefore, the epistatic analysis was facilitated by Trex2’s location on the X chromosome.
For our previously published studies we used AB2.2 cells. We made a null by deleting a single exon that contains all coding sequence (trex2null and trex2null-2) (Chen et al. 2007a; Dumitrache et al. 2009). In addition, by knock-in we introduced the wild-type human short isoform of TREX2 (Trex2hTX2) and two variants with a single-amino-acid change adjacent to the Trex2 promoter (Chen et al. 2007b; Dumitrache et al. 2009). One variant is mutated in the catalytic domain (Trex2H188A) while the other variant is mutated in the DNA-binding domain (Trex2R167A). IB10 is the genetic background used for immunostaining. These cells were used because they are better than AB2.2 cells at forming monolayers on plastic chamber slides. TC1 cells were used to generate the h2ax−/− cells (Bassing et al. 2002) and J1 cells were used to generate the ku70−/− cells (Gu et al. 1997). AB2.2 cells were used to generate the blmtm3Brd/tm4Brd cells (Luo et al. 2000); for simplicity these cells are referred to as blm−/− cells for the remainder of the text.
Gene targeting was very similar for each ES cell type except different selection cassettes were used. The Hprt minigene was used for AB2.2 cells since they are Hprt negative. However, the other strains are Hprt positive. For IB10 cells we mutated the Hprt gene (below) and therefore used the same targeting vector as described for AB2.2 cells (Chen et al. 2007a). However, Hprt was not mutated for TC1 and J1 cells; therefore, we modified the Trex2 targeting vector by using a different selection cassette, puΔtk (Figure 1A and below). We also used a different selection cassette to delete Trex2 in blm−/− cells, puromycin phosphotransferase (Figure 1B and below). Even though these different selection cassettes were used, the mutation is the same, deletion of the single exon that contains all Trex2 coding sequences.
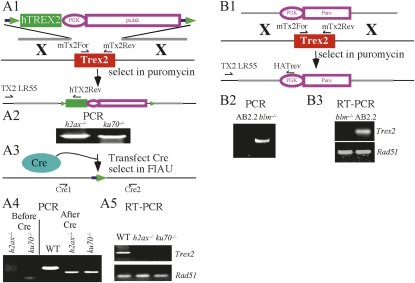
Figure 1 .
Trex2 knockouts. (A1) Targeting Trex2 with the conditional vector in h2ax−/− and ku70−/− cells. The targeting vector contains floxed puΔtk (Chen and Bradley 2000) and the human short TREX2 isoform (Chen et al. 2007b). After gene targeting the mouse Trex2 coding sequences (red) were replaced with the human short TREX2 isoform (green). (A2) Screen for targeted clones. PCR amplification with TX2LR55 and hTX2Rev is shown. (A3) Cre-mediated deletion of human TREX2 and puΔtk. (A4) Screen for Trex2-deleted clones by PCR amplification with Cre1 and Cre2. (A5) Verification of Trex2 deletion by RT-PCR with mTx2For and mTx2Rev primers shown in A1. Detection of Rad51 mRNA was used as a loading control (Holcomb et al. 2007). WT, wild-type control. (B1) Replacing Trex2 in blm−/− cells with puromycin acetyltransferase expressed by the phosphoglycerate kinase (PGK) promoter. (B2) Screen for targeted clones. PCR amplification with TX2LR55 and HATrev is shown. (B3) Verification of targeted clones with RT-PCR using mTx2For and mTx2Rev primers shown in A1. Detection of Rad51 mRNA was used a loading control.
Trex2 was deleted in IB10 control cells using the same targeting vector and targeting strategy as previously described (Chen et al. 2007a). This targeting vector has a floxed miniHPRT cassette (Holcomb et al. 2007); therefore, the Hprt gene was first mutated in control IB10 ES cells. We used a replacement targeting vector that is similar to the one previously described (Thomas et al. 1992). However, a floxed puΔtk cassette was used for selection instead of neomycin phosphotransferase (not shown). The puΔtk cassette is a positive/negative selection cassette that fuses puromycin N-acetyltransferase (pu) to a truncated version of herpes simplex virus type 1 thymidine kinase (Δtk) (Chen and Bradley 2000). Cells expressing puΔtk are resistant to puromycin and sensitive to 1-(-2-deoxy-2-fluoro-1-β-D-arabino-furanosyl)-5-iodoracil (FIAU). RE mutant loxPs (Araki et al. 1997) are located on each side of puΔtk so it can be removed upon Cre expression. The 5′ end of RE mutant loxP (TACCGTTCGTATAATGTATGCTATACGAAGTTAT) differs from that of wild-type loxP (ATAACTTCGTATAATGTATGCTATACGAAGTTAT); the difference is in boldface type. Targeted clones were selected in tissue culture with 3–9 μg/ml puromycin for 5 days and then 10 μM 6-thioguanine + 3 μg/ml puromycin for another 5 days, using previously described conditions (Hasty et al. 1991).
Trex2 was targeted with a conditional vector in cells mutated for H2ax (Bassing et al. 2002) and Ku70 (Gu et al. 1997). This targeting vector introduces cDNA coding for the human short isoform of TREX2 (Chen et al. 2007b), similar to a previously described knock-in vector (Chen et al. 2007a) except puΔtk was used instead of miniHPRT for selection since these cells are Hprt positive (Figure 1A). SfiI sites flank this selection cassette to allow sticky directional cloning as previously described (Holcomb et al. 2007).
Trex2 was targeted with a deletion vector in cells mutated for Blm (Luo et al. 2000). This deletion vector is the same as the one previously described (Chen et al. 2007a) except a puromycin acetyltransferase selection cassette was used for selection instead of miniHPRT (Figure 1B). SfiI sites also flank this selection cassette as previously described (Holcomb et al. 2007).
The floxed puΔtk in Hprt and Trex2 were deleted using a Cre-recombinase expression vector (pPGKcrepA) that was transiently transfected into ES cells using previously described conditions (Holcomb et al. 2007; Kim et al. 2008). PCR was performed on FIAU-resistant clones to verify puΔtk deletion (Figure 1A). Primers for Trex2 deletion are Tx2Cre2 (5′-CCAAAGGCCTCATGAGATGG-3′) and Tx2CreRev3 (5′-AAGGCATGGACTAGCTCTCTGC-3′). The PCR conditions are as follows: 1 cycle of 98° for 5 min; then 35 cycles of 98° for 1 min, 62° for 1 min, and 72° for 30 sec; and then 1 cycle of 72° for 10 min.
Dose response to camptothecin and γ-radiation
The camptothecin and γ-radiation dose response was performed as described (Marple et al. 2004).
Immunostaining
IB10 cells were used as opposed to AB2.2 cells for immunostaining because they are better at forming monolayers soon after seeding when cultured on plastic chamber slides (AB2.2 cells proliferate on top of each other even immediately after seeding). IB10 ES cells were plated at 5 × 104 cells per well in a four-well chamber slide and cultured at atmospheric pressure for 1 day as described for murine fibroblasts (Parrinello et al. 2003). Cells were washed in PBS, fixed with 2% formaldehyde in PBS for 10 min at room temperature, washed three times with PBS, permeabilized with 0.5% Triton X-100 for 10 min, washed three times with PBS, blocked for 30 min, and incubated overnight at 4° with primary antibody. After washing three times with PBS, cells were incubated with secondary antibody for 45 min at room temperature, washed three times with PBS, and stained with DAPI (1 μg/ml). The cells were photographed using a Zeiss Axioplan2 with a 60× objective and a Nikon Digital Camera DXM1200. The primary antibody for 53BP1 was a rabbit polyclonal from Bethyl (BL182) and the secondary antibody was a goat–anti-rabbit conjugated to Alexa fluor 488 from Molecular Probes.
Three-color FISH to measure DSBs
ES cells were seeded onto plastic tissue culture plates pretreated with 0.1% gelatin for 1 hr. The next day cells were exposed to 100 nM camptothecin for 5 hr and 540 nM colcemid for 4 hr and then trypsinized. The remainder of the procedure has been described (Chen et al. 2007a).
Sister chromatid exchange assay
ES cells (1 × 106) were seeded onto a 10-cm plate pretreated with 0.1% gelatin for 1 hr. The next day BrdU was added (10 μM final concentration) and cells were incubated for 24 hr. Then the media were removed and new media added, cells were incubated for an additional 12 hr, and then 540 nM colcemid was added for 4 hr. Cells were then trypsinized and prepared for slides using the same procedure for three-color FISH as previously described (Chen et al. 2007a). Once slides were prepared, they were immersed into a 40ul solution of 0.5% Tw20, 10 μl FITC-conjugated anti-BrdU antibody (Becton Dickinson), and 0.25 mg/ml major satellite repeat (CY-3 5′-TGG AAT ATG GCG AGA AAA CTG AAA ATC ATG GAA AAT GAG A-3′) for 10 min, at 37°, in the dark. The slides were washed with PBS (pH 7) for 30 sec, treated with a DAPI (Vectashield), and analyzed under a microscope (Axioplan).
I-SceI assays
Integration of DR-GFP and EJ5-GFP into the Pim1 locus of AB2.2 control and trex2null cells was performed as described (Bennardo et al. 2009). Transfection of the I-SceI expression plasmid was performed as described (Bennardo et al. 2009). Repair junctions were analyzed as described (Bennardo and Stark 2010).
Results and Discussion
Trex2-deleted cells are not hypersensitive to camptothecin
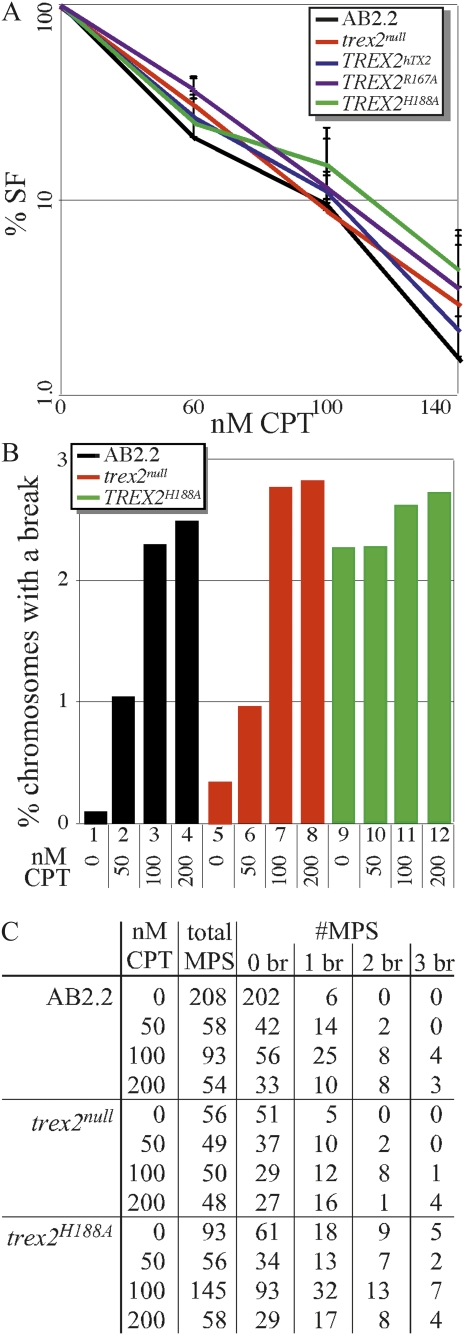
To test the possibility that Trex2 is important for repairing agent-induced DSBs at DNA replication forks, we compared control cells to Trex2-altered cells for sensitivity to camptothecin, a type 1 topoisomerase inhibitor. Camptothecin blocks rejoining of the cleavage/religation reaction and causes the accumulation of covalent reaction intermediates that generate DSBs after collision with a replication fork in S phase (Liu et al. 2000). We found all Trex2-altered cells exhibit the same level of sensitivity to camptothecin as control cells (Figure 2A). These data indicate that Trex2 is dispensable for chromosome break repair since mouse ES cells defective for HDR are hypersensitive to camptothecin (Marple et al. 2006). In addition, the survival fraction curve suggests the dominant negative-like effects of the H188A mutation are not due to interference with DSB repair pathways.
Figure 2 .
Camptothecin (CPT)-induced broken chromosomes. (A) Trex2-altered cells exhibited the same dose response to camptothecin as control cells (AB2.2). Four clones of Trex2hTX2, Trex2H188A, and Trex2R167A were compared to the parental clones of trex2null and trex2null-2 cells (Dumitrache et al. 2009) and control cells. These experiments were done three times. The four clones from each experiment were averaged and then the three experiments were averaged. Standard deviation is shown. SF, survival fraction. (B) The percentage of metaphase spreads (MPS) with microscopically visible spontaneous and camptothecin-induced broken chromosomes. AB2.2, control cells. (C) Summary of results. Shown is the number of MPS with zero broken (br) chromosomes, one broken chromosome, two broken chromosomes, or three broken chromosomes. No metaphase spread had more than three broken chromosomes. Statistics (Student’s t-test) refer to lanes in B for comparisons: 1 and 2, P = 0.0001; 1 and 3, P < 0.0001; 1 and 4, P = 0.0003; 2 and 3. P = 0.0141; 2 and 4, P = 0.026; 3 and 4, P = 0.398; 5 and 6, P = 0.061; 5 and 7, P = 0.0003; 5 and 8, P = 0.0008; 6 and 7, P = 0.033; 6 and 8, P = 0.013; 7 and 8, P = 0.391; 9 and 10, P = 0.708; 9 and 11, P = 0.321; 9 and 12, P = 0.481; 10 and 11, P = 0.517; 10 and 12, P = 0.847; 11 and 12, P = 0.608; 1 and 5, P = 0.037; 1 and 9, P = 0.0002; 2 and 6, P = 0.872; 2 and 10, P = 0.027; 3 and 7, P = 0.511; 3 and 11, P = 0.206; 4 and 8, P = 0.546; 4 and 12, P = 0.872; 5 and 9, P < 0.0001; 6 and 10, P = 0.037; 7 and 11, P = 0.569; and 8 and 12, P = 0.411.
We further investigated trex2null and Trex2H188A cells for camptothecin-induced chromosomal breaks by looking at metaphase spreads using three-color fluorescence in situ hybridization (FISH). Metaphase spreads from these cells were stained with a telomeric probe (green), a MSR probe in the pericentromere (red), and DAPI (blue) (Guenatri et al. 2004). Previously we showed trex2null and Trex2H188A cells exhibited increased levels of spontaneous broken chromosomes compared to control cells. These breaks were almost exclusively in the pericentromere. In addition, the Trex2H188A cells showed higher levels of spontaneous broken chromosomes than the trex2null cells, suggesting a dominant negative-like effect (Dumitrache et al. 2009). We looked at the level of broken chromosomes in cells after 5 hr exposure to camptothecin at 50 nM, 100 nM, or 200 nM followed by 4 hr exposure to colcemid. For control and trex2null cells we found a progressive increase in chromosomal breaks with dose, yet deleting Trex2 did not increase the number of camptothecin-induced breaks (Figure 2, B and C; see legend for statistics). Camptothecin exposure did not significantly increase the number of chromosomal breaks for Trex2H188A cells beyond the high level of spontaneous broken chromosomes (Figure 2, B and C). These data show Trex2 does not suppress camptothecin-induced broken chromosomes and suggest it is unessential for DSB repair.
The breaks were almost exclusively in the pericentromere for all genotypes at all CPT doses; thus, it is possible that mouse ES cells are susceptible to broken pericentromeres. However, our recent publication shows FancB-deleted mouse ES cells exhibited elevated levels of broken chromosomes in the long arm and the crosslinking agent mitomycin exacerbated this phenotype (Kim et al. 2011). Therefore, the pericentromere seems to be vulnerable to Trex2 deletion and type-1 topoisomerase inhibition. This phenotype suggests Trex2 and type-1 topoisomerase play an important role in maintaining highly repetitive regions since the pericentromere is composed of major satellite repeats (Guenatri et al. 2004).
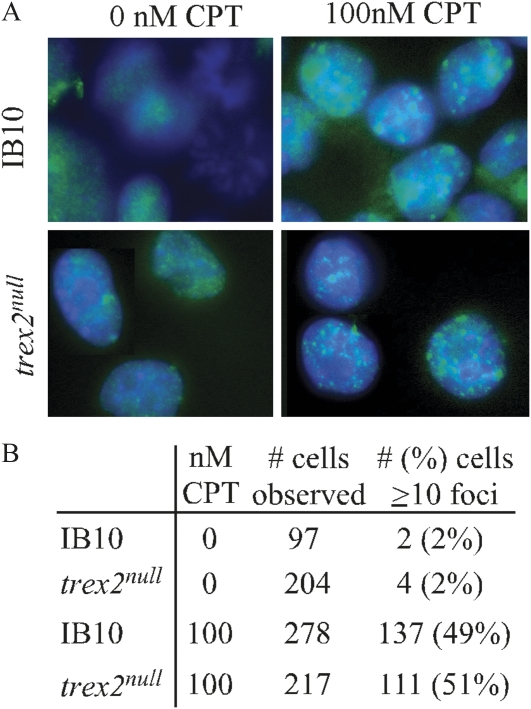
To evaluate DNA lesions, we measured the number of nuclear foci containing p53-binding protein 1 (53BP1) in ES cells. 53BP1 rapidly localizes to DNA DSBs and is a marker of unrepaired DNA damage and an important early component of the DNA damage response (Mochan et al. 2004). For this experiment, we deleted Trex2 in IB10 cells since immunostaining is easier in these cells compared to AB2.2 cells (see Materials and Methods), the parental cells described in our previous publications. We found control and trex2null cells exhibited the same level of spontaneous and camptothecin-induced 53BP1 foci (Figure 3), suggesting Trex2 deletion does not alter the level of DSBs or the early DNA damage response to DSBs. This observation indicates Trex2 deletion does not alter the early stages of the DNA damage response.
Figure 3 .
Camptothecin-induced 53BP1 foci. (A) trex2null cells exhibit the same level of camptothecin-induced 53BP1 foci as control cells (IB10). (B) Summary of results.
Trex2-altered cells are not hypersensitive to γ-radiation
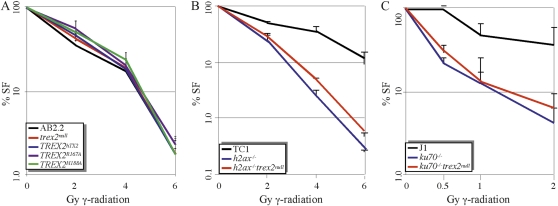
Camptothecin causes DSBs associated with replication forks, so we tested the impact γ-radiation has on Trex2-altered cells since it causes DSBs throughout the cell cycle. γ-Radiation is a high-frequency electromagnetic wave that interacts directly with DNA or with surrounding molecules to make reactive species that cause DSBs (Friedberg et al. 1995). Cells defective for either HDR (Essers et al. 1997; Morimatsu et al. 1998) or classical NHEJ (Burma et al. 2006) are hypersensitive to γ-radiation. We found Trex2-altered cells exhibited the same level of sensitivity to γ-radiation as control cells, suggesting Trex2 does not participate in repairing γ-radiation–induced DSBs (Figure 4A). Again, the survival fraction curve suggests the dominant negative-like effects of the H188A mutation are not due to interference with DSB repair pathways.
Figure 4 .
Exposure to γ-radiation. (A) Trex2-altered cells exposed to γ-radiation. Four clones of Trex2hTX2, Trex2H188A, and Trex2R167A were compared to the parental clones of trex2null and trex2null-2 cells (Dumitrache et al. 2009) and control cells. These experiments were done three times. Standard deviation is shown. SF, survival fraction. (B) trex2null h2ax−/− cells exposed to γ-radiation. Average of two h2ax−/− trex2null clones is shown. These experiments were done three times and standard deviation is shown. TC1, control cells. (C) trex2null ku70−/− cells exposed to γ-radiation. Average of two trex2null ku70−/− clones is shown. These experiments were done three times and standard deviation is shown. J1, control cells.
It is possible Trex2 has an important, but nonessential role in DSB repair. Therefore, we deleted Trex2 in ES cells defective for either HDR or NHEJ to look for an additive or synergistic phenotype. Such a phenotype may be revealed in DSB repair-compromised cells. To observe HDR-defective cells, Trex2 was deleted in control TC1 cells and TC1 cells defective for H2ax (Figure 1A). H2ax is a histone phosphorylated in response to DSBs (γ-H2ax) (Bassing et al. 2002). γ-H2ax recognizes DNA DSBs (Pilch et al. 2003), recruits DNA repair proteins to DNA damage (Paull et al. 2000), and controls recombination between sister chromatids (Xie et al. 2004, 2010). Therefore, the h2ax−/− cells are compromised for HDR and are therefore more likely dependent on NHEJ for repairing DSBs (Couedel et al. 2004). However, deleting Trex2 did not further sensitize these cells to γ-radiation (Figure 4B). To look at classical NHEJ-defective cells, we deleted Trex2 in control J1 cells and J1 cells mutated for Ku70 (Gu et al. 1997) (Figure 1A). Ku70 forms a heterodimer with Ku80, called Ku that binds to DNA ends as an essential component of classical NHEJ (Burma et al. 2006). Therefore, ku70−/− cells are more dependent on HDR (Pierce et al. 2001; Couedel et al. 2004). However, deleting Trex2 did not further sensitize these cells to γ-radiation (Figure 4C). Thus, Trex2 does not appear to be important for repairing general DSBs even in cells compromised for either HDR or classical NHEJ.
Trex2-deleted cells were not defective for repairing I-SceI–induced DSBs by either HDR or NHEJ
Previously we observed the impact ectopically expressed human TREX2 had on the repair of I-SceI–induced DSBs, using multiple substrates in a variety of genetically altered mouse ES cells (Bennardo et al. 2009). The level of ectopically expressed human TREX2 was 10-fold greater than that of endogenous mouse Trex2 at the mRNA level. We found this high level of ectopically expressed TREX2 reduced persistent I-SceI cutting through its nonprocessive exonuclease activity. Specifically, high levels of TREX2 lead to I-SceI–resistant NHEJ products with modified 3′ overhangs, which also reduced the frequency of NHEJ between distal ends of two tandem DSBs, but did not affect the frequency of HDR. On the basis of these results, it is possible that endogenous Trex2 levels are sufficient to modify the I-SceI–cut 3′ ends. Therefore, we tested whether endogenous levels of Trex2 had an impact on the frequency of HDR, the frequency of distal NHEJ, or end processing during NHEJ.
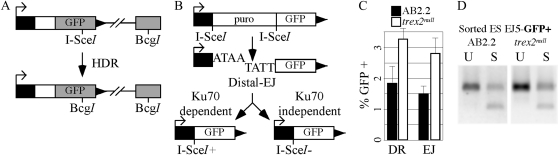
To begin with, we tested whether endogenous Trex2 levels affect the repair of an I-SceI–induced DSB in a substrate specific for either HDR (DR-GFP, Figure 5A) or NHEJ between distal ends of two tandem DSBs (EJ5-GFP, Figure 5B); both substrates were targeted to Pim1 (Bennardo et al. 2009). The generation of GFP+ cells with DR-GFP is specific for HDR, since it is facilitated by Rad51 (Stark et al. 2004; Bennardo et al. 2008). In addition, DR-GFP does not detect single-strand annealing since the iGFP donor fragment lacks essential 3′ elements of the GFP expression cassette. The generation of GFP+ cells with EJ5-GFP can involve classical NHEJ factors (e.g., Ku70 and Xrcc4), which are important for I-SceI site restoration, but also can be generated independent of classical NHEJ when the I-SceI site is destroyed (Bennardo et al. 2008, 2009). We found the frequency of HDR and NHEJ was increased in trex2null cells relative to control (Figure 4C; Student’s t-test, P < 0.001), supporting the possibility these DSB repair pathways are elevated in trex2null cells. We also found the relative HDR/NHEJ ratio was similar between trex2null (1.17) and control (1.23) cells, suggesting that Trex2 deletion increased DSB repair efficiency without affecting pathway choice. These observations support the possibility that HDR and NHEJ are elevated in trex2null cells.
Figure 5 .
I-SceI–specific DSBs. (A) The HDR-specific substrate, DR-GFP. The I-SceI site interrupts the GFP sequence while the BcgI site is the wild-type sequence at this location. After generation of an I-SceI DSB, the free ends invade the adjacent truncated GFP substrate that contains the wild-type BcgI site. Nonreciprocal homologous recombination (gene conversion) restores GFP. (B) The NHEJ-specific substrate, EJ5-GFP. There are two I-SceI sites separated by a puromycin phosphotransferase (puro) selection cassette. After generation of two I-SceI–induced DSBs, distal end joining restores the GFP cassette. There are two potential outcomes. First, the I-SceI site is restored. This detects classical NHEJ (Ku70 dependent). Second, the I-SceI site is lost. This detects Alt-NHEJ (Ku70 independent). (C) trex2null cells repaired I-SceI–induced DSBs more efficiently than control cells (AB2.2) for the DR-GFP (DR, P < 0.001, Student’s t-test) and EJ5-GFP (EJ, P < 0.0001) substrates. The mean of six to eight experiments is shown. (D) I-SceI digestion of PCR-amplified DNA from GFP+ cells with the EJ5-GFP substrate. After I-SceI digestion, the amplified DNA is either uncut (U) or cut with I-SceI (S).
Elevation in HDR and NHEJ in trex2null cells could be due to compensation for increased spontaneous DSBs or due to loss of Trex2-mediated processing of I-SceI overhangs prior to NHEJ. To address the latter possibility, we characterized distal NHEJ repair junctions from control and trex2null cells. We sorted GFP+ cells from the EJ5-GFP reporter experiment and amplified the region flanking the I-SceI site. Since the two tandem I-SceI sites in EJ5-GFP are in the same orientation, one possible distal NHEJ repair product is restoration of the I-SceI site (Figure 5B, left). The generation of GFP+ cells with EJ5-GFP can involve classical NHEJ factors (e.g., Ku70 and Xrcc4), which are important for I-SceI site restoration (I-SceI+), but can also be generated independent of classical NHEJ when the I-SceI site is destroyed (I-SceI−) (Bennardo et al. 2008, 2009). Even though formation of I-SceI− NHEJ products is independent of Ku70 and Xrcc4, we do not mean to imply that classical NHEJ factors never participate in these repair events in wild-type cells. Accordingly, loss of the I-SceI site (Figure 5B, right), can be independent of both Ku70 and Xrcc4 and promoted by ectopic expression of Trex2 (Bennardo et al. 2008, 2009). Hence, we first examined the amplification products by I-SceI digestion analysis. From this experiment, we found that restoration of the I-SceI site was the same between control and trex2null cells: 45 ± 7% I-SceI restoration for control and 43 ± 5% I-SceI restoration for trex2null (Figure 5D). Therefore, Trex2 deletion did not affect loss of the I-SceI site, which suggests that endogenous Trex2 does not affect processing of 3′ ends during NHEJ.
To further examine repair junctions, we isolated the I-SceI–resistant products for subcloning into a plasmid and then sequenced individual clones. From this analysis, we found no significant difference between control and trex2null in the sizes of deletions (Table 1). For example, in both cell lines, the majority of junctions showed 6–10 nucleotide deletions (14/23 for control and 12/23 for trex2null). These experiments show that Trex2 deletion did not affect either the efficiency of distal NHEJ or the nature of the repair products. Hence, these data suggest that endogenous Trex2 levels are unlikely to modify the 3′ ends of I-SceI–induced DSBs.
Table 1 . I-Sce1 resistant end joining junctions.
| Microhomology (nt) | WT: no. of clones | Trex2null: no. of clones | |
|---|---|---|---|
| I-SceI site (CAPS) | |||
| attcTAGGGATAACAGGGTAATggat | |||
| Insertions/deletions | |||
| Insertion + 1 | 1/23 | 1/23 | |
| TAGGGAATAACAGGGTAAT + 1 insertion | 0 | 1 | |
| TAGGGataacATAACAGGGTAAT + 5 insertions | NA | 1 | |
| Deletion, 1–5 nt | 2/23 | 2/23 | |
| TAGGGAT…ACAGGGTAAT, 1-nt deletion | 0 | 1 | |
| TAGGG…TAACAGGGTAAT, 1-nt deletion | 0 | 1 | |
| TAGGGA…ACAGGGTAAT, 2-nt deletion | 1 | 1 | |
| TAGGG…AACAGGGTAAT, 2-nt deletion | 1 | 1 | |
| Deletion, 6–10 nt | 14/23 | 12/23 | |
| ttc…TAACAGGGTAAT, 6-nt deletion | 2 | 1 | |
| TAGGGA…TAATGGAT, 8-nt deletion | 3 | 3 | |
| TAGGG...TAAT, 9-nt deletion | 4 | 9 | 9 |
| agaa…ATAACAGGGTAAT, 9-nt deletion | 2 | 1 | |
| TAGGGAT…Atgg, 9-nt deletion | 1 | 1 | |
| aatt…ACAGGGTAAT, 9-nt deletion | 0 | 1 | |
| ttc...AGGGTAT, 10-nt deletion | 1 | 1 | |
| Deletion, >10 nt | 6/23 | 8/23 | |
| agaa…CAGGGTAAT, 12-nt deletion | 2 | 1 | |
| ttcTA…ggatcc, 16-nt deletion | 2 | 1 | |
| agaa…TAAT, 17-nt deletion | 1 | 1 | |
| ttgg…CAGGGTAAT, 19-nt deletion | 2 | 1 | |
| TAGGG…tcatggtcg, 22-nt deletion, 4-nt insertion | 0 | 1 | |
| catc…AACAGGGTAAT, 24-nt deletion | 1 | 1 | |
| TAGGG…cgcc, 25-nt deletion | 0 | 1 | |
| atca…GGGTAATGG, 27-nt deletion | 2 | 1 | |
| attt…Tggat, 30-nt deletion | 2 | 1 | |
| TAGGGA…atgg, 31-nt deletion | 0 | 1 | |
| tcat…cacc, 41-nt deletion | 2 | 1 | |
| tgct…GGGTAAT, 48-nt deletion | 2 | 1 | |
| tttt…CAGGGTAAT, 70-nt deletion | 1 | 1 | |
| gcaa…AGGGTAAT, 79-nt deletion | 2 | 1 | |
Boldface type, inserted sequence; underlined, microhomology; NA, not applicable.
Why do trex2null cells exhibit elevated levels of DSB repair? While the I-SceI reporter systems provide insight into the relative frequency of different repair outcomes, they do not assess the mechanism by which a genetic mutation affects each repair outcome equivalently. Accordingly, the main insight from these experiments indicates that Trex2 deficiency does not affect the relative frequency of diverse DSB repair outcomes (HR vs. NHEJ and the types of NHEJ products). To explain the increase in DSB repair, we suggest DSB repair is elevated in trex2null cells as a general compensatory response to increased spontaneous chromosomal breaks (Dumitrache et al. 2009). In any case, these data do not support a role for endogenous Trex2 in promoting DSB repair.
Trex2 facilitates spontaneous SCEs, but not SCEs induced by Blm deletion
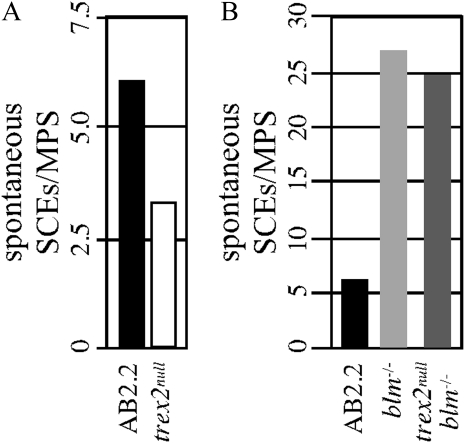
SCE is the process by which a single chromatid breaks and then recombines with the intact sister chromatid during replication. During this process, regions of parental strands in the duplicated chromosomes are reciprocally exchanged (Wilson and Thompson 2007). HDR of DSBs induces SCE (Sonoda et al. 1999; Dronkert et al. 2000; Takata et al. 2000, 2001; Dong and Fasullo 2003). Therefore, we observed spontaneous SCEs by FISH. We found trex2null cells exhibited reduced levels of spontaneous SCEs (Figure 6A, Yates-corrected chi-square test: P < 0.0001). Spontaneous SCEs may result from HDR-dependent crossing over or perhaps an HDR-independent pathway.
Figure 6 .
The average number of spontaneous SCEs per metaphase spread (MPS). (A) Spontaneous SCEs in control (AB2.2) and trex2null cells. Trex2 deletion reduced SCEs in control AB2.2 cells (Yates-corrected chi-square test, P < 0.0001). (B) Spontaneous SCEs in control (AB2.2), blm−/−, and trex2null blm−/− cells. Trex2 deletion did not reduce SCEs in blm−/− cells (P = 0.11). Total numbers: AB2.2, 374 SCEs from 1965 chromosomes (61 metaphase spreads); trex2null, 354 SCEs from 4020 chromosomes (106 metaphase spreads); blm−/−, 1348 SCEs from 1900 chromosomes (50 metaphase spreads); and trex2null blm−/−, 1348 SCEs from 1894 chromosomes (50 metaphase spreads).
To determine whether these SCEs were HDR dependent, we deleted Trex2 in blm−/− cells (Figure 1B and Materials and Methods) (Luo et al. 2000). Blm is a RecQ helicase that suppresses HDR-mediated SCEs by Holliday junction dissolution to inhibit crossing over (Ira et al. 2003; Wu and Hickson 2003; Liberi et al. 2005; Raynard et al. 2006). Additionally, the role of Blm in suppressing HDR-mediated SCEs was shown in mouse ES cells (Chu et al. 2010). Trex2 deletion did not alter the level of spontaneous SCEs in blm−/− cells (Figure 6B, P = 0.11). This observation suggests that Trex2 does not participate in Blm-regulated SCEs but instead participates in a pathway that enables HDR-independent SCEs. Therefore, the SCEs present in the trex2null cells are likely HDR dependent since we know HDR is responsible for at least some spontaneous SCEs and since HDR is elevated in these cells. This presents the possibility that two pathways cause spontaneous SCEs, one that is dependent on HDR and another that is dependent on Trex2.
Conclusion
Previously we showed Trex2 deletion caused increased levels of spontaneous chromosome breaks and rearrangements. In addition we found human TREX2H188A further increased the level of spontaneous broken chromosomes compared to no expression or to expression of wild-type human TREX2. These phenotypes suggest defective DSB repair so we hypothesized Trex2 participates in either HDR or NHEJ. This hypothesis is especially attractive since ectopic expression of human TREX2 modified the 3′ ends of I-SceI–induced DSBs in mouse ES cells. TREX2’s 3′ → 5′ exonuclease activity was essential for end modification. However, ectopic expression of human TREX2 was 10-fold greater than endogenous expression of mouse Trex2. Thus, we did not know whether this endogenous level was sufficient to affect DSB repair. Contrary to our hypothesis, our data suggest Trex2 suppresses the formation of chromosomal breaks as opposed to repairing them. In fact, Trex2 deletion significantly increased the repair of I-SceI–induced DSBs by both HDR and NHEJ. Therefore, these pathways might be enhanced to compensate for the increased levels of spontaneous broken chromosomes.
We present a conceptual model that suggests Trex2 suppresses the formation of spontaneous chromosomal breaks known to occur at collapsed replication forks (Petermann and Helleday 2010). Our model begins with a replication fork stalling as it encounters a defect. This defect may be the consequence of alternative structures found in repetitive DNA (like a hairpin) but could also be some form of damage. Trex2 then enables replication fork progression to prevent collapse and break formation. In Trex2’s absence chromosomal breaks increase along with a compensatory upregulation of HDR and NHEJ. TREX2H188A enhances break formation by interfering with another maintenance pathway. Thus, this model proposes Trex2 suppresses the formation of chromosomal breaks as opposed to repairing them.
The following data support this conceptual model:
Trex2 appears to be important for cell proliferation since Trex2 was expressed during S-G2, but not mitosis (Chen et al. 2007b) and since reduced TREX2/Trex2 levels inhibited proliferation in HeLa cells (Chen et al. 2007b) and ES cells (Chen et al. 2007a).
Trex2 may play a specialized role in maintaining highly repetitive regions since the majority of spontaneous chromosomal breaks found in Trex2-altered cells occurred in the pericentromere, a region rich in major satellite repeats (Guenatri et al. 2004). This phenotype is especially obvious for cells expressing TREX2H188A and suggests this exonuclease mutant interferes with proper replication fork maintenance.
Trex2 may enable postreplication repair since it was reported to associate with Polδ (Shevelev et al. 2002). In addition we show trex2null cells exhibit diminished spontaneous HDR-independent SCEs, suggesting Trex2 participates in a strand exchange pathway that is independent of HDR. For example in prokaryotes, diminished spontaneous SCEs were indicative of a defect in newly synthesized DNA strands switching templates during replication (Goldfless et al. 2006). Furthermore, defects in error-free postreplication repair resulted in gross chromosomal rearrangements in yeast (Smith et al. 2004) and inactivation of HDR reduced the level of these rearrangements (Motegi et al. 2006).
These observations support the possibility that Trex2 suppresses the formation of DSBs by maintaining replication forks perhaps in association with polδ or perhaps by enabling a strand exchange mechanism that is HDR independent. In the absence of Trex2 it is possible that replication forks stall and then collapse to form a DSB that is then a substrate for chromosomal rearrangements.
Acknowledgments
We thank Charnae Williams for technical support and Fred Alt (Ku70) and Allan Bradley (Blm) for mutant cells and their control cells. We also thank Allan Bradley for puΔtk. This work was supported by grants from the National Institutes of Health [U01 ES11044 and 1 R01 CA123203-01A1 (to P.H.), R01 CA120954 (to J.S.), and T32 CA86800-03 (to L.C.D.)].
Literature Cited
- Araki K., Araki M., Yamamura K., 1997. Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res. 25: 868–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassing C. H., Chua K. F., Sekiguchi J., Suh H., Whitlow S. R., et al. , 2002. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 99: 8173–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennardo N., Stark J. M., 2010. ATM limits incorrect end utilization during non-homologous end joining of multiple chromosome breaks. PLoS Genet. 6: e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennardo N., Cheng A., Huang N., Stark J. M., 2008. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 4: e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennardo N., Gunn A., Cheng A., Hasty P., Stark J. M., 2009. Limiting the persistence of a chromosome break diminishes its mutagenic potential. PLoS Genet. 5: e1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S., Chen B. P., Chen D. J., 2006. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst.) 5: 1042–1048 [DOI] [PubMed] [Google Scholar]
- Chen M. J., Dumitrache L. C., Wangsa D., Ma S. M., Padilla-Nash H., et al. , 2007a Cisplatin depletes TREX2 and causes Robertsonian translocations as seen in TREX2 knockout cells. Cancer Res. 67: 9077–9083 [DOI] [PubMed] [Google Scholar]
- Chen M. J., Ma S. M., Dumitrache L. C., Hasty P., 2007b Biochemical and cellular characteristics of the 3′ → 5′ exonuclease TREX2. Nucleic Acids Res. 35: 2682–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T., Bradley A., 2000. A new positive/negative selectable marker, puDeltatk, for use in embryonic stem cells. Genesis 28: 31–35 [DOI] [PubMed] [Google Scholar]
- Chu W. K., Hanada K., Kanaar R., Hickson I. D., 2010. BLM has early and late functions in homologous recombination repair in mouse embryonic stem cells. Oncogene 29: 4705–4714 [DOI] [PubMed] [Google Scholar]
- Couedel C., Mills K. D., Barchi M., Shen L., Olshen A., et al. , 2004. Collaboration of homologous recombination and nonhomologous end-joining factors for the survival and integrity of mice and cells. Genes Dev. 18: 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Fasullo M., 2003. Multiple recombination pathways for sister chromatid exchange in Saccharomyces cerevisiae: role of RAD1 and the RAD52 epistasis group genes. Nucleic Acids Res. 31: 2576–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkert M. L., Beverloo H. B., Johnson R. D., Hoeijmakers J. H., Jasin M., et al. , 2000. Mouse RAD54 affects DNA double-strand break repair and sister chromatid exchange. Mol. Cell. Biol. 20: 3147–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrache L. C., Hu L., Hasty P., 2009. TREX2 exonuclease defective cells exhibit double-strand breaks and chromosomal fragments but not Robertsonian translocations. Mutat. Res. 662: 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J., Hendriks R. W., Swagemakers S. M., Troelstra C., de Wit J., et al. , 1997. Disruption of mouse RAD54 reduces ionizing radiation resistance and homologous recombination. Cell 89: 195–204 [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., Walker G. C., Siede W., 1995. DNA Repair and Mutagenesis. American Society of Microbiology, Washington, DC. [Google Scholar]
- Goldfless S. J., Morag A. S., Belisle K. A., Sutera V. A., Jr, Lovett S. T., 2006. DNA repeat rearrangements mediated by DnaK-dependent replication fork repair. Mol. Cell 21: 595–604 [DOI] [PubMed] [Google Scholar]
- Gu Y., Jin S., Gao Y., Weaver D. T., Alt F. W., 1997. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc. Natl. Acad. Sci. USA 94: 8076–8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenatri M., Bailly D., Maison C., Almouzni G., 2004. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J. Cell Biol. 166: 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Rivera-Perez J., Chang C., Bradley A., 1991. Target frequency and integration pattern for insertion and replacement vectors in embryonic stem cells. Mol. Cell. Biol. 11: 4509–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Campisi J., Hoeijmakers J., van Steeg H., Vijg J., 2003. Aging and genome maintenance: Lessons from the mouse? Science 299: 1355–1359 [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., 2001. Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374 [DOI] [PubMed] [Google Scholar]
- Holcomb V. B., Kim T. M., Dumitrache L. C., Ma S. M., Chen M. J., et al. , 2007. HPRT minigene generates chimeric transcripts as a by-product of gene targeting. Genesis 45: 275–281 [DOI] [PubMed] [Google Scholar]
- Ira G., Malkova A., Liberi G., Foiani M., Haber J. E., 2003. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115: 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., 2000. Chromosome breaks and genomic instability. Cancer Invest. 18: 78–86 [DOI] [PubMed] [Google Scholar]
- Kim T. M., Choi Y. J., Ko J. H., Hasty P., 2008. High-throughput knock-in coupling gene targeting with the HPRT minigene and Cre-mediated recombination. Genesis 46: 732–737 [DOI] [PubMed] [Google Scholar]
- Kim T. M., Ko J. H., Choi Y. J., Hu L., Hasty P., 2011. The phenotype of FancB-mutant mouse embryonic stem cells. Mutat. Res. 712: 20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberi G., Maffioletti G., Lucca C., Chiolo I., Baryshnikova A., et al. , 2005. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 19: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. R., Ma Y., Pannicke U., Schwarz K., 2004. The mechanism of vertebrate nonhomologous DNA end joining and its role in V(D)J recombination. DNA Repair (Amst.) 3: 817–826 [DOI] [PubMed] [Google Scholar]
- Liu L. F., Desai S. D., Li T. K., Mao Y., Sun M., et al. , 2000. Mechanism of action of camptothecin. Ann. N Y Acad. Sci. 922: 1–10 [DOI] [PubMed] [Google Scholar]
- Lombard D. B., Chua K. F., Mostoslavsky R., Franco S., Gostissa M., et al. , 2005. DNA repair, genome stability, and aging. Cell 120: 497–512 [DOI] [PubMed] [Google Scholar]
- Luo G., Santoro I. M., McDaniel L. D., Nishijima I., Mills M., et al. , 2000. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat. Genet. 26: 424–429 [DOI] [PubMed] [Google Scholar]
- Marple T., Li H., Hasty P., 2004. A genotoxic screen: rapid analysis of cellular dose-response to a wide range of agents that either damage DNA or alter genome maintenance pathways. Mutat. Res. 554: 253–266 [DOI] [PubMed] [Google Scholar]
- Marple T., Kim T. M., Hasty P., 2006. Embryonic stem cells deficient for Brca2 or Blm exhibit divergent genotoxic profiles that support opposing activities during homologous recombination. Mutat. Res. 602: 110–120 [DOI] [PubMed] [Google Scholar]
- Mazur D. J., Perrino F. W., 2001. Excision of 3′ termini by the Trex1 and TREX2 3′→5′ exonucleases. Characterization of the recombinant proteins. J. Biol. Chem. 276: 17022–17029 [DOI] [PubMed] [Google Scholar]
- Mochan T. A., Venere M., DiTullio R. A., Jr, Halazonetis T. D., 2004. 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst.) 3: 945–952 [DOI] [PubMed] [Google Scholar]
- Morimatsu M., Donoho G., Hasty P., 1998. Cells deleted for Brca2 COOH terminus exhibit hypersensitivity to gamma-radiation and premature senescence. Cancer Res. 58: 3441–3447 [PubMed] [Google Scholar]
- Motegi A., Kuntz K., Majeed A., Smith S., Myung K., 2006. Regulation of gross chromosomal rearrangements by ubiquitin and SUMO ligases in Saccharomyces cerevisiae. Mol. Cell. Biol. 26: 1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., et al. , 2003. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5: 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull T. T., Rogakou E. P., Yamazaki V., Kirchgessner C. U., Gellert M., et al. , 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10: 886–895 [DOI] [PubMed] [Google Scholar]
- Petermann E., Helleday T., 2010. Pathways of mammalian replication fork restart. Nat. Rev. Mol. Cell Biol. 11: 683–687 [DOI] [PubMed] [Google Scholar]
- Pierce A. J., Hu P., Han M., Ellis N., Jasin M., 2001. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev. 15: 3237–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch D. R., Sedelnikova O. A., Redon C., Celeste A., Nussenzweig A., et al. , 2003. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem. Cell Biol. 81: 123–129 [DOI] [PubMed] [Google Scholar]
- Raynard S., Bussen W., Sung P., 2006. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIalpha, and BLAP75. J. Biol. Chem. 281: 13861–13864 [DOI] [PubMed] [Google Scholar]
- Roberts S. A., Strande N., Burkhalter M. D., Strom C., Havener J. M., et al. , 2010. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature 464: 1214–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Filippo J., Sung P., Klein H., 2008. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 77: 229–257 [DOI] [PubMed] [Google Scholar]
- Shevelev I. V., Ramadan K., Hubscher U., 2002. The TREX2 3′→5′ exonuclease physically interacts with DNA polymerase delta and increases its accuracy. ScientificWorldJournal 2: 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. M., Linder C. C., Sargent E. E., Davisson M. T., Mobraaten L. E., et al. , 1997. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat. Genet. 16: 19–27 [DOI] [PubMed] [Google Scholar]
- Smith S., Hwang J. Y., Banerjee S., Majeed A., Gupta A., et al. , 2004. Mutator genes for suppression of gross chromosomal rearrangements identified by a genome-wide screening in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 101: 9039–9044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E., Sasaki M. S., Morrison C., Yamaguchi-Iwai Y., Takata M., et al. , 1999. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol. Cell. Biol. 19: 5166–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J. M., Pierce A. J., Oh J., Pastink A., Jasin M., 2004. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell. Biol. 24: 9305–9316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Sasaki M. S., Sonoda E., Fukushima T., Morrison C., et al. , 2000. The Rad51 paralog Rad51B promotes homologous recombinational repair. Mol. Cell. Biol. 20: 6476–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Sasaki M. S., Tachiiri S., Fukushima T., Sonoda E., et al. , 2001. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol. Cell. Biol. 21: 2858–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Deng C., Capecchi M. R., 1992. High-fidelity gene targeting in embryonic stem cells by using sequence replacement vectors. Mol. Cell. Biol. 12: 2919–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threadgill D. W., Yee D., Matin A., Nadeau J. H., Magnuson T., 1997. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm. Genome 8: 390–393 [DOI] [PubMed] [Google Scholar]
- Vijg J., Dolle M. E., 2002. Large genome rearrangements as a primary cause of aging. Mech. Ageing Dev. 123: 907–915 [DOI] [PubMed] [Google Scholar]
- West S. C., 2003. Molecular views of recombination proteins and their control. Nat. Rev. Mol. Cell Biol. 4: 435–445 [DOI] [PubMed] [Google Scholar]
- Wilson D. M., 3rd, Thompson L. H., 2007. Molecular mechanisms of sister-chromatid exchange. Mutat. Res. 616: 11–23 [DOI] [PubMed] [Google Scholar]
- Wu L., Hickson I. D., 2003. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870–874 [DOI] [PubMed] [Google Scholar]
- Xie A., Puget N., Shim I., Odate S., Jarzyna I., et al. , 2004. Control of sister chromatid recombination by histone H2AX. Mol. Cell 16: 1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A., Odate S., Chandramouly G., Scully R., 2010. H2AX post-translational modifications in the ionizing radiation response and homologous recombination. Cell Cycle 9: 3602–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]