Abstract
The development of an organism depends on individual cells receiving and executing their specific fates, although how this process is regulated remains largely unknown. Here, we identify a mechanism by which a specific cell fate, apoptosis, is determined through the cooperative efforts of Hox and E2F proteins. E2F transcription factors are critical, conserved regulators of the cell cycle and apoptosis. However, little is known about the two most recently discovered mammalian E2Fs—E2F7 and E2F8. In the nematode Caenorhabditis elegans, we identify a novel E2F7/8 homolog, EFL-3, and show that EFL-3 functions cooperatively with LIN-39, providing the first example in which these two major developmental pathways—E2F and Hox—are able to directly regulate the same target gene. Our studies demonstrate that LIN-39 and EFL-3 function in a cell type-specific context to regulate transcription of the egl-1 BH3-only cell death gene and to determine cell fate during development.
THE generation of a complex, multicellular organism from a single cell is an immensely complicated process. Cell fate must be specified by factors such as contexts, mechanisms, and regulatory complexes for the proper number and types of cells to be generated. Here, we identified a mechanism governed by Hox and E2F genes in which cell fate is determined in a highly specific manner, according to cellular lineage and spatial positioning.
The genetics of development focus on how cells are directed to adopt specific fates. To this work Caenorhabditis elegans has brought the advantage of a known and essentially invariant lineage, making possible the discovery and analysis of developmental pathways that direct—with single-cell resolution—the precise patterns of cell fates, a feat that cannot be achieved in mammalian systems. The genetic pathway that underlies programmed cell death in all animals was first identified in C. elegans and includes four genes—egl-1, ced-9, ced-4, and ced-3 (Ellis and Horvitz 1986; Hengartner et al. 1992; Conradt and Horvitz 1998; Horvitz 1999).
In many cells, apoptosis is initiated by transcriptional regulation of the BH3 domain-encoding gene egl-1 (Conradt and Horvitz 1998). The EGL-1 protein binds the BCL-2 family member CED-9, releasing the APAF-1 homolog CED-4 to activate the caspase CED-3, killing the cell (Hengartner et al. 1992; Yuan et al. 1993; Chinnaiyan et al. 1997a,b; Seshagiri and Miller 1997; Wu et al. 1997a,b; Zou et al. 1997; Conradt and Horvitz 1998). The central cell death machinery is conserved across species, including Drosophila, mice, and humans (Danial and Korsmeyer 2004). How the apoptotic pathway is controlled in individual cells is not well understood, although it is of great importance to human disease, as the pathway is frequently abnormally regulated in cancer (see review by Chonghaile and Letai 2008). Here, we have characterized how an E2F transcription factor directly regulates the cell death pathway, providing insight into a mechanism of apoptosis regulation that is likely similar across species, given the conservation of the cell death pathway and E2F family functions.
E2Fs have been extensively studied in vitro and in cell culture models as regulators of the cell cycle and apoptosis (see review by Chen et al. 2009). The mammalian E2F family is composed of eight members, termed E2F1–8, with each member categorized according to structure and function—as an activator and/or repressor of transcription. It is thought that E2F7 and E2F8 are repressor E2Fs (De Bruin et al. 2003; Di Stefano et al. 2003; Logan et al. 2005) mostly on the basis of in vitro assays. Structurally, E2F7 and E2F8 are distinct from other family members, specifically lacking domains for binding E2Fs’ dimerization partner (DP) and pocket proteins, including the retinoblastoma (RB) protein (De Bruin et al. 2003; Di Stefano et al. 2003; Logan et al. 2005). E2F7/8 are the most recently identified E2F family members. Thus far, only one murine study has examined the function of E2F7 and E2F8 in vivo. In that study, E2F7 and E2F8 double-knockout mice display widespread TUNEL staining in embryos, suggesting that E2F7 and E2F8 prevent apoptosis (Li et al. 2008), although currently the mechanisms and contexts by which they regulate target genes are unknown.
C. elegans has previously been employed to study how the E2F family functions in vivo. Like mammals, C. elegans possess a DP homolog, dpl-1 (“DP-like”); an RB homolog, lin-35 (Reddien et al. 2007); and E2Fs: efl-1, efl-2 (Ceol and Horvitz 2001; Reddien et al. 2007), and efl-3 (“E2F-like, 3rd family member,” introduced here). In a study by Reddien and colleagues (2007), efl-1 was identified as a promoter of cell death in the anterior pharynx, functioning in the same pathway as dpl-1 and lin-35. In the hermaphrodite germline, lin-35, dpl-1, efl-1, and efl-2 possess context-specific pro-apoptotic functions (Schertel and Conradt 2007). EFL-1 can be considered an activator E2F and shares a proposed structure similar to mammalian E2F4 and E2F5, while EFL-2 is more similar to mammalian E2F3 and E2F6 (Ceol and Horvitz 2001).
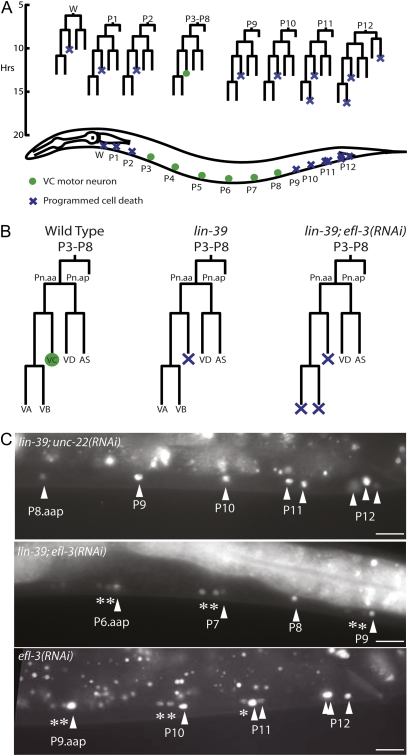
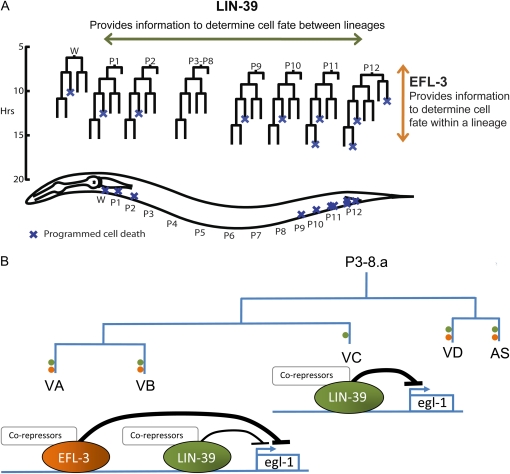
C. elegans has also been used to study the Hox family of transcription factors, particularly in the context of the ventral nerve cord. The ventral nerve cord is a model for studying how cells differentiate and determine cell death based on varying developmental cues. The ventral nerve cord is generated from 12 P cell and 1 W blast cell lineages (Figure 1, A and B), each of which produces up to five types of motor neurons—VA, VB, VC, VD, and AS—and one hypodermal cell (Sulston 1976). All ventral nerve cord lineages are similar in terms of the number of progeny that they create and what types of neurons are generated, but because the lineages are spread across the length of the body, each is under the control of different spatial cues, leading to variations in cell fate based on developmental context. In the midbody, the survival of one neuron type, the VC neuron, allows for the innervation of egg-laying muscles, but in the anterior and posterior lineages, where the VC-lineal equivalents are unnecessary, the neurons undergo apoptosis (Clark et al. 1993). The Hox gene lin-39 provides the spatial information to determine VC neuron survival vs. death; without lin-39 to tell the midbody VC neurons where within the animal they are located, the neurons undertake the fate (death) of their lineal equivalents in the anterior or posterior of the animal (Clark et al. 1993; Potts et al. 2009) (Figure 1).
Figure 1 .
LIN-39 and EFL-3 regulate programmed cell death in the ventral nerve cord. (A) The ventral nerve cord is derived from 12 P cell and 1 W cell lineages across the length of the animal. In the anterior (W, P1–P2) and posterior (P9–P12) lineages, some neurons undergo programmed cell death. (B) Each midbody (P3–P8) lineage is composed of five neuron types, which all survive in wild-type animals. Loss of lin-39 induces one neuron type, the VC, to express the cell death gene egl-1 and die in each midbody lineage, in addition to the seven that typically die in the posterior. In our screen, we identified that lin-39; efl-3(RNAi) animals have two additional neurons per midbody lineage expressing egl-1. (C) On unc-22 control RNAi, lin-39 animals express Pegl-1gfp in cells that undergo programmed cell death. In lin-39; efl-3(RNAi) animals, there is an increase in the number of neurons in the midbody and posterior expressing Pegl-1gfp. In efl-3(RNAi) animals, there is an increase in posterior neurons expressing Pegl-1gfp. Arrowheads indicate neurons expressing Pegl-1gfp in a lin-39 mutant background, and asterisks indicate additional neurons expressing Pegl-1gfp. All animals shown are also homozygous for ced-1; ced-3; Pegl-1gfp. Scale bars, 10 μm.
Materials and Methods
Worm strains and scoring
“Wild type” refers to the Bristol N2 strain. All worms were maintained under standard conditions (Brenner 1974) at 20°, unless otherwise noted. The RNA interference (RNAi) screen background was ced-1(e1735) I; lin-39(n709ts) III; ced-3(n717) IV; mxIs14 X. mxIs14 is an integrated Pegl-1histone:gfp transgene (Liu et al. 2006). The strains used to identify VA, VB, and VC neurons included wdIs4[Punc-4gfp] II (Pflugrad et al. 1997), wdIs6[Pdel-1gfp] II (Winnier et al. 1999), nIs106[Plin-11gfp] X (Reddien et al. 2001), lin-39(n709ts), and ced-3(n717). wdIs4 and wdIs6 were generously provided by David Miller. Strains used to study how cell death is affected by E2Fs include efl-1(se1) V that was raised at 26°, dpl-1(n2994) II, and efl-3(gk835)/mIn1[mIs14dpy-10(e128)] II. From strains used for scoring—including Pegl-1gfp, Punc-4gfp, Pdel-1gfp, and Plin-11gfp reporter strains—between 17 and 31 individuals were scored for each experiment. Raw P-values from the scoring results were used to calculate significance in Figure 2 and Figure 4.
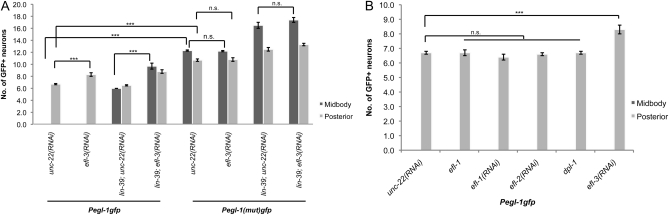
Figure 2 .
egl-1 is repressed by efl-3 and lin-39 in a context-specific manner in the ventral nerve cord. (A) A repressive E2F, likely EFL-3, directly binds Pegl-1. (B) Loss of efl-3 induces an increase in the number of neurons expressing egl-1. All strains are homozygous for ced-3. Standard errors are shown. ***P < 0.0001; “n.s.” indicates P > 0.05.
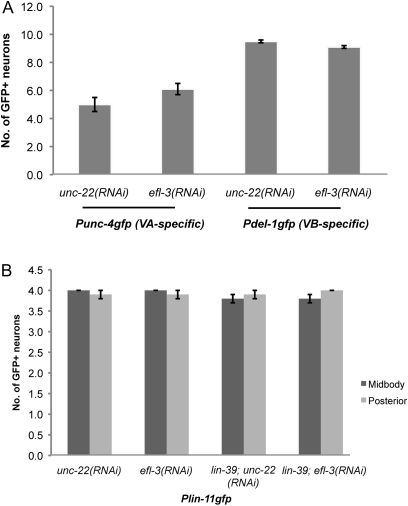
Figure 4 .
efl-3(RNAi) does not alter the identity of the VA, VB, and VC neurons. (A) Differentiation of the VA and VB neurons is unaffected with efl-3(RNAi). (B) Differentiation of the VC neurons is unaffected with efl-3(RNAi). The Plin-11gfp strain is homozygous for ced-3. Standard errors are shown.
RNAi analyses and imaging
dsRNA-expressing bacteria from the Ahringer RNAi library were fed to worms according to the procedure described by Kamath et al. (2001). L4 animals were fed on RNAi bacteria and their L2 progeny were scored 2–3 days later. For RNAi clones used after the initial screen, the validity of gene targets was confirmed by sequencing. dpl-1 RNAi was constructed by PCR, amplifying a 1.1-kb segment of dpl-1 ORF from N2 genomic DNA. The dpl-1 gene fragment was then dropped into the L4440 RNAi backbone, using the following primers to introduce NcoI and PstI sites for cloning: 5′-TAGCCATGGACAAACTACGATCCCCGTATC-3′ and 5′-CTACTGCAGCTTACTGGCAATGATTTCGTC-3′. For RNAi screening and for all images, we used a Zeiss Axiophot microscope.
Conservation across species
To identify putative E2F-binding sites in Pegl-1, the 7.67-kb BamHI-StuI promoter of egl-1 (Liu et al. 2006) was first searched using MatInspector (http://www.genomatix.de) for general core promoter binding sites, with a matrix similarity of 5, indicating similarity across many species. All sequences identified as possible E2F- or E2F/DP-binding sites were then examined for conservation with Caenorhabditis briggsae and Caenorhabditis remanei using Family Relations II and Cartwheel (Caltech; http://cartwheel.caltech.edu/).
To search for domains conserved between human and C. elegans E2Fs, human E2F proteins were examined in Uniprot (http://www.uniprot.org) for their respective domains. Each E2F domain was aligned with C. elegans E2F protein sequences (Wormbase http://www.wormbase.org) using MacVector Pustell Protein Matrix software.
Reporter constructs
Pegl-1gfp is Pegl-1histone:gfp (Liu et al. 2006). To create Pegl-1(mut)gfp, the Pegl-1gfp construct was first split into two parts. A 3.5-kb PstI fragment was dropped out, creating pJW001, and the 3.5-kb PstI fragment was cloned into pBluescript II KS, creating pMP024. Mutations were made using Phusion (Finnzymes) site-directed mutagenesis. In pJW001, two mutations were made by changing 5′-TTTCCCGCATGAA-3′ to 5′-TTTAGGCCTTGAA-3′ and 5′-AGTTCCCGCGTTT-3′ to 5′-AGTACTAGTGTTT-3′. In pMP024, three mutations were made, changing 5′-TTTCGCGCATT-3′ to 5′-TTTCGATCATT-3′, 5′-TTTCGCGCATTTC-3′ to 5′-TTTAGGCCTTTTC-3′, and 5′-ATTGCGCGAGACC-3′ to 5′-ATTACTAGTGACC-3′. Mutations were confirmed by sequencing. The pMP024 PstI fragment was then excised and recombined with pJW001 to create the final Pegl-1(mut)gfp construct. Pegl-1(mut)gfp (5 ng/μL) was injected into ced-1(e1735) I; ced-3(n717) IV; lin-15(n765) X with the lin-15 rescuing construct pL15EK (50 ng/μl). Non-Muv transgenic progeny were maintained. Three stable transgenic lines gave similar gfp expression. Mutations of single sites induced no change in egl-1 expression, indicating that a combination of multiple binding sites is required for E2F regulation of egl-1. One line was crossed with lin-39(n1760) III to give lin-39; Pegl-1(mut)gfp.
Pefl-3mCherry was created by first amplifying a 3.2-kb fragment upstream of efl-3’s ATG start. To this fragment, SphI and XmaI sites were added by PCR using the following primers: 5′-TAGTAGGCATGCCCAGCAGTGTGACTGTACATGTTC-3′ and 5′-CTACTACCCGGGATTTGTTGAGCTCAATTACCAGATG-3′. Pefl-3 was cloned into a pPD95.70 construct in which the gfp coding sequence was replaced with mCherry. The final Pefl-3mCherry construct was confirmed by sequencing. Pefl-3mCherry (50 ng/μl) was injected with a Pmyo-2gfp (30 ng/μl) co-injection marker into N2 worms. Three stable transgenic lines gave similar mCherry expression.
Results
EFL-3 is identified in a screen for repressors of cell death redundant with LIN-39
Although the Hox transcription factor LIN-39 is expressed in all five neuron types generated by each midbody P3-8 lineage, only the VC neurons express egl-1 and subsequently undergo apoptosis in a lin-39 mutant (Maloof and Kenyon 1998) (Figure 1). With loss of LIN-39, what causes the VC neurons to die and the other four neuron types to remain alive? One possibility is that there is a repressor of egl-1 that functions redundantly with lin-39 in the VA, VB, VD, and/or AS neurons.
Using an RNAi screen of 387 transcription factors and 263 chromatin-remodeling factors (Kamath and Ahringer 2003), we sought repressors of egl-1 that are redundant with Hox function in the non-VC motor neurons. egl-1 expression was determined by a Pegl-1gfp reporter in a cell death-defective (ced-3) background so cells that initiate the cell death cascade would express Pegl-1gfp, but the execution of death would be blocked by a downstream mutation in the caspase ced-3, allowing the cells to remain alive for scoring by fluorescence microscopy. In the screening background of lin-39; ced-3; Pegl-1gfp, we discovered that the gene F49E12.6, which we refer to as efl-3, represses egl-1 expression in a subset of ventral nerve cord neurons in a manner that is at least partially redundant with lin-39 (Figure 1).
EFL-3 and LIN-39 repress egl-1 in the ventral nerve cord in a partially redundant manner
Additional neurons expressed egl-1 on efl-3(RNAi) in a lin-39 background (Figure 2A). In the midbody, loss of LIN-39 was sufficient to derepress egl-1 in the VC neurons. Additional loss of EFL-3 led to derepression of egl-1 in the VA and VB neurons. Loss of EFL-3 alone was not sufficient to induce any change in egl-1 expression in the midbody. In the posterior, where LIN-39 is not expressed, loss of EFL-3 alone was sufficient to result in ectopic egl-1 expression. These findings suggest that, in the midbody, EFL-3 and LIN-39 act partially redundantly to repress egl-1 transcription in the VA and VB motor neurons.
EFL-3 is the C. elegans homolog of mammalian E2F7 and E2F8
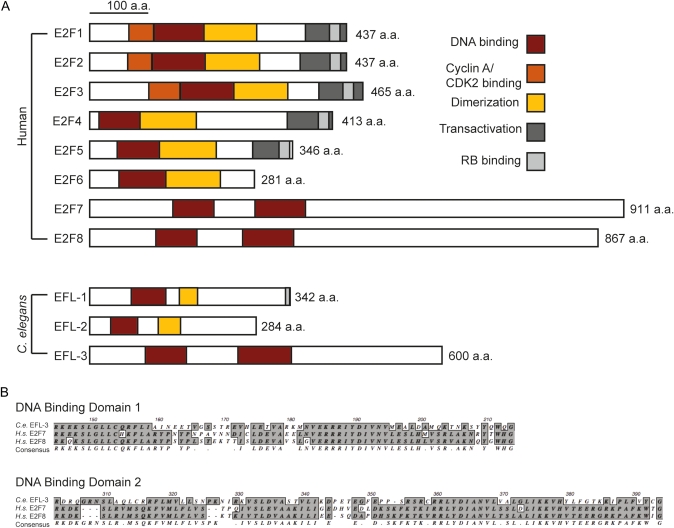
The predicted protein structure of EFL-3 includes two DNA-binding domains characteristic of the E2F family of transcription factors (Figure 3). Like E2F7 and E2F8, EFL-3 lacks a transactivation domain and binding domains for RB and cyclin A. EFL-3 is the only predicted protein in the completely sequenced C. elegans genome that shares all these characteristics with E2F7 and E2F8.
Figure 3 .
EFL-3 is a homolog of mammalian E2F7 and E2F8. (A) The mammalian and C. elegans E2F families of transcription factors. (B) Alignment of the DNA-binding domains of C. elegans EFL-3 and Homo sapiens E2F7 and E2F8.
EFL-3 induces cell death without altering VA and VB neuron differentiation
To examine whether a differentiation defect might explain the additional egl-1-expressing neurons that we observed in the screen, we examined the expression of cell type-specific gfp markers after treatment with efl-3(RNAi) (Figure 4). We found that the VA, VB, and VC cell-type differentiations were apparently unaltered with efl-3(RNAi), supporting the hypothesis that efl-3 represses egl-1 expression without altering the identity of the VA, VB, and VC neurons.
EFL-3 directly represses egl-1 in the VA and VB motor neurons
In the sole murine E2F7/8 study (Li et al. 2008), widespread apoptosis was observed in embryos with loss of E2F7/8, but it is unclear whether the apoptosis occurred indiscriminately or in a cell type-specific manner. Furthermore, whether E2F7/8 regulation of cell death is direct (E2Fs binding to the promoters of cell death genes to repress transcription) or indirect (possibly as the result of cell cycle deregulation) is unclear. In C. elegans, we sought to investigate these two questions. Our hypothesis was that EFL-3 may function directly at the site of a cell death gene, egl-1, to repress its transcription in the VA and VB neurons.
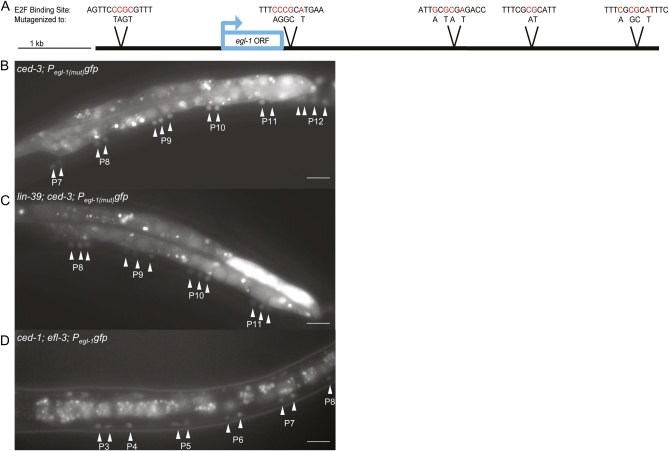
To determine whether EFL-3 is able to directly bind Pegl-1 in vivo, we mutagenized two to five nucleotides at each of the five consensus E2F-binding sites across the 7.6-kb egl-1 promoter to give Pegl-1(mut)gfp. In ced-3; Pegl-1(mut)gfp transgenic animals, we observed an increase in the number of ventral nerve cord neurons expressing egl-1 (Figure 5) when compared with ced-3; Pegl-1gfp animals (Figure 1 and Figure 2A). Thus, an E2F likely binds egl-1 to directly regulate its transcription.
Figure 5 .
A repressive E2F binds Pegl-1. (A) Five E2F consensus sites were identified in Pegl-1. At each site, the nucleotides shown in red were mutated to abolish E2F binding. All five mutated sites were combined into one construct to give Pegl-1(mut). (B) Loss of E2F-binding sites in Pegl-1 results in ectopic egl-1 expression in midbody P3–P8 and posterior P9–P12 lineages. Mutations in Pegl-1gfp induce a doublet pattern of neurons in the midbody and an increase of neurons in the posterior expressing egl-1. (C) In lin-39; Pegl-1(mut)gfp animals, GFP is expressed in a triplet pattern in the midbody and most posterior lineages. (D) efl-3(null); Pegl-1gfp animals exhibit ectopic egl-1 expression. Scale bars, 10 μm. Animals in B and C are homozygous for ced-3, and the animal in D is homozygous for ced-1.
It is thought that all E2Fs are capable of binding a similar E2F consensus sequence (Zheng et al. 1999; De Bruin et al. 2003; Di Stefano et al. 2003; Logan et al. 2005), so it is possible that efl-1 or efl-2 is capable of repressing egl-1. However, loss of efl-1 and/or efl-2 or their putative obligate dimerization partner dpl-1 did not affect cell death in the ventral nerve cord, although efl-3 did (Figure 2B). Furthermore, ced-3; Pegl-1(mut)gfp animals expressed GFP in two neurons per midbody lineage (Figure 5), similarly to the two additional neurons per midbody lineage that expressed egl-1 in lin-39; ced-3; Pegl-1gfp; efl-3(RNAi) animals, as compared to control RNAi (Figure 1). In addition, although efl-3(RNAi) induced additional neurons to express egl-1 in ced-3; Pegl-1gfp animals, efl-3(RNAi) induced no change in expression in ced-3; Pegl-1(mut)gfp animals, suggesting that efl-3 repression had been lost by removing Pegl-1’s E2F-binding sites (Figure 2A).
EFL-3 is a stronger repressor of egl-1 than LIN-39 in the midbody VA and VB neurons
In the midbody of Pegl-1gfp transgenic animals, no neurons express egl-1, but in the midbody of Pegl-1(mut)gfp animals, there are two neurons per lineage that expressed egl-1 (Figure 5). We knew these to be VA and VB neurons on basis of differential interference contrast analysis. Furthermore, in lin-39; ced-3; Pegl-1(mut)gfp animals, we observed three cells per midbody lineage expressing egl-1—the VA, VB, and VC neurons (Figure 5). The pattern of egl-1 expression in lin-39; ced-3; Pegl-1(mut)gfp animals was similar to the pattern observed in lin-39; ced-3; Pegl-1gfp; efl-3(RNAi) animals, but more consistent (see ranges of gfp-expressing neuons in supporting information, Table S1). One likely reason for the inconsistency of egl-1 expression in lin-39; ced-3; Pegl-1gfp; efl-3(RNAi) animals is that efl-3(RNAi) provides incomplete knockdown of efl-3, as supported by the observation that efl-3(RNAi) worms are viable, but deletion of alleles efl-3—gk835 and gk896—are larval lethal.
ced-3; Pegl-1(mut)gfp animals (compared with ced-3; Pegl-1gfp animals) exhibited an increase of midbody neurons expressing egl-1 independently of lin-39. Previously, we had shown that lin-39 loss was required for efl-3(RNAi) to have an effect on egl-1 expression in the midbody (Figure 2A). However, with E2F-binding sites removed, we created an essentially null condition in terms of EFL-3 binding Pegl-1gfp, indicating that, in the midbody VA and VB neurons, LIN-39 functions as a weak repressor and EFL-3 functions as a strong repressor of egl-1. That is, complete loss of LIN-39 (via a null allele) and partial loss of EFL-3 (via RNAi) is sufficient to derepress egl-1, while complete loss of EFL-3 alone (via Pegl-1 mutagenesis) is sufficient to derepress egl-1 (Figure 2A).
One possible mechanism to explain the partial redundancy of LIN-39 and EFL-3 in the midbody VA and VB neurons is that, by abolishing E2F sites, we inadvertently disrupted one or more Hox sites as well, leading to derepression of egl-1. However, this hypothesis seems unlikely, as a previous study has shown that, of the 116 putative Pegl-1 Hox/Pbx-binding sites, LIN-39 is predicted to repress egl-1 at multiple sites or indirectly by binding one or more Pegl-1-bound transcription factors (Potts et al. 2009).
A second hypothesis is that LIN-39 represses egl-1 by forming a complex with EFL-3 bound to Pegl-1. Partial knockdown of EFL-3 (via RNAi) would lead to variable expression of egl-1 due to a minimal amount of EFL-3 being present to allow for inconsistent LIN-39/EFL-3 repression of egl-1, and elimination of E2F-binding sites would lead to a more predictable pattern of egl-1 expression, which is what we observed (Figure 2 and Table S1).
To directly test whether complete loss of efl-3 function is sufficient to cause ectopic expression of egl-1, we attempted to look at efl-3(gk835); ced-3; Pegl-1gfp animals. This experiment failed because the majority of these worms die as larvae before completing cell deaths in the ventral nerve cord. We found, however, that a rare few ced-1; efl-3; Pegl-1gfp animals are able to survive long enough to display a GFP pattern in the ventral nerve cord matching that of ced-3; Pegl-1(mut)gfp animals (Figure 5). (ced-1 is necessary for the normal engulfment of cell corpses.) In ced-1; efl-3; Pegl-1gfp animals, similar to ced-3; Pegl-1(mut)gfp animals, two neurons per midbody lineage expressed egl-1, supporting the hypothesis that efl-3 is a repressor of egl-1 in the VA and VB neurons.
EFL-3 is expressed in the VA and VB motor neurons
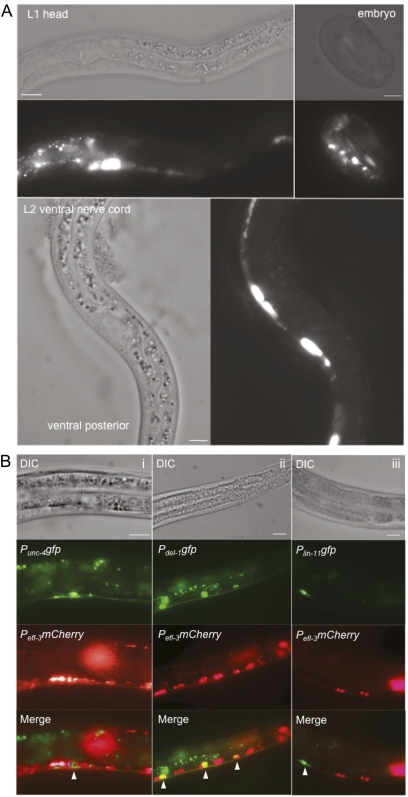
EFL-3 is required in the VA and VB neurons to repress egl-1 in a manner partially redundant with LIN-39, but loss of LIN-39 alone is sufficient to derepress egl-1 in the VC neurons. Why is EFL-3 not required in the VC neurons to repress egl-1? One possibility is that EFL-3 is not present in the VC neurons. To determine the pattern of efl-3 expression, we created a Pefl-3mCherry reporter. Pefl-3mCherry transgenic worms displayed expression beginning in multiple cells in embryos (Figure 6A). The expression continued throughout the life span of the worm, with maximal expression appearing in the L1–L2 larval stages. In addition, EFL-3 expression was observed consistently in head neurons from L1 to adult stage (Figure 6A).
Figure 6 .
efl-3 is expressed in a subset of cells during development. (A) Pefl-3mCherry is expressed throughout development in embryos, L1 animals (with highest expression in the head), and L2 animals (with highest expression in the head and ventral nerve cord). Three independent transgenic lines exhibit the same expression pattern. (B) Pefl-3mCherry is expressed in the (i) VA neurons and (ii) VB neurons. (iii) EFL-3 is not expressed in the VC neurons. Images are false-colored epifluorescence of L2–L3 animals. Scale bars, 10 μm.
In the ventral nerve cord, EFL-3 was most clearly expressed during the L2 stage. By crossing Pefl-3mCherry transgenic animals with integrated lines of VA (Punc-4)-, VB (Pdel-1)-, and VC (Plin-11)-specific GFP markers, we determined that EFL-3 is expressed in the VA and VB neurons but not in the VC neurons (Figure 6B). This finding provides insight as to why EFL-3 represses egl-1 in the VA and VB but not in the VC neurons.
Discussion
Here we have examined how a newly identified E2F, efl-3, is able to regulate transcription of the egl-1 cell death gene. We found that efl-3 functions in the ventral nerve cord VA and VB cells to repress egl-1 in a manner partially redundant with a Hox, lin-39, but that efl-3 function is not required to repress egl-1 in a third cell type, the VC neuron (Figure 7). In the posterior, where lin-39 is not expressed, efl-3 alone is sufficient to repress egl-1. Across the ventral nerve cord, lin-39 provides information to developing lineages that tells them where they are located, and within each lineage, efl-3 provides information to the developing VA and VB neurons that tells them how their fate should be different from that of the VC neurons. Thus, spatial information from lin-39 Hox is integrated with lineage-specific information from efl-3 at the site of the egl-1 cell death gene to determine cell fate (Figure 7).
Figure 7 .
EFL-3 and LIN-39 interact to specify ventral nerve cord development. (A) LIN-39 provides spatial-specific information to developing lineages. EFL-3 provides information within each lineage to specify cell fate between the different cell types. (B) In the midbody P3–P8 lineages, EFL-3 and LIN-39 integrate their lineage and spatial-specific cues on the egl-1 promoter. EFL-3 and LIN-39 co-repress egl-1 in the VA and VB neurons, with LIN-39 functioning as a weaker repressor. In the VC neurons, LIN-39 is necessary to repress egl-1. EFL-3 is not expressed in the VC neurons and has no apparent affect on egl-1 expression in the VCs. Green dots represent neurons that express lin-39; orange dots represent neurons that express efl-3.
Our findings demonstrate a context in which two major developmental pathways—Hox and E2F—cooperatively regulate a target gene, egl-1. This interaction is dependent on context, with the transcription of egl-1 being determined in a spatial- and cell type-specific manner. Although our studies examined how Hox and E2F interact within the limited setting of the C. elegans ventral nerve cord, it is possible that the mechanism of cell fate regulation by Hox and E2F is relevant across species. Hox proteins have previously demonstrated broad functional conservation, with Drosophila Hox proteins able to function in C. elegans (Hunter and Kenyon 1995) and vertebrate Hox proteins able to function in Drosophila (Malicki et al. 1990; McGinnis et al. 1990; Zhao et al. 1993; Lutz et al. 1996). In the E2F family, mechanistic similarities can also be drawn between species, particularly between mouse, Drosophila, and C. elegans, although precise functional comparisons are more difficult given the variety and redundancy of E2F family proteins and their extensive range of functions (Van Den Heuvel and Dyson 2008). For future studies, it would be interesting to determine how the E2F and Hox families may interact in different species and whether the cooperative action of the two families is limited to cell fate regulation, or if their interaction affects other developmental processes as well.
Acknowledgments
We thank the Caenorhabditis Genetics Center at the University of Minnesota for providing some of the C. elegans strains and the National Institutes of Health (grant #HL46154) for funding.
Literature Cited
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceol C. J., Horvitz H. R., 2001. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol. Cell 7: 461–473 [DOI] [PubMed] [Google Scholar]
- Chen H. Z., Tsai S. Y., Leone G., 2009. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat. Rev. Cancer 9: 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan A. M., Chaudhary D., O’Rourke K., Koonin E. V., Dixit V. M., 1997a Role of CED-4 in the activation of CED-3. Nature 388: 728–729 [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A. M., O’Rourke K., Lane B. R., Dixit V. M., 1997b Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science 275: 1122–1126 [DOI] [PubMed] [Google Scholar]
- Chonghaile T. N., Letai A., 2008. Mimicking the BH3 domain to kill cancer cells. Oncogene 27(Suppl 1): S149–S157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. G., Chisholm A. D., Horvitz H. R., 1993. Control of cell fates in the central body region of C. elegans by the homeobox gene lin-39. Cell 74: 43–55 [DOI] [PubMed] [Google Scholar]
- Conradt B., Horvitz H. R., 1998. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93: 519–529 [DOI] [PubMed] [Google Scholar]
- Danial N. N., Korsmeyer S. J., 2004. Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- de Bruin A., Maiti B., Jakoi L., Timmers C., Buerki R., et al. , 2003. Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J. Biol. Chem. 278: 42041–42049 [DOI] [PubMed] [Google Scholar]
- Di Stefano L., Jensen M. R., Helin K., 2003. E2F7, a novel E2F featuring DP-independent repression of a subset of E2F-regulated genes. EMBO J. 22: 6289–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R., 1986. Genetic control of programmed cell death in the nematode C. elegans. Cell 44: 817–829 [DOI] [PubMed] [Google Scholar]
- Hengartner M. O., Ellis R. E., Horvitz H. R., 1992. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356: 494–499 [DOI] [PubMed] [Google Scholar]
- Horvitz H. R., 1999. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 59: 1701s–1706s [PubMed] [Google Scholar]
- Hunter C. P., Kenyon C., 1995. Specification of anteroposterior cell fates in Caenorhabditis elegans by Drosophila Hox proteins. Nature 377: 229–232 [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Ahringer J., 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30: 313–321 [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Martinez-Campos M., Zipperlen P., Fraser A. G., Ahringer J., 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2: RESEARCH0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ran C., Li E., Gordon F., Comstock G., et al. , 2008. Synergistic function of E2F7 and E2F8 is essential for cell survival and embryonic development. Dev. Cell 14: 62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Strauss T. J., Potts M. B., Cameron S., 2006. Direct regulation of egl-1 and of programmed cell death by the Hox protein MAB-5 and by CEH-20, a C. elegans homolog of Pbx1. Development 133: 641–650 [DOI] [PubMed] [Google Scholar]
- Logan N., Graham A., Zhao X., Fisher R., Maiti B., et al. , 2005. E2F–8: an E2F family member with a similar organization of DNA-binding domains to E2F–7. Oncogene 24: 5000–5004 [DOI] [PubMed] [Google Scholar]
- Lutz B., Lu H. C., Eichele G., Miller D., Kaufman T. C., 1996. Rescue of Drosophila labial null mutant by the chicken ortholog Hoxb-1 demonstrates that the function of Hox genes is phylogenetically conserved. Genes Dev. 10: 176–184 [DOI] [PubMed] [Google Scholar]
- Malicki J., Schughart K., McGinnis W., 1990. Mouse Hox-2.2 specifies thoracic segmental identity in Drosophila embryos and larvae. Cell 63: 961–967 [DOI] [PubMed] [Google Scholar]
- Maloof J. N., Kenyon C., 1998. The Hox gene lin-39 is required during C. elegans vulval induction to select the outcome of Ras signaling. Development 125: 181–190 [DOI] [PubMed] [Google Scholar]
- McGinnis N., Kuziora M. A., McGinnis W., 1990. Human Hox-4.2 and Drosophila deformed encode similar regulatory specificities in Drosophila embryos and larvae. Cell 63: 969–976 [DOI] [PubMed] [Google Scholar]
- Pflugrad A., Meir J. Y., Barnes T. M., Miller D. M., 3rd, 1997. The Groucho-like transcription factor UNC-37 functions with the neural specificity gene unc-4 to govern motor neuron identity in C. elegans. Development 124: 1699–1709 [DOI] [PubMed] [Google Scholar]
- Potts M. B., Wang D. P., Cameron S., 2009. Trithorax, Hox, and TALE-class homeodomain proteins ensure cell survival through repression of the BH3-only gene egl-1. Dev. Biol. 329: 374–385 [DOI] [PubMed] [Google Scholar]
- Reddien P. W., Andersen E. C., Huang M. C., Horvitz H. R., 2007. DPL-1 DP, LIN-35 Rb and EFL-1 E2F act with the MCD-1 zinc-finger protein to promote programmed cell death in Caenorhabditis elegans. Genetics 175: 1719–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P. W., Cameron S., Horvitz H. R., 2001. Phagocytosis promotes programmed cell death in C. elegans. Nature 412: 198–202 [DOI] [PubMed] [Google Scholar]
- Schertel C., Conradt B., 2007. C. elegans orthologs of components of the RB tumor suppressor complex have distinct pro-apoptotic functions. Development 134: 3691–3701 [DOI] [PubMed] [Google Scholar]
- Seshagiri S., Miller L. K., 1997. Caenorhabditis elegans CED-4 stimulates CED-3 processing and CED-3-induced apoptosis. Curr. Biol. 7: 455–460 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., 1976. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275: 287–297 [DOI] [PubMed] [Google Scholar]
- van den Heuvel S., Dyson N. J., 2008. Conserved functions of the pRB and E2F families. Nat. Rev. Mol. Cell Biol. 9: 713–724 [DOI] [PubMed] [Google Scholar]
- Winnier A. R., Meir J. Y., Ross J. M., Tavernarakis N., Driscoll M., et al. , 1999. UNC-4/UNC-37-dependent repression of motor neuron-specific genes controls synaptic choice in Caenorhabditis elegans. Genes Dev. 13: 2774–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wallen H. D., Inohara N., Nunez G., 1997a Interaction and regulation of the Caenorhabditis elegans death protease CED-3 by CED-4 and CED-9. J. Biol. Chem. 272: 21449–21454 [DOI] [PubMed] [Google Scholar]
- Wu D., Wallen H. D., Nunez G., 1997b Interaction and regulation of subcellular localization of CED-4 by CED-9. Science 275: 1126–1129 [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R., 1993. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75: 641–652 [DOI] [PubMed] [Google Scholar]
- Zhao J. J., Lazzarini R. A., Pick L., 1993. The mouse Hox-1.3 gene is functionally equivalent to the Drosophila Sex combs reduced gene. Genes Dev. 7: 343–354 [DOI] [PubMed] [Google Scholar]
- Zheng N., Fraenkel E., Pabo C. O., Pavletich N. P., 1999. Structural basis of DNA recognition by the heterodimeric cell cycle transcription factor E2F-DP. Genes Dev. 13: 666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Henzel W. J., Liu X., Lutschg A., Wang X., 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90: 405–413 [DOI] [PubMed] [Google Scholar]