Abstract
Quality protein maize combines a high-lysine trait with kernel hardness, for which a new, simpler genetic selection was designed.
Keywords: dominance, maize, RNA interference (RNAi), GFP, high-lysine
CORN seeds are used as food for humans and as feed for livestock. However, essential amino acids like lysine and tryptophan are deficient because of the abundance of major storage proteins, called zeins. As a consequence, in countries where corn is consumed as the primary or sole protein source, malnutrition is common (Osborne and Mendel 1914). In those countries, demand for high-lysine corn is on the rise. Actually, high-lysine corn has been reported in which the transcriptional activator of the 22-kDa α-zein gene Opaque 2 (O2) is mutated (Schmidt et al. 1992) and non-zein proteins are proportionally increased, resulting in twice the lysine content than in normal maize (Mertz et al. 1964, 1965). However, this o2 mutant has several drawbacks, such as reduced grain yield, soft chalky endosperm, greater susceptibility to pests and diseases, and higher moisture content at harvest time, which precluded its direct commercialization. Fortunately, there are quantitative trait loci, referred to as o2 modifiers (Mo2s), that convert soft into hard endosperm without loosing the high lysine trait; this combination is called quality protein maize (QPM) (Vasal et al. 1980). Interestingly, transcript and protein of 27-kDa γ-zein accumulate two- to threefold higher in QPM than in normal inbreds and in unmodified o2 mutants (Geetha et al. 1991; Holding et al. 2008), and silencing 27- and 16-kDa γ-zein genes resulted in clumping of protein bodies and opacity of QPM seeds, demonstrating hypostasis of γ-zeins over o2 modifiers (Wu et al. 2010).
QPM has been introduced to many developing countries in South America, Africa, and Asia (Prasanna et al. 2001). However, conversion of QPM into local germplasm is a lengthy process that discourages the spread of the benefits of QPM because breeders have to monitor a high-lysine level, the recessive o2 mutant allele, and the Mo2s. Here, we present a simpler and accelerated QPM selection. Instead of using the recessive o2 mutation, we use an RNA interference (RNAi) construct directed against both 22- and 19-kDa zeins, but linked to the visible green fluorescent protein (GFP) marker gene. Indeed, when such a green and nonvitreous phenotype was crossed with QPM lines, the Mo2s produced a vitreous green kernel, demonstrating that high lysine and kernel hardness can be selected in a dominant fashion.
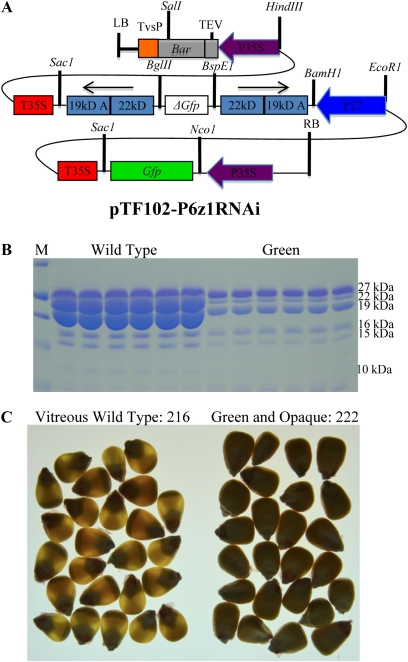
Visible Marker for α-Zein Reduction
The transgenic high-lysine corn was reported to be generated by endosperm-specific silencing of lysine catabolism (Houmard et al. 2007) or accumulation of α-zeins with RNAi (Segal et al. 2003; Huang et al. 2006), with the latter showing a similar phenotype to o2 mutant. We therefore chose inverted repeats of members of each α-zein class to assemble a double RNAi construct. However, if Mo2s restore vitreousness of the kernel, we could not use the opaque phenotype of the RNAi construct for selection. Therefore, we placed a chimeric GFP gene under the control of the cauliflower mosaic virus 35S promoter, immediately upstream of the double RNAi cassette, resulting in pTF102-P6z1RNAi (abbreviated as P6z1RNAi) (Figure 1A). The hybrid of B73 × Mo17 was pollinated with T0 transgenic pollen to produce T1 seeds for genetic analysis. Six normal and “green” immature kernels were sorted and zein proteins were extracted. Indeed, the 22- and 19-kDa α-zeins were dramatically reduced in the six green kernels compared to the six normal kernels (Figure 1B). At maturity, the vitreous and nongreen kernels were 216, while the opaque and green kernels were 222, exhibiting a ratio of 1:1 segregation (Figure 1C).
Figure 1.
Silencing of the 22- and 19-kDa α-zeins linked with the GFP marker. (A) pTF102-P6z1RNAi construct structure. The Zp22/6 gene was used for the 22-kDa and z1A2 gene for the 19-kDa inverted repeat (Song and Messing 2002, 2003). The binary vector pTF102 was used for all intermediate construct building and Agrobacterium-mediated transformation (Frame et al. 2002). (B) SDS-PAGE analysis of zein accumulation in progeny from (B73 × Mo17) × P6z1RNAi/+. Six normal and “green” kernels at 18 days after pollination (DAP) were selected with a fluorescence dissection microscope (Leica MZ16 F) for zein extraction. Total zein loaded in each lane was equal to 500 mg of fresh endosperm at 18 DAP. Protein markers (M) from top to bottom are 37, 25, 20, 15, and 10 kDa. (C) Segregation of vitreous and opaque kernels scored with a light box.
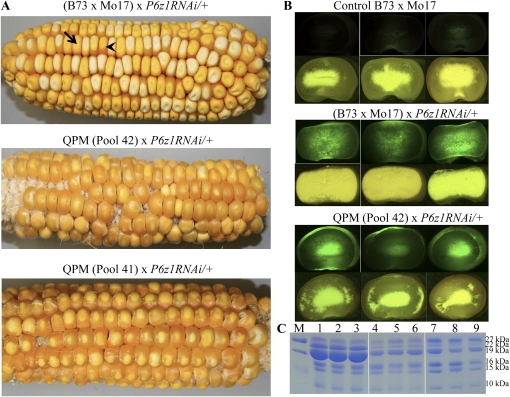
Dominance of Mo2s
A hybrid of B73 × Mo17 with P6z1RNAi was used to pollinate the QPM lines pool 41 and 42. While half of the kernels from (B73 × Mo17) × P6z1RNAi/+ were opaque, most progeny from pool 41 × P6z1RNAi/+ and pool 42 × P6z1RNAi/+ had a vitreous phenotype (Figure 2A) because the Mo2s overcame the action of the RNAi, which was monitored with GFP (Figure 2B). Control kernels of B73 × Mo17 were vitreous and nonfluorescent. As expected, the green and vitreous seeds from pool 42 × P6z1RNAi/+ with reduced accumulation of α-zeins at maturity (Figure 2C) contained an increased lysine level of >25% over a control (Table 1), exceeding the 18.5% achieved previously with RNAi against the 22-kDa zeins only (Segal et al. 2003).
Figure 2.
Modification of RNAi mutant with Mo2s. (A) Ear phenotypes. The opaque and vitreous kernels from the cross of (B73 × Mo17) × P6z1RNAi/+ (Top) showed a ratio of 1:1 segregation. Two representative kernels with opaque and vitreous phenotypes are indicated by arrow and arrowhead, respectively; Most progeny from pool 42 × P6z1RNAi/+ (Middle) and pool 41 × P6z1RNAi/+ (Bottom) showed the vitreous phenotype. (B) Kernels were truncated for observation under a fluorescence dissection microscope (Top) and natural light (Bottom) in each panel. (Top) B73 × Mo17 control. (Middle) Green and opaque kernels from (B73 × Mo17) × P6z1RNAi/+. (Bottom) Green and vitreous kernels from pool 42 × P6z1RNAi/+. (C) α-Zein accumulation pattern in mature seeds. Lanes 1–3, B73 × Mo17; lanes 4–6, green and opaque kernels from (B73 × Mo17) × P6z1RNAi/+; lanes 7–9, green and vitreous kernels from pool 42 × P6z1RNAi/+. Protein markers (M) from top to bottom are 25, 20, and 15 kDa.
TABLE 1.
Amino acid composition analysis in ground cornmeal
| Amino acida | B73 × Mo17 (%) | Pool 42 × P6z1RNAi/+ (%) |
| Lysine | 0.30 | 0.38 |
| Phenylalanine | 0.73 | 0.48 |
| Leucine | 2.01 | 0.71 |
| Isoleucine | 0.41 | 0.27 |
| Threonine | 0.36 | 0.32 |
| Valine | 0.44 | 0.40 |
| Histidine | 0.35 | 0.29 |
| Arginine | 0.55 | 0.49 |
| Glycine | 0.43 | 0.40 |
| Aspartic Acid | 0.63 | 0.79 |
| Serine | 0.56 | 0.37 |
| Glutamic Acid | 2.68 | 1.56 |
| Proline | 1.61 | 0.85 |
| Hydroxyproline | 0.04 | 0.03 |
| Alanine | 0.88 | 0.55 |
| Tyrosine | 0.59 | 0.31 |
| Total ground kernels (g) | 25.28 | 25.77 |
Samples were ground cornmeal of bulked mature kernels from B73 × Mo17 and mature green and vitreous kernels from pool 42 × P6z1RNAi/+.
Amino acid levels were expressed as the percentage of total cornmeal.
QPM Conversion
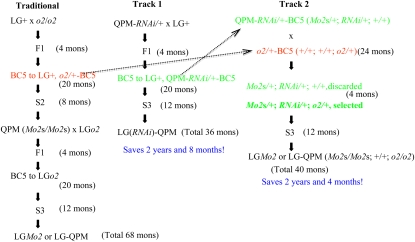
There are two main steps in conversion of QPM into local germplasm wild type (LG+) by traditional means. First, one crosses LG+ with the o2 mutant and then backcrosses with LG+ for five generations and self-crosses for three generations to obtain a homozygous LG o2 mutant (LGo2). Because the o2 allele is recessive, one cannot use the opaque phenotype as a selective marker during backcrosses and has to follow the allele by selfing or with a molecular marker. After introgression of o2, the LGo2 is used to pollinate a QPM donor line. The resultant progeny are backcrossed again with LGo2 for five generations and then self-crossed for at least three generations to obtain a modified LG o2 mutant (LGMo2) (Figure 3). This lengthy and laborious method would take at least 5 years and 8 months. In contrast, the QPM-RNAi (Figure 2B) can be directly pollinated with LG+, simply selecting green and vitreous progeny, which can easily be followed during recurring backcrosses with LG+. After five backcross generations, resultant progeny are self-crossed for three generations to generate LG(RNAi)-QPM. During the entire process, green and vitreous kernels are selected in each generation, which could save 2 years and 8 months (Figure 3). Even if one would like to create LG without any transgene by replacing it with the o2 mutation, introgression of Mo2s would still be faster. The P6z1RNAi transgene could be segregated and the o2 allele introduced. By crossing QPM-RNAi/+−BC5(Mo2s/+; RNAi/+; +/+) and o2/+−BC5 (+/+; +/+; o2/+), half of the green and vitreous kernels should carry the o2 allele, which could be screened by the specific o2 allele markers; then the vitreous and green progeny with genotype Mo2s/+; RNAi/+; o2/+ are selected and self-crossed for three generations to generate LGMo2 without the P6z1RNAi transgene, still saving >2 years.
Figure 3.
Comparison of traditional and accelerated methods for QPM conversion. LG+, local germplasm wild type; LGo2, LG o2 mutant; Mo2s, o2 modifiers; LGMo2, modified LGo2 or LG-QPM; and LG(RNAi)-QPM, modified LG with P6z1RNAi transgene;
Conclusion
The dominance of Mo2s over an RNAi phenotype has been used to accelerate backcrossing a multigenic trait into maize cultivars for enhanced nutrition.
Acknowledgments
The research described in this article was supported by the Selman A. Waksman Chair in Molecular Genetics at Rutgers University.
Literature Cited
- Frame B. R., Shou H., Chikwamba R. K., Zhang Z., Xiang C., et al. , 2002. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 129: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha K. B., Lending C. R., Lopes M. A., Wallace J. C., Larkins B. A., 1991. opaque-2 modifiers increase γ-zein synthesis and alter its spatial distribution in maize endosperm. Plant Cell 3: 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding D. R., Hunter B. G., Chung T., Gibbon B. C., Ford C. F., et al. , 2008. Genetic analysis of opaque2 modifier loci in quality protein maize. Theor. Appl. Genet. 117: 157–170 [DOI] [PubMed] [Google Scholar]
- Houmard N. M., Mainville J. L., Bonin C. P., Huang S., Luethy M. H., et al. , 2007. High-lysine corn generated by endosperm-specific suppression of lysine catabolism using RNAi. Plant Biotechnol. J. 5: 605–614 [DOI] [PubMed] [Google Scholar]
- Huang S., Frizzi A., Florida C. A., Kruger D. E., Luethy M. H., 2006. High lysine and high tryptophan transgenic maize resulting from the reduction of both 19- and 22-kD alpha-zeins. Plant Mol. Biol. 61: 525–535 [DOI] [PubMed] [Google Scholar]
- Mertz E. T., Bates L. S., Nelson O. E., 1964. Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145: 279–280 [DOI] [PubMed] [Google Scholar]
- Mertz E. T., Veron O. A., Bates L. S., Nelson O. E., 1965. Growth of rats fed on opaque-2 maize. Science 148: 1741–1742 [DOI] [PubMed] [Google Scholar]
- Osborne T. B., Mendel L. B., 1914. Nutritional properties of proteins of the maize kernel. J. Biol. Chem. 18: 1–16 [Google Scholar]
- Prasanna B. M., Vasal S. K., Kassahun B., Singh N. N., 2001. Quality protein maize. Curr. Sci. 81(10): 1308–1319 [Google Scholar]
- Schmidt R. J., Ketudat M., Aukerman M. J., Hoschek G., 1992. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell 4: 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G., Song R., Messing J., 2003. A new opaque variant of maize by a single dominant RNA-interference-inducing transgene. Genetics 165: 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R., Messing J., 2002. Contiguous genomic DNA sequence comprising the 19-kD zein gene family from maize. Plant Physiol. 130: 1626–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R., Messing J., 2003. Gene expression of a gene family in maize based on noncollinear haplotypes. Proc. Natl. Acad. Sci. USA 100: 9055–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasal S. K., Villegas E., Bjarnason M., Gelaw B., Goertz P., 1980. Genetic Modifiers and Breeding Strategies in Developing Hard Endosperm opaque-2 Materials, pp. 37–73 in Improvement of Quality Traits of Maize for Grain and Silage Use, edited by Pollmer W. G., Phipps R. H. Martinus Nijhoff, London [Google Scholar]
- Wu Y., Holding D. R., Messing J., 2010. γ-Zeins are essential for endosperm modification in quality protein maize. Proc. Natl. Acad. Sci. USA 107: 12810–12815 [DOI] [PMC free article] [PubMed] [Google Scholar]