Abstract
Heterotrimeric (αβγ) G proteins are crucial components of eukaryotic signal transduction pathways. G-protein-coupled receptors (GPCRs) act as guanine nucleotide exchange factors (GEFs) for Gα subunits. Recently, facilitated GDP/GTP exchange by non-GPCR GEFs, such as RIC8, has emerged as an important mechanism for Gα regulation in animals. RIC8 is present in animals and filamentous fungi, such as the model eukaryote Neurospora crassa, but is absent from the genomes of baker’s yeast and plants. In Neurospora, deletion of ric8 leads to profound defects in growth and asexual and sexual development, similar to those observed for a mutant lacking the Gα genes gna-1 and gna-3. In addition, constitutively activated alleles of gna-1 and gna-3 rescue many defects of Δric8 mutants. Similar to reports in Drosophila, Neurospora Δric8 strains have greatly reduced levels of G-protein subunits. Effects on cAMP signaling are suggested by low levels of adenylyl cyclase protein in Δric8 mutants and suppression of Δric8 by a mutation in the protein kinase A regulatory subunit. RIC8 acts as a GEF for GNA-1 and GNA-3 in vitro, with the strongest effect on GNA-3. Our results support a role for RIC8 in regulating GNA-1 and GNA-3 in Neurospora.
EUKARYOTIC cells sense many hormones, growth factors, neurotransmitters, and the presence of light via G-protein-signaling pathways. The G-protein heterotrimer consists of a Gα subunit, which binds and hydrolyzes GTP, and of tightly associated Gβ and Gγ subunits. G proteins interact with seven-transmembrane helix G-protein-coupled receptors (GPCRs) to regulate downstream signaling pathways (reviewed in Neves et al. 2002 and Wilkie and Kinch 2005). GDP-bound Gα subunits are associated with the Gβγ dimer and the GPCR (Wilkie and Kinch 2005). Ligand binding to the receptor leads to exchange of GDP for GTP on the Gα, leading to dissociation of the heterotrimer into Gα-GTP and Gβγ units, which can both interact with effector proteins to generate changes in cellular physiology (Neves et al. 2002). GPCRs thus act as guanine nucleotide-exchange factors (GEFs) for heterotrimeric Gα proteins. The activation cycle is terminated by hydrolysis of GTP to GDP by the Gα subunit and reassociation with Gβγ and the GPCR.
Recently a non-GPCR protein, RIC8, has been implicated as a positive regulator of Gα proteins in several animal species, including Caenorhabditis elegans, Drosophila melanogaster, and mammalian cells (reviewed in Wilkie and Kinch 2005). Invertebrates such as C. elegans and Drosophila contain one RIC8 protein, while vertebrates contain two homologs, Ric-8A and Ric-8B (Tall et al. 2003). RIC8 is required for asymmetric cell division in zygotes and priming of synaptic vesicles in C. elegans (Miller and Rand 2000; Wilkie and Kinch 2005). In Drosophila, RIC8 is essential for responses to extracellular ligands and for maintenance of polarity during asymmetric cell division in embryogenesis (Hampoelz et al. 2005). Furthermore, RIC8 is also required for stability of a Gα and Gβ protein in Drosophila (Hampoelz et al. 2005). Of the two mammalian RIC8 proteins, only Ric-8A has demonstrated GEF activity toward the Gα proteins Gαq, Gαi, Gαo, and Gα13 (Tall et al. 2003). Ric-8A binds the GDP-bound Gα in the absence of Gβγ, and GDP is then released, forming a stable Ric-8A:Gα complex (Tall et al. 2003). Subsequently, GTP binds the Gα protein and Ric-8A is released. Similar to Drosophila RIC8, Ric-8B regulates the stability of a Gα subunit in mammalian cells (Nagai et al. 2010).
Neurospora crassa possesses three Gα subunits (GNA-1, GNA-2, and GNA-3), one Gβ (GNB-1), one Gγ (GNG-1), and >25 putative GPCRs (reviewed in Li et al. 2007). We have previously demonstrated that loss of gna-1 and gna-3 or of all three Gα genes leads to a severe decrease in extension of both basal and aerial hyphae. The relatively subtle phenotypes of GPCR mutants in comparison to strains lacking one or more Gα genes (Kim and Borkovich 2004; Krystofova and Borkovich 2006; Li and Borkovich 2006; Bieszke et al. 2007) prompted us to explore the possibility of additional non-GPCR regulators of G proteins in Neurospora. It was recently reported that a single protein homologous to RIC8 is present in Neurospora and other filamentous fungi, but is absent from the genomes of Saccharomyces cerevisiae and plants (Wilkie and Kinch 2005). It has been hypothesized that RIC8 was acquired by animals and certain fungi after the evolution of G proteins and GPCRs and is therefore a fairly recent addition to G-protein regulatory pathways (Wilkie and Kinch 2005). The only other study of a RIC8 homolog in filamentous fungi was reported very recently in the fungal rice pathogen Magnaporthe oryzae (Li et al. 2010). MoRic8 was shown to bind to the Gα subunit MagB in the yeast two-hybrid assay and is highly expressed in the appressorium, a specialized structure that invades plant tissue during pathogenesis. MoRic8 was also found to act upstream of the cAMP pathway, which regulates appressorium formation. However, this study did not investigate possible GEF activity for MoRic8.
Here we provide evidence that RIC8 is a Gα GEF that regulates growth and development in Neurospora. Loss of ric8 leads to several phenotypes also displayed by strains lacking the Gα genes gna-1 and gna-3, including extremely impaired growth, short aerial hyphae, inappropriate conidiation in submerged culture, and loss of female fertility. We also demonstrate that all three Neurospora Gα proteins bind GTP and that RIC8 interacts with and has GEF activity toward GNA-1 and GNA-3 in N. crassa.
Materials and Methods
Strains and growth conditions
A list of Neurospora strains is provided in Table 1. Strains were maintained on Vogel’s minimal medium (VM) (Vogel 1964). Synthetic crossing medium was used to induce formation of female reproductive structures (protoperithecia) (Westergaard and Mitchell 1947). All submerged liquid and VM plate cultures were inoculated with conidia and grown as described previously (Krystofova and Borkovich 2006; Li and Borkovich 2006). Where indicated, ampicillin, histidine, and inositol were used at 100 μg/ml, hygromycin at 200 μg/ml, and phosphinothricin at 400 μg/ml.
Table 1 . Strains used in this study.
| Strain | Relevant genotype | Comments | Source |
|---|---|---|---|
| 74A-OR23-1A | Wild type, matA | FGSC 987 | FGSC |
| 74a-OR8-1a | Wild type, mata | FGSC 988 | FGSC |
| 74-OR23-IVA | Wild type, matA | FGSC 2489 | FGSC |
| ORS-SL6a | Wild type, mata | FGSC 4200 | FGSC |
| Y234M723 | his-3 matA | FGSC 6103 | FGSC |
| am1 | am1 ad3B cyh-1 | FGSC 4564 | FGSC |
| R81a | Δric8::hph+mata | Δric8 mutant | This study |
| R81-5a | Δric8::hph+mata | Δric8 mutant | This study |
| R8H3 | Δric8::hph+his3 matA | Δric8 his-3 strain | This study |
| R8 am1 | Δric8::hph+am1 ad3B cyh-1 | R8H3 + am1 heterokaryon | This study |
| R8GFP | Δric8::hph+ric8-gfp::his-3+ | ric8-gfp strain | This study |
| R81* | Δric8::hph+gna-1Q204L::his-3+ | gna-1Q204L in Δric8 background | This study |
| R82* | Δric8::hph+gna-2Q205L::his-3+ | gna-2Q205L in Δric8 background | This study |
| R83* | Δric8::hph+gna-3Q208L::his-3+ | gna-3Q208L in Δric8 background | This study |
| R81*3* | Δric8::hph+gna-1Q204L::bar+gna-3Q208L::his-3+ | gna-1Q204L and gna-3Q208L in Δric8 background | This study |
| mcb | mcb inl | FGSC 7094 | FGSC |
| R8 mcb | Δric8::hph+mcb::inl | R8 am1 × mcb progenya | This study |
| 3B10 | Δgna-1::hph+mata | Δgna-1 mutant | Ivey et al. (1999) |
| a29-1 | Δgna-2::pyrG+matA | Δgna-2 mutant | Baasiri et al. (1997) |
| 43c2 | Δgna-3::hph+mata | Δgna-3 mutant | Kays et al. (2000) |
| g1.3 | Δgna-1::hph+ Δgna-3::hph+matA | Δgna-1 Δgna-3 double mutant | Kays and Borkovich (2004) |
| noa | Δgna-1::hph+ Δgna-2::pyrG+ Δgna-3::hph+matA | Δgna-1 Δgna-2 Δgna-3 triple mutant | Kays and Borkovich (2004) |
FGSC, Fungal Genetics Stock Center (Kansas City, MO).
“X” denotes a genetic cross.
Identification and characterization of the ric8 gene
The existence of a ric8 homologue in fungi was suggested by Wilkie and Kinch (2005). Homology searches (BLAST) (Cookson et al. 1997) of the N. crassa genome database at the Broad Institute (http://www.broad.mit.edu/annotation/fungi/neurospora_crassa_7/index.html) (Galagan et al. 2003) using the Xenopus laevus Ric8 protein sequence (accession no. NP_989159) revealed that the Neurospora RIC8 homolog corresponds to NCU02788. A ric8 subclone, pSM1, was generated by digesting cosmid pMOcosX G19 C10 with EcoRI, releasing a 12.6-kb fragment (corresponding to contig 7.7, nucleotides 24,473–37,042) that was subcloned into pGEM4 (Promega, Madison, WI). The clone was sequenced at the Institute for Integrative Genome Biology, Core Sequencing Facility, University of California, Riverside using primers T7 and SP6.
Strain construction
A transformant containing the ric8 deletion mutation was obtained from the Neurospora genome project (Colot et al. 2006). This Δric8 strain was crossed as a male to wild-type strain 74A (female), and progeny were plated on VM plates containing hygromycin to select for Δric8::hph+ strains. The Δric8 his-3 (R8H3) strain was isolated in a similar manner by crossing a his-3 strain [Fungal Genetics Stock Center (FGSC) 6103] as a male to the Δric8 heterokaryon (female) with selection on VM–hygromycin plates containing histidine. The presence of the Δric8 mutation in progeny was verified by growth on hygromycin and Southern analysis, and his-3 auxotrophs were identified by spot testing on VM medium lacking histidine.
To complement the Δric8 mutation in trans, the ric8 open reading frame (ORF) was cloned into vector pMF272 (Folco et al. 2003; Freitag et al. 2004). pMF272 contains the ccg-1 promoter and allows expression of an ORF with a 3′ GFP fusion. The ric8 ORF (including introns) was amplified from pSM1 using primers designed to add XbaI (5′ end, R8 GFP fw) and BamHI (3′ end, R8 GFP rv) sites to the ric8 gene (supporting information, Table S1). The fragment was ligated into pMF272 digested with BamHI and EcoRI, yielding plasmid pSM2. This vector was used to target the ric8–gfp fusion to the his-3 locus of the R8H3 strain (R8GFP, Table 1) (Aramayo et al. 1996). Transformants were plated on VM–hygromycin medium, and isolates were analyzed for integration of ric8-gfp at the his-3 locus using Southern analysis. Transformants with the ric8-gfp Southern pattern were purified by three rounds of colony isolation from VM–hygromycin plates.
Predicted GTPase-deficient, constitutively activated alleles of gna-1 (Q204L) (Yang and Borkovich 1999), gna-2 (Q205L) (Baasiri et al. 1997), and gna-3 (Q208L) were introduced into the Δric8 background to determine epistatic relationships between ric8 and the three Gα genes. These vectors will be described elsewhere (S. Krystofova, S. Won, and K. Borkovich, unpublished results). Briefly, the ORFs corresponding to the three Gα alleles were subcloned into vector pMF272, with eviction of the GFP gene, producing plasmids pSVK51 (gna-1Q204L), pSVK52 (gna-2Q205L), and pSVK53 (gna-3Q208L). These plasmids were targeted to the his-3 locus in R8H3 as described above, generating strains R81*, R82*, and R83*. Correct integration at the his-3 locus was verified by Southern analysis.
To construct a strain containing both gna-1Q204L and gna-3Q208L alleles in the Δric8 background, the insert from plasmid pSVK51 (carrying gna-1Q204L) was introduced into a vector that would allow ectopic integration and confer resistance to phosphinothricin (Pall 1993). A 2.2-kb fragment from pSVK51 containing the ccg-1 promoter and gna-1Q204L allele was released using NotI and EcoRI. This fragment was cloned into pBARGEM7-2, which contains the bar+ gene and confers phosphinothricin resistance. The resulting vector, pSM3, was transformed into the R83* strain using electroporation. Transformants were plated on sorbose medium containing hygromycin and phosphinothricin, and colonies were analyzed for the ectopic integration of gna-1Q204L using Southern analysis.
The Δric8 mcb double mutant was generated from a cross with R8 am1 (Δric8::hph+ am1 heterokaryon, Table S1) as a female and mcb inl as a male. Strain am1 is a mating-type mutant that allows crossing of female-sterile strains, but does not passage its nuclei in a cross (Griffiths 1982). Since mcb is tightly linked to inl, inositol auxotrophy can be used to score the presence of the mcb mutation. Progeny were plated on VM medium containing hygromycin and inositol to isolate Δric8 mcb inl mutants (R8 mcb, Table 1).
RT-PCR and Northern and Western analysis
There are two predicted introns in ric8 (see gene structure in Figure S1A). The presence of the 3′ predicted intron was confirmed by the sequence of a cDNA clone (NCW06G3T7 cDNA clone W06G3 3′; Broad Institute). The 5′ predicted intron and ric8 gene deletion were verified by semiquantitative RT-PCR (Kays and Borkovich 2004). Expression of ric8 was analyzed using 2 μg total RNA extracted from conidia (Puregene RNA isolation kit, Gentra Systems, Minneapolis) (Krystofova and Borkovich 2006). RT-PCR analysis was performed using the Access RT-PCR system (Promega) with gene specific primers (R8I1fw and R8I1rv) designed to flank the 5′ intron (Table S1). Each reaction included 2 μg DNase-treated RNA and both AMV reverse transcriptase and Tfl polymerase (Promega). The genomic control contained the same primers with plasmid pSM2 (genomic fragment) as the template. The RT reaction was incubated at 48° for 45 min and at 94° for 2 min. The PCR thermocycling conditions were the following: 35 cycles at 94° for 30 sec, at 50° for 1 min, and at 68° for 90 sec with a final elongation step at 68° for 7 min. The reactions were electrophoresed on an agarose gel, blotted onto a nylon membrane, and subjected to Southern analysis as described (Krystofova and Borkovich 2005), using the radiolabeled 410-bp fragment from the genomic control PCR as the probe. A control reaction for RNA quality was also run using 18S rRNA-specific primers as previously described (Bieszke et al. 2007).
For Northern analysis, total RNA was isolated and samples containing 20 μg were analyzed as described (Krystofova and Borkovich 2005). Probes for Northern detection of gna-1, gna-2, gna-3, and gnb-1 were generated as described previously (Krystofova and Borkovich 2005).
For Western analysis, the particulate fraction (GNA-1, GNA-2, GNA-3, and GNB-1) or whole-cell extracts (CR-1, R8GFP) were isolated as described previously (Ivey et al. 1996; Kays et al. 2000; Krystofova and Borkovich 2005; Li and Borkovich 2006). Samples containing 100 μg of total protein were subjected to Western analysis as described (Yang et al. 2002; Krystofova and Borkovich 2005). The primary polyclonal antisera for GNA-1, GNA-2, and GNB-1 were used at dilutions of 1:1000, while GNA-3 and CR-1 were diluted 1:750 and 1:5000, respectively. The primary polyclonal antibody against GFP (Abcam, Cambridge, MA) was used at a dilution of 1:2000. A horseradish peroxidase conjugate (Bio-Rad) was used as the secondary antibody at a dilution of 1:4000 (GNA-1 and GNA-2), 1:2000 (GNA-3), or 1:10,000 (CR-1), and chemiluminescent detection was performed as described previously (Krystofova and Borkovich 2005).
Phenotypic analysis and microscopy
Plate cultures were imaged by scanning. Intracellular localization of the RIC8 protein in conidia and vegetative hyphae was achieved by microscopic observation of GFP fluorescence from a Δric8 strain carrying a RIC8–GFP fusion protein at the his-3 locus (see Strain construction). Conidia were harvested as described (Krystofova and Borkovich 2006; Li and Borkovich 2006) and imaged directly or used to inoculate 30-ml liquid cultures at a concentration of 1 × 106 cell/ml, followed by incubation for 16 hr at 30° with centrifugation at 200 × g (for vegetative hyphae). Conidia and vegetative hyphae were viewed using differential interference contrast (DIC) and GFP fluorescence microscopy using an Olympus IX71 inverted microscope with a ×60 oil immersion objective (numerical aperture = 1.42). Images were captured using a QIClick digital CCD camera (QImaging) and analyzed using Metamorph software (Molecular Devices).
Yeast two-hybrid assay
A ric8 ORF clone lacking introns (pSM6) was constructed using homologous recombination of PCR fragments in S. cerevisiae (Colot et al. 2006). All DNA fragments for cDNA were amplified by PCR using Pfu turbo DNA polymerase (Stratagene) according to the manufacturer’s protocols. To remove the first intron, the 5′ UTR up to the first intron was amplified (574-bp product; primers R8YR11fw and R8YR11rv, Table S1) as well as the first part of the second exon (756-bp product; primers R8YR12fw and R8YR12rv), using pSM1 as a template. The second intron was removed by amplifying the last part of the second exon (727-bp product; R8YR21fw and R8YR21rv) and the third exon and 3′ UTR (579 bp; R8YR22fw and R8YR22rv, Table S1).
The two sets of fragments were transformed separately into S. cerevisiae strain FY834 (Winston et al. 1995; Colot et al. 2006) along with vector pRS416 (Christianson et al. 1992; Gera et al. 2002) gapped with XbaI and EcoRI, with selection on SC medium lacking uracil. DNA was extracted from transformants, and plasmids were recovered by electroporation into Escherichia coli and plating on LB + Amp and sequenced.
PCR was used to amplify the ric8 cDNA and introduce restriction sites for yeast two-hybrid cloning. The 5′ fragment was amplified with primers R8Y2Hfw and R8YR12rv (744 bp), introducing the EcoRI restriction site 5′ to the initiator ATG and extending to include the restriction site XhoI. The 3′ fragment was amplified with primers R8YR21fw and R8Y2Hrv (836 bp), which include the XhoI site, and a BamHI site was inserted in place of the TGA stop codon. These fragments were digested and ligated into EcoRI- and BamHI-digested pGEM4 to create pSM6. pSM6 was digested using EcoRI and BamHI, and the ric8 insert was subsequently ligated into pGBKT7 and pGAD424 (Clontech) to yield plasmids pSM7 and pSM8, respectively.
The yeast two-hybrid assay was performed as described previously (Li and Borkovich 2006). Yeast strains containing RIC8 gene plasmids (pSM7 or pSM8) were mated to the appropriate Gα-vector-containing yeast to isolate diploids. Diploids were then tested on SC medium lacking leucine, tryptophan, and histidine ± adenine to select for the expression of the HIS3 or HIS3 and ADE2 reporter genes, respectively. A colony lift assay for β-galactosidase activity was performed according to the manufacturer’s recommendations to screen for the lacZ reporter (Clontech).
Expression and purification of RIC8 and Gα proteins from E. coli
For amplification of the Gα genes, primer pairs (NΔ13G1fw/NΔ13G1rv, NΔ13G2fw/NΔ13G2rv, and NΔ12G3fw/ NΔ12G3rv; Table S1) were used to introduce a 36 (gna-3) or 39 (gna-1 and gna-2) nucleotide deletions at the 5′ end of each gene, along with restriction sites for cloning into pET16b, which expresses a 10-histidine N-terminal fusion protein (Novagen, Gibbstown, NJ). The ric8 primers (R8 NdeI fw/R8 BamHI rv; Table S1) were designed to clone full-length ric8 into pET16b.
E. coli strain BL21 (DE3) plysS was used to express HIS-tagged proteins. LB medium supplemented with 100 μg/ml ampicillin and 35 μg/ml chloramphenicol was inoculated with 1/50 volume of an overnight E. coli culture and grown at 37° for 2–3 hr with centrifugation at 225 × g. Heterologous protein expression was induced by the addition of IPTG to a final concentration of 100 μM, and incubation continued for an additional 2–3 hr at 30° with centrifugation at 80 × g. The cells were centrifuged at 4,000 × g in an Avanti J-26 XP floor centrifuge using rotor JS-4.0 for 20 min at 4°, and pellets were stored at −20°.
For RIC8, 40 ml of His-Pur Lysis buffer [50 mM NaH2PO4 (pH 7.4), 300 mM NaCl, 10 mM imidazole (Sigma), 10% glycerol, 0.1 mM PMSF] containing 0.2 mg/ml lysozyme was added to cell pellets from a 1-liter culture, followed by incubation on ice for 20 min. The cell suspension was then sonicated (Sonic Dismembrator, model 500, Fisher Scientific) at 30% power for 15–30 sec, two to six times with 1-min rests on ice. The extract was centrifuged at 20,000 × g using rotor JA-25.5 at 4° for 20 min. The supernatant was added to a 2-ml bed volume of His-Pur Cobalt beads (Thermo Scientific, Rockford, IL) in a conical tube and incubated for 1 hr at 4° with rotation. The slurry was centrifuged at 700 × g for 30 sec in an IEC Centra CL3 centrifuge with an IEC 243 rotor, the supernatant was removed, and the beads were transferred to a poly-prep column using 5 ml wash buffer (lysis buffer containing 20 mM imidazole). The column was washed with 10–20 ml wash buffer (until protein concentration was near zero as determined using the Bradford protein assay). Protein was eluted in 1-ml fractions with lysis buffer containing increasing amounts of imidazole: 50, 75, and 100 mM (3 ml of each concentration).
The Gα proteins were purified similarly to RIC8 with the few differences noted below. The Gα proteins were purified using Ni-NTA resin, and all buffers contained 50 mM NaH2PO4 (pH 7.4), 500 mM NaCl, 10% glycerol, 0.1 mM PMSF. In addition, the lysis and elution buffers contained 20 μM GDP and 1 mM MgSO4. Lysis buffer contained 20 mM imidazole, wash buffer contained 40 mM imidazole, and protein was eluted in 4-ml fractions with lysis buffer containing increasing amounts of imidazole: 60, 80, 100, and 120 mM.
For both RIC8 and Gα proteins, fractions with the highest protein concentration (typically 50–75 mM imidazole for RIC8 and 80–120 mM for Gα proteins) were pooled and simultaneously desalted and concentrated. The protein was transferred to an Amicon Ultra 30 column (Millipore, Billerica, MA) and centrifuged for 20 min at 4000 × g in an Avanti J-26 XP floor centrifuge using rotor JS-5.3. Exchange buffer contained 200 mM HEPES (pH 7.5), 100 mM NaCl, 10% glycerol, 1 mM EDTA, and 1 mM DTT. Gα exchange buffer also contained 1 mM MgSO4, and 20 μM GDP was added to the concentrated protein (500–1000 μl) in 3- to 4-ml portions and centrifuged each time for 4–10 min for a total 10–12 ml of buffer exchange. The protein was then divided into aliquots and stored at −80°. The concentration of RIC8 and Gα proteins was determined by comparing to BSA after electrophoresis on an SDS-PAGE gel, followed by Coomassie staining. Samples containing 1–20 μl of purified protein were subjected to Western analysis according to the manufacturer’s suggestions. The hexahistidine monoclonal antibody raised in rabbit (Bethyl Laboratories, Montgomery, TX) was used at a 1:1000 dilution, and goat anti-rabbit secondary antibody (Bio-Rad) was used at 1:10,000 dilution.
GTP-binding assays were modeled after protocols described previously (Tall et al. 2003). Reactions were carried out in exchange buffer [200 mm HEPES (pH 7.5), 100 mm NaCl, 10% glycerol, 1 mM EDTA, and 1 mM DTT] with a total volume of 20 μl. Each reaction contained 200 nM Gα protein and 0 or 200 nM RIC8. Solutions containing purified Gα proteins (400 nM) and 20 mM MgSO4 in a total volume of 10 μl were prepared and kept on ice until reaction start. Mixtures containing RIC8 (0 or 400 nM), 4 μm GTPγS, and 1 μCi GTPγ35S (Perkin Elmer, Waltham MS) were also prepared and kept on ice until reaction start. Reactions were initiated by mixing 10 μl of the RIC8 solution with 10 μl of each Gα solution and then incubated at 30° for up to 60 min. Reactions were quenched using 100 μl of cold stop solution [20 mM Tris–Cl (pH 8.0), 100 mM NaCl, 2 mM MgSO4 and 1 mM GTP]. Proteins were separated from unbound GTPγS by vacuum filtration using 0.45 μM of HA mixed cellulose membranes (Millipore), and filters were washed three times with 5 ml cold wash solution [50 mM Tris–Cl (pH 8.0), 100 mM NaCl, and 2 mM MgSO4] using vacuum filtration. Filters were dried at room temperature and placed in vials, and scintillation fluid (Scintiverse BD cocktail, Fisher Scientific, 4–5 ml) was added prior to counting using a Beckman LS 6500 liquid scintillation counter.
Results
ric8 isolation, gene structure analysis, and protein alignment
RIC8 belongs to a unique protein family that does not exhibit any conserved domains when compared to known proteins (Wilkie and Kinch 2005; Figueroa et al. 2009). However, analysis of the primary sequence of Xenopus laevis xRic-8 reveals an armadillo-type folding pattern, which is a group of three α-helices folded into a triangle structure (Coates 2003; Figueroa et al. 2009). This armadillo fold is repeated several times in the xRic-8 protein to form a compact armadillo repeat structure. This is of interest because armadillo proteins are known to interact with several protein partners and to be involved in multiple cellular pathways (Coates 2003). These in silico results were corroborated by circular dichroism studies that revealed a high number of α-helices in the xRic-8 protein (Figueroa et al. 2009).
RIC8 is found only in fungi and animals (Wilkie and Kinch 2005). In N. crassa, the closest homolog to animal RIC8 is a predicted 53-kDa protein designated NCU02788 (http://www.broad.mit.edu/annotation/fungi/neurospora_crassa_7/index.html). The predicted gene structure in the Broad Institute database was confirmed by sequence analysis and verification of the predicted introns. The presence of the 3′ intron was confirmed by cDNA sequence NCW06G3T7 (cDNA clone W06G3 3′; Broad Institute), and the 5′ predicted intron was verified by RT-PCR (Figure S1).
Of the animal RIC8 homologs, Neurospora RIC8 is most similar to Xenopus tropicalis Ric-8A and also exhibits significant identity to human RIC-8A (Figure 1). Although RIC8 proteins are highly conserved among filamentous fungi (Figure S2), there are no significant (E < 1e−4) homologs in the yeast S. cerevisiae, and RIC8 is also absent from the genomes of plants.
Figure 1 .
RIC8 homologs. ClustalW (http://www.ch.embnet.org/software/ClustalW.html) was used to align RIC8 sequences from N. crassa (Nc; accession NCU02788.3), Homo sapiens (Hs; accession NP_068751, E = 5e−8), X. tropicalis (Xt; accession NP_989159, E = 2e−10), C. elegans (Ce; accession Q9GSX9), and D. melanogaster (Dm; accession Q9W358). Background indicates identical (solid) and similar (shaded) amino acid residues (http://www.ch.embnet.org/software/BOX_form.html).
Epistatic relationships between RIC8 and Gα subunits
Vegetative growth in Neurospora involves apical polar growth of basal hyphae away from the point of inoculation and parallel to the solid medium surface, with branching occurring back from the tips of the hyphae (reviewed in Springer 1993). Under nutrient limiting conditions or in the presence of an air/water interface, specialized aerial hyphae branch perpendicularly from basal hyphae and produce asexual spore-forming structures called conidiophores, which in turn elaborate strings of asexual spores called macroconidia or conidia (Springer 1993). Once mature, conidia can be dispersed by wind currents or animals in nature. In nutrient-rich submerged cultures, wild-type Neurospora does not form conidiophores.
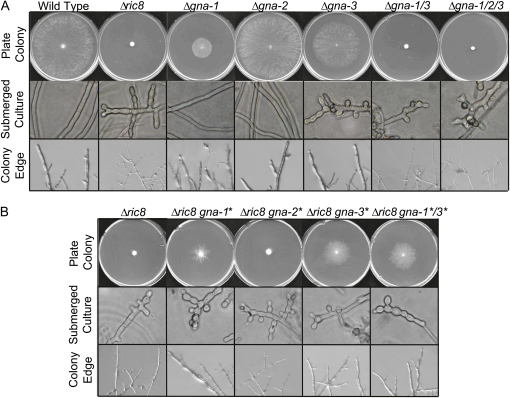
Since RIC8 has been shown to be a GEF for Gα proteins in other systems, we investigated a possible role for Neurospora RIC8 as an upstream regulator of the Gα subunits GNA-1, GNA-2, and GNA-3. A gene deletion mutant was created by replacement of the ric8 gene with the hph gene and analyzed for growth and/or developmental phenotypes (Figure 2A). Our laboratory has previously demonstrated that simultaneous loss of the Gα genes gna-1 and gna-3 causes severe defects in vegetative growth, absence of female sexual structures (protoperithecia), and inappropriate formation of conidia in submerged cultures (Kays and Borkovich 2004). Mutation of ric8 leads to phenotypes similar to those observed for Δgna-1 Δgna-3 double mutants. Δric8 mutants have thin basal hyphae that grow extremely slowly and produce short aerial hyphae, accumulate less mass, and inappropriately form conidia in submerged cultures (Figure 2A). During the sexual cycle, Δric8 strains do not differentiate protoperithecia and are thus female-sterile (data not shown).
Figure 2 .
Epistatic relationships between ric8 and Gα genes. (A) Phenotypes of Gα deletion and Δric8 strains. Strains were inoculated on VM plates and cultured for 1 day at 25° (“Plate Colony” and “Colony Edge”) and in VM liquid for 16 hr at 30° with shaking (“Submerged Culture”). Strains used for analysis were wild type (FGSC 2489), Δric8 (R81a), Δgna-1 (3B10), Δgna-2 (a29-1), Δgna-3 (43c2), Δgna-1 Δgna-3 (Δgna-1/3, g1.3), and Δgna-1 Δgna-2 Δgna-3 (Δgna-1/2/3, noa). Submerged Culture and Colony Edge photos were taken at ×400 and ×560 magnification, respectively. (B) Phenotypes after introduction of GTPase-deficient, constitutively activated Gα alleles (indicated by asterisks) into the Δric8 background. Strains used for analysis were Δric8 (R81a), Δric8 gna-1Q204L (R81*), Δric8 gna-2Q205L (R82*), Δric8 gna-3Q208L (R83*), and Δric8 gna-1Q204L gna-3Q208L (R81*3*). Culture conditions are as described in A.
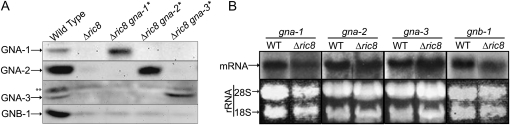
The shared defects of Δric8 and Gα mutants prompted us to measure Gα protein levels in the Δric8 mutant. Loss of ric8 causes a decrease in GNA-1, GNA-2, GNA-3, and GNB-1 protein levels (Figure 3A). In contrast, transcript amounts for the respective genes are similar in wild type and the Δric8 strain (Figure 3B), suggesting a post-transcriptional control mechanism. Interestingly, a similar requirement for ric8 in maintaining normal levels of a Gαi protein and an associated Gβ subunit has been observed in Drosophila (Hampoelz et al. 2005). In mammals, Ric-8B inhibits ubiquitination and proteasomal turnover of Gαs (Nagai et al. 2010). We have previously shown that loss of the Neurospora Gβ gene gnb-1 leads to decreased levels of GNA-1 and GNA-2, while GNA-3 protein levels are unaffected in submerged cultures (Yang et al. 2002). Although a decreased level of GNB-1 in the Δric8 mutant could account for low amounts of GNA-1 and GNA-2, the reduction in GNA-3 levels in Δric8 mutants cannot be explained by the loss of GNB-1. This suggests that GNA-3 stability is regulated by RIC8 independently of GNB-1.
Figure 3 .
Expression of G proteins in a Δric8 mutant. (A) Gα− and Gβ-protein levels. Samples containing 100 μg of protein from particulate fractions isolated from 16-hr submerged cultures were subjected to Western analysis using the GNA-1, GNA-2, and GNA-3 antibodies. The asterisk (*) denotes strains containing activated alleles of Gα genes. The double asterisk (**) indicates a background band observed in all GNA-3 Western blots. Strains used for analysis were wild type (FGSC 2489), Δric8 (R81a), Δric8 gna-1Q204L (R81*), Δric8 gna-2Q205L (R82*), and Δric8 gna-3Q208L (R83*). (B) Levels of Gα and Gβ transcripts. Samples containing 20 μg of total RNA isolated from 16-hr submerged cultures were subjected to Northern analysis using gene-specific probes. Strains used for analysis were wild type (FGSC 2489) and Δric8 (R81a).
During the G-protein cycle, GTP-bound Gα is released from Gβγ and the GPCR and is then free to interact with downstream effectors. Hydrolysis of GTP to GDP leads to reassociation of the Gα protein with Gβγ and termination of signaling. Because of these attributes, a GTPase-deficient Gα allele is constitutively active and independent of Gβγ. We therefore tested whether introduction of GTPase-deficient alleles of the three Gα genes (under control of the ccg-1 promoter) into the Δric8 background would rescue Δric8 phenotypes.
The results show that Δric8 strains carrying a GTPase-deficient activated Gα allele restored the encoded protein to wild-type levels (Figure 3A). This suggests that overexpression and/or a constitutively GTP-bound Gα can override the reduced protein levels observed in Δric8 mutants. Transformation of the gna-2Q205L activated allele into the Δric8 background had no effect. However, the presence of the gna-1Q204L- or gna-3Q208L-activated allele partially rescued the slow growth and reduced mass and thin basal hyphal phenotypes of Δric8 (Figure 2B), with gna-3Q208L having the greatest effect on growth. When both gna-1Q204L and gna-3Q208L are introduced into the Δric8 background, the phenotype resembles that obtained after transformation with gna-3Q208L alone (Figure 2B). Taken together, these results suggest that RIC8 acts upstream of and is a positive regulator of GNA-1 and GNA-3, but not of GNA-2 in Neurospora.
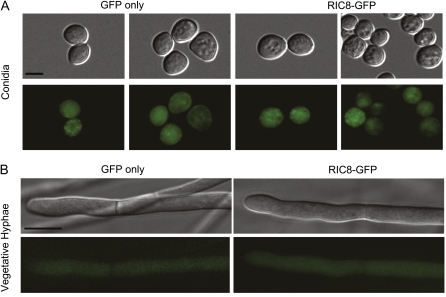
RIC8 is cytoplasmic in conidia and vegetative hyphae
To determine the localization of RIC8 in Neurospora, we produced a vector containing a C-terminal fusion of GFP to RIC8. This construct complemented Δric8 phenotypes and restored expression of the ric8 transcript (Figure S1, B and C). Production of a full-length RIC8-GFP fusion protein was observed by Western analysis using whole-cell extracts and GFP antiserum (Figure S1D). Analysis of strains carrying the GFP fusion showed that RIC8 is cytosolic in mature conidia and vegetative hyphae; this was confirmed by comparison to a control strain expressing a cytosolic GFP protein (Figure 4). GFP fluorescence images for a wild-type strain without a GFP construct were completely black using the same settings, indicating negligible background fluorescence (data not shown).
Figure 4 .
Localization of RIC8-GFP in Neurospora. Cultures were grown as indicated in Materials and Methods. Images were obtained using an Olympus IX71 microscope with a QIClickTM digital CCD camera and analyzed using Metamorph software. (A) DIC and GFP fluorescence micrographs showing localization of RIC8-GFP in conidia. Bar, 5 μm. (B) DIC and GFP fluorescence micrographs showing localization of RIC8-GFP in vegetative hyphae. For both A and B, GFP fluorescence images for a wild-type strain without a GFP construct were completely black. Bar, 10 μm.
This observation of a cytosolic location for RIC8 is consistent with observations in animals (Hampoelz et al. 2005) and in M. oryzae (Li et al. 2010). In higher eukaryotes, RIC8 has been shown to have cytoplasmic localization, which moves to the mitotic machinery at specific times during embryonic mitosis. In Drosophila embryos, RIC8 is located in the cytoplasm, with concentrated amounts at the mitotic spindle (Hampoelz et al. 2005). In C. elegans embryos, RIC8 is localized at the cortex, mitotic spindle, nuclear envelope, and around chromatin (Couwenbergs et al. 2004). The absence of nuclear localization for RIC8 in the two fungi analyzed could result from a number of factors, including growth conditions, time in the life cycle, or absence of an environmental trigger.
Loss of ric8 affects the cAMP-signaling pathway, which is regulated by Gα proteins in Neurospora
Adenylyl cyclase has been extensively characterized in eukaryotes and has been shown to be activated and repressed by G-protein signaling in various systems (reviewed in Neves et al. 2002). Activated adenylyl cyclase produces cAMP, which binds to the regulatory subunits of protein kinase A (PKA), releasing the catalytic subunits of PKA to phosphorylate a wide variety of target proteins (Neves et al. 2002). In the C. elegans synaptic signaling pathway, RIC8 has been linked to cAMP metabolism through the identification of mutations that suppress defects of ric8 loss-of-function mutants (Schade et al. 2005; Charlie et al. 2006). These mutations include dominant activated alleles of gsa-1 (Gαs) and acy-1 (adenylyl cyclase) and reduction-of-function alleles of kin-2 (regulatory subunit of PKA) and pde-4 (cAMP phosphodiesterase). These results are consistent with RIC8 as an upstream activator of the cAMP pathway during synaptic signal transmission in C. elegans. In Neurospora, previous work from our group suggests that adenylyl cyclase (CR-1) is activated by GTP-bound GNA-1, while GNA-3 is required to maintain normal levels of CR-1 protein (Ivey et al. 1999; Kays et al. 2000). Furthermore, a mutant allele of the PKA regulatory subunit (mcb) that is predicted to lead to hyperactivation of the catalytic subunit is epistatic to gna-3 and suppresses the Δgna-1 mutation (Bruno et al. 1996; Kays and Borkovich 2004).
On the basis of the known relationship between RIC8 and cAMP metabolism in C. elegans as well as the genetic relationship between RIC8, GNA-1 and GNA-3 in Neurospora, we investigated whether cAMP signaling is affected in Neurospora Δric8 mutants. We first assessed levels of the CR-1 adenylyl cyclase using western analysis with a CR-1 antiserum (Figure 5A). The amount of CR-1 protein was reduced in the Δric8 background (Figure 5A), similar to results observed for Δgna-3 mutants and consistent with low cAMP levels (Kays et al. 2000). However, expression of the activated forms of GNA-1, GNA-2, or GNA-3 did not return the CR-1 protein levels to normal (Figure 5A). These results suggest a Gα-independent role for RIC8 in modulation of CR-1 protein levels.
Figure 5 .
Relationship to the cAMP pathway. (A) CR-1 (adenylyl cyclase) protein levels. Samples containing 100 μg of protein from whole-cell extracts from 16-hr submerged cultures were subjected to Western analysis using the CR-1 antibody. Strains used were the same as in Figure 3A. (B) Effects of the mcb mutation. Strains Δric8 (R81a), mcb inl, and Δric8 mcb inl were cultured on VM + inositol plates for 3 days at 25°.
We followed up on the above finding by testing the effect of the mcb PKA regulatory subunit mutation described above (Table 1). The mcb mutation partially suppresses the Δric8 growth and conidiation phenotypes (Figure 5B). These results support a role for RIC8 as a positive regulator of the cAMP-signaling pathway through the activation of Gα subunits and at least in part via a Gα-independent pathway that influences the stability of cAMP-signaling pathway components.
RIC8 interacts with and acts as a GEF toward Gα subunits
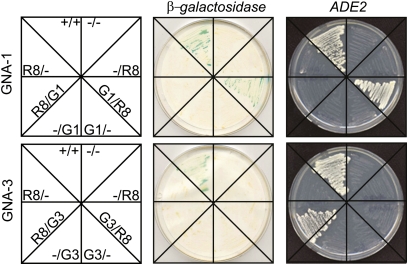
Results from the genetic epistasis experiments suggest that RIC8 is a positive regulator of GNA-1 and GNA-3. We next tested for a possible physical interaction between Gα proteins and RIC8 using the yeast two-hybrid assay. RIC8 was observed to interact with both GNA-1 and GNA-3, but not with GNA-2 (Figure 6). Interestingly, RIC8 interacts with GNA-1 only as a Gal4p DNA-binding domain fusion and with GNA-3 only as a Gal4p activation domain fusion (Figure 6). This may indicate a different physical relationship and/or binding site for RIC8 on these two Gα subunits.
Figure 6 .
Interaction between RIC8 and Gα subunits. Yeast two-hybrid analysis was employed using diploid strains containing the GAL4 activation domain (pGAD424) and the DNA-binding domain (pGBKT7) vectors. Labels represent inserts in the pGAD424/pGBKT7 plasmids, respectively (G1, GNA-1; G3, GNA-3; R8, RIC8). Minus sign (−), no insert in vector; plus sign (+), positive control interactors. β-Galactosidase activity shows strains containing vectors with the indicated genes that were cultured on medium lacking tryptophan and leucine for 3 days at 30°. Plates were scanned 24 hr after the colony lift assay for β-galactosidase. The adenine growth assay (ADE2) shows strains that were grown on medium lacking tryptophan, leucine, and adenine for 5 days.
Having established a physical association between RIC8 and a subset of Neurospora Gα subunits using the yeast two-hybrid assay, we next performed experiments to directly test whether RIC8 can accelerate exchange of GDP for GTP on Gα proteins. Since Gα proteins had not been previously purified or assayed for GTP binding in any filamentous fungal species, we had to devise methods to facilitate expression of soluble, active proteins and their subsequent purification. A previous study reported that efficient purification of mammalian Gα proteins from E. coli and subsequent protein crystallization were facilitiated either by removal of the disordered N-terminal region or by replacement with the N terminus of another Gα subunit (Kimple et al. 2002). These proteins were shown to retain normal GTP binding (Kreutz et al. 2006). We predicted that the first 12 (GNA-3) or 13 (GNA-1 and GNA-2) amino acids of Neurospora Gα proteins are disordered using the DisEMBL Intrinsic Protein Disorder Prediction program (http://dis.embl.de/). We therefore produced E. coli vectors expressing deca-histidine (10H) N-terminal tagged and N-terminal truncated versions of all three Gα subunits, termed 10H-NΔ13-GNA-1, 10H-NΔ13-GNA-2, and 10H-NΔ12-GNA-3. For clarity, we refer to these proteins as GNA-1, GNA-2, and GNA-3. A vector encoding full-length 10H-tagged RIC8 was also produced for expression in E. coli. Proteins were overexpressed in E. coli and then purified using nickel or cobalt affinity chromatography (see Materials and Methods).
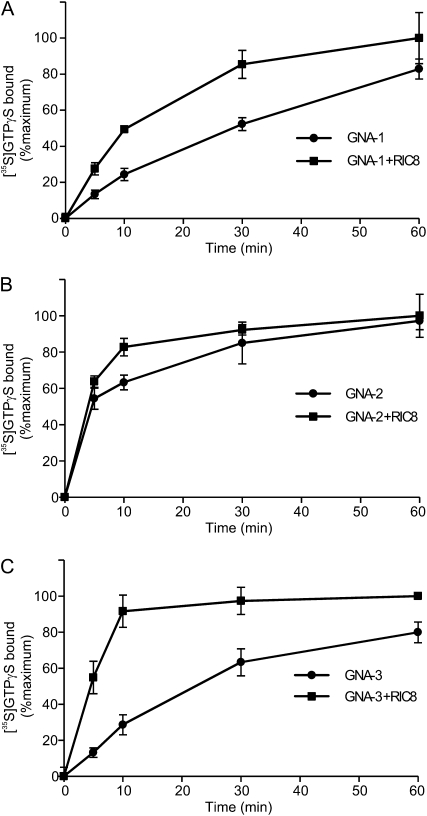
GTPγS-binding assays were conducted in the absence and presence of RIC8. All three Gα proteins bind GTP in the absence of RIC8, demonstrating that the fusions are active and confirming the identity of the three Neurospora proteins as G proteins (Figure 7). Addition of RIC8 had different effects on GTPγS binding to the three Gα proteins. RIC8 does not appreciably influence binding of GTPγS to GNA-2 (Figure 7B). However, binding of GTPγS by GNA-1 is increased twofold by the addition of RIC8 at early time points (5–30 min, Figure 7A), consistent with GEF activity for RIC8. A more dramatic effect is observed with GNA-3, where binding is significantly greater in the presence of RIC8 at all time points, with a maximum stimulation of more than fourfold at 5 min (Figure 7C). These results suggest that RIC8 acts as a GEF for GNA-1 and GNA-3, playing a major role in the activation of GNA-3. Importantly, the findings from the GEF assays are consistent with those obtained from the genetic epistasis analysis and yeast two-hybrid assays and support a model in which RIC8 activates GNA-1 and GNA-3, but not GNA-2, in Neurospora.
Figure 7 .
GTP binding of Gα proteins and GEF activity of RIC8. GNA-1 (A), GNA-2 (B), and GNA-3 (C) were incubated with [35S]GTPγS in the absence (●) or presence (▪) of RIC8. All protein concentrations were 200 nM. Reactions were performed in triplicate, as indicated in Materials and Methods. A representative result from several independent assays is shown for each protein.
Discussion
The discovery and subsequent study of RIC8 in metazoan cells has shown that this protein is required for major cellular processes, including asymmetric cell division and synaptic signaling. Here we demonstrate that RIC8 is involved in critical functions, including vegetative growth and sexual and asexual development in Neurospora. Mutations that activate the GNA-1 and GNA-3 Gα proteins in the Δric8 background partially suppress Δric8 phenotypes, and RIC8 interacts with GNA-1 and GNA-3 in the yeast two-hybrid assay. Most importantly, we show that RIC8 acts as a GEF for GNA-1 and GNA-3 in vitro. The data suggest that RIC8 participates in pathways involving GNA-1 and GNA-3 to regulate growth and development.
Loss of ric8 leads to lower levels of G-protein subunits in Neurospora, Drosophila, C. elegans, and mammalian cells. In Drosophila, RIC8 is required for proper plasma membrane localization and normal protein stability of Gαi, but only correct membrane localization of Gαo (Hampoelz et al. 2005). In C. elegans, RIC8 is essential for membrane localization and normal protein stability of Gα16, while Gαo does not depend on RIC8 for protein stability or localization (Afshar et al. 2004, 2005). In Drosophila, it has been proposed that effects on Gα-protein localization and stability in the absence of RIC8 may stem from defective assembly of the heterotrimer and inhibited transport to the plasma membrane (Hampoelz et al. 2005). In mammalian cells, it has been demonstrated that Ric-8B inhibits ubiquitination of Gαs, thus preventing degradation of the protein (Nagai et al. 2010). The mechanism underlying how loss of RIC8 leads to lower levels of Gα proteins in Neurospora is unknown, but may be a consequence of both mislocalization and accelerated protein turnover (A. Michkov, S. Won, and K. Borkovich, unpublished observations).
Work in C. elegans has demonstrated the importance of cAMP metabolism to RIC8-related functions in the synaptic signaling pathway. In addition, stabilization of Gαs by Ric-8B in mouse embryonic fibroblast cells is required for increased cAMP in response to isoproterenol (Nagai et al. 2010). The similarities between the genetically tractable C. elegans and Neurospora with regards to positive regulation of the cAMP pathway by RIC8 are particularly striking. In both systems, a mutation in the PKA regulatory subunit suppresses a ric8 mutation. Importantly, we also demonstrate that loss of RIC8 leads to a reduced level of adenylyl cyclase protein, a finding that has not been previously reported in any system. This observation may explain why a hyperactive allele of adenylyl cyclase was recovered in a ric8 suppressor screen in C. elegans (Schade et al. 2005). As mentioned above, RIC8 is also required for normal levels of various G proteins in Neurospora, Drosophila, C. elegans, and mammalian cells. Taken together, these results suggest that maintenance of normal levels of G proteins and adenylyl cyclase (and perhaps other yet-unknown regulatory components) is an important conserved function of the RIC8 protein and one that directly impacts cAMP signaling.
A prediction based on our results is that RIC8 regulates Gα activity and subsequent cAMP production in response to a specific developmental signal. However, the mechanism by which RIC8 is regulated at the pre- or post-translational level is currently unknown. Regarding transcriptional regulation, microarray analysis indicates that ric8 is expressed ubiquitously throughout the vegetative colony in Neurospora (Kasuga and Glass 2008). Results from additional microarray studies using Neurospora grown on medium that mimics either symbiotic or pathogenic plant relationships suggest that ric8 expression is not induced during fungal/plant interactions (http://bioinfo.townsend.yale.edu/browse.jsp?scope=4&tabid=1&query=ncu02788).
Heterotrimeric Gα proteins are activated by the exchange of GDP for GTP. This exchange is facilitated by a GEF, such as a GPCR or RIC8. Our results provide the first evidence that filamentous fungal Gα subunits do indeed bind GTP and that RIC8 functions as an apparent GEF by increasing GDP–GTP exchange on two of the three subunits. The main target of RIC8 appears to be GNA-3, as GNA-3 is activated most strongly by RIC8 in vitro, accelerating the GTP binding by fourfold. This activation is comparable to the more than sevenfold activation of Gαi1 by RIC8 in mammals (Tall et al. 2003) and to the fourfold activation of Gpa1 by another GEF, Arr4, in S. cerevisiae (Lee and Dohlman 2008). The twofold activation of GNA-1 by RIC8 is similar to that observed for mammalian Gαo, while mammalian Gαs is not affected by RIC8 (Tall et al. 2003). The finding that RIC8 acts preferentially on GNA-3 is supported by the observations that the GTPase-deficient allele of GNA-3 suppresses the Δric8 mutation to the greatest extent and that CR-1 protein levels are reduced in both Δric8 and Δgna-3 mutants.
Our results reveal important parallels between RIC8 functions during cell division, cAMP signaling, and perhaps regulated protein turnover in Neurospora and metazoans. Neurospora is a haploid organism that has a relatively short doubling time, well-developed genetic tools, and a sequenced genome. There is also a comprehensive collection of knockout mutants for most genes, and it is relatively easy to isolate and clone suppressor mutations. Future investigations will capitalize on these tools to reveal roles for known proteins, as well as to screen for novel components that participate in RIC8-regulated processes, thus illuminating conserved functions for RIC8 during growth and development in Neurospora and animals.
Acknowledgments
We thank Gyungsoon Park, Liande Li, Carol Jones, James Kim, Patrick Schacht, Hildur Colot, and Gloria Turner for helpful discussions and manuscript critique; Jacqueline Servin and Ilva Cabrera for microscopy controls; and David Carter for microscopy training. This work was supported by National Institutes of Health grant GM086565 (to K.A.B.).
Literature Cited
- Afshar K., Willard F. S., Colombo K., Johnston C. A., McCudden C. R., et al. , 2004. RIC-8 is required for GPR-1/2-dependent Gα function during asymmetric division of C. elegans embryos. Cell 119: 219–230 [DOI] [PubMed] [Google Scholar]
- Afshar K., Willard F. S., Colombo K., Siderovski D. P., Gonczy P., 2005. Cortical localization of the Gα protein GPA-16 requires RIC-8 function during C. elegans asymmetric cell division. Development 132: 4449–4459 [DOI] [PubMed] [Google Scholar]
- Aramayo R., Peleg Y., Addison R., Metzenberg R., 1996. Asm-1+, a Neurospora crassa gene related to transcriptional regulators of fungal development. Genetics 144: 991–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baasiri R. A., Lu X., Rowley P. S., Turner G. E., Borkovich K. A., 1997. Overlapping functions for two G protein α subunits in Neurospora crassa. Genetics 147: 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszke J. A., Li L., Borkovich K. A., 2007. The fungal opsin gene nop-1 is negatively-regulated by a component of the blue light sensing pathway and influences conidiation-specific gene expression in Neurospora crassa. Curr. Genet. 52: 149–157 [DOI] [PubMed] [Google Scholar]
- Bruno K. S., Aramayo R., Minke P. F., Metzenberg R. L., Plamann M., 1996. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 15: 5772–5782 [PMC free article] [PubMed] [Google Scholar]
- Charlie N. K., Thomure A. M., Schade M. A., Miller K. G., 2006. The Dunce cAMP phosphodiesterase PDE-4 negatively regulates Gαs-dependent and Gαs-independent cAMP pools in the Caenorhabditis elegans synaptic signaling network. Genetics 173: 111–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P., 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122 [DOI] [PubMed] [Google Scholar]
- Coates J. C., 2003. Armadillo repeat proteins: beyond the animal kingdom. Trends Cell Biol. 13: 463–471 [DOI] [PubMed] [Google Scholar]
- Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M., et al. , 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 103: 10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson S. T., Lopardo H., Marin M., Arduino R., Rial M. J., et al. , 1997. Study to determine the ability of clinical laboratories to detect antimicrobial-resistant Enterococcus spp. in Buenos Aires, Argentina. Diagn. Microbiol. Infect. Dis. 29: 107–109 [DOI] [PubMed] [Google Scholar]
- Couwenbergs C., Spilker A. C., Gotta M., 2004. Control of embryonic spindle positioning and Gα activity by C. elegans RIC-8. Curr. Biol. 14: 1871–1876 [DOI] [PubMed] [Google Scholar]
- Figueroa M., Hinrichs M. V., Bunster M., Babbitt P., Martinez-Oyanedel J., et al. , 2009. Biophysical studies support a predicted superhelical structure with armadillo repeats for Ric-8. Protein Sci. 18: 1139–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco H. D., Freitag M., Ramon A., Temporini E. D., Alvarez M. E., et al. , 2003. Histone H1 is required for proper regulation of pyruvate decarboxylase gene expression in Neurospora crassa. Eukaryot. Cell 2: 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag M., Hickey P. C., Raju N. B., Selker E. U., Read N. D., 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41: 897–910 [DOI] [PubMed] [Google Scholar]
- Galagan J. E., Calvo S. E., Borkovich K. A., Selker E. U., Read N. D., et al. , 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422: 859–868 [DOI] [PubMed] [Google Scholar]
- Gera J. F., Hazbun T. R., Fields S., 2002. Array-based methods for identifying protein-protein and protein-nucleic acid interactions. Methods Enzymol. 350: 499–512 [DOI] [PubMed] [Google Scholar]
- Griffiths A. J. F., 1982. Null mutants of the A and a mating-type alleles of Neurospora crassa. Can. J. Genet. Cytol. 24: 167–176 [Google Scholar]
- Hampoelz B., Hoeller O., Bowman S. K., Dunican D., Knoblich J. A., 2005. Drosophila Ric-8 is essential for plasma-membrane localization of heterotrimeric G proteins. Nat. Cell Biol. 7: 1099–1105 [DOI] [PubMed] [Google Scholar]
- Ivey F. D., Hodge P. N., Turner G. E., Borkovich K. A., 1996. The Gαi homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 7: 1283–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey F. D., Yang Q., Borkovich K. A., 1999. Positive regulation of adenylyl cyclase activity by a Galphai homolog in Neurospora crassa. Fungal Genet. Biol 26: 48–61 [DOI] [PubMed] [Google Scholar]
- Kasuga T., Glass N. L., 2008. Dissecting colony development of Neurospora crassa using mRNA profiling and comparative genomics approaches. Eukaryot. Cell 7: 1549–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays A. M., Borkovich K. A., 2004. Severe impairment of growth and differentiation in a Neurospora crassa mutant lacking all heterotrimeric Gα proteins. Genetics 166: 1229–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays A. M., Rowley P. S., Baasiri R. A., Borkovich K. A., 2000. Regulation of conidiation and adenylyl cyclase levels by the Gα protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20: 7693–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Borkovich K. A., 2004. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 52: 1781–1798 [DOI] [PubMed] [Google Scholar]
- Kimple R. J., Kimple M. E., Betts L., Sondek J., Siderovski D. P., 2002. Structural determinants for GoLoco-induced inhibition of nucleotide release by Gα subunits. Nature 416: 878–881 [DOI] [PubMed] [Google Scholar]
- Kreutz B., Yau D. M., Nance M. R., Tanabe S., Tesmer J. J., et al. , 2006. A new approach to producing functional Gα subunits yields the activated and deactivated structures of Gα(12/13) proteins. Biochemistry 45: 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystofova S., Borkovich K. A., 2005. The heterotrimeric G-protein subunits GNG-1 and GNB-1 form a Gβγ dimer required for normal female fertility, asexual development, and Gα protein levels in Neurospora crassa. Eukaryot. Cell 4: 365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystofova S., Borkovich K. A., 2006. The predicted G-protein-coupled receptor GPR-1 is required for female sexual development in the multicellular fungus Neurospora crassa. Eukaryot. Cell 5: 1503–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Dohlman H. G., 2008. Coactivation of G protein signaling by cell-surface receptors and an intracellular exchange factor. Curr. Biol. 18: 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Borkovich K. A., 2006. GPR-4 is a predicted G-protein-coupled receptor required for carbon source-dependent asexual growth and development in Neurospora crassa. Eukaryot. Cell 5: 1287–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wright S. J., Krystofova S., Park G., Borkovich K. A., 2007. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 61: 423–452 [DOI] [PubMed] [Google Scholar]
- Li Y., Yan X., Wang H., Liang S., Ma W. B., et al. , 2010. MoRic8 is a novel component of G-protein signaling during plant infection by the rice blast fungus Magnaporthe oryzae. Mol. Plant Microbe Interact. 23: 317–331 [DOI] [PubMed] [Google Scholar]
- Miller K. G., Rand J. B., 2000. A role for RIC-8 (Synembryn) and GOA-1 (Gαo) in regulating a subset of centrosome movements during early embryogenesis in Caenorhabditis elegans. Genetics 156: 1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Nishimura A., Tago K., Mizuno N., Itoh H., 2010. Ric-8B stabilizes the alpha subunit of stimulatory G protein by inhibiting its ubiquitination. J. Biol. Chem. 285: 11114–11120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves S. R., Ram P. T., Iyengar R., 2002. G protein pathways. Science 296: 1636–1639 [DOI] [PubMed] [Google Scholar]
- Pall M. L., 1993. The use of ignite (basta; glufosinate; phosphinothricin) to select transformants of bar-containing plasmids in Neurospora crassa. Fungal Genet. Newsl. 40: 58 [Google Scholar]
- Schade M. A., Reynolds N. K., Dollins C. M., Miller K. G., 2005. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the Gαs pathway and define a third major branch of the synaptic signaling network. Genetics 169: 631–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. L., 1993. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays 15: 365–374 [DOI] [PubMed] [Google Scholar]
- Tall G. G., Krumins A. M., Gilman A. G., 2003. Mammalian Ric-8A (synembryn) is a heterotrimeric Gα protein guanine nucleotide exchange factor. J. Biol. Chem. 278: 8356–8362 [DOI] [PubMed] [Google Scholar]
- Vogel H. J., 1964. Distribution of lysine pathways among fungi: evolutionary implications. Am. Nat. 98: 435–446 [Google Scholar]
- Westergaard M., Mitchell H. K., 1947. Neurospora-V: a synthetic medium favoring sexual reproduction. Am. J. Bot. 34: 573–577 [Google Scholar]
- Wilkie T. M., Kinch L., 2005. New roles for Gα and RGS proteins: communication continues despite pulling sisters apart. Curr. Biol. 15: R843–R854 [DOI] [PubMed] [Google Scholar]
- Winston F., Dollard C., Ricupero-Hovasse S. L., 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11: 53–55 [DOI] [PubMed] [Google Scholar]
- Yang Q., Borkovich K. A., 1999. Mutational activation of a Gαi causes uncontrolled proliferation of aerial hyphae and increased sensitivity to heat and oxidative stress in Neurospora crassa. Genetics 151: 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Poole S. I., Borkovich K. A., 2002. A G-protein β subunit required for sexual and vegetative development and maintenance of normal Gα protein levels in Neurospora crassa. Eukaryot. Cell 1: 378–390 [DOI] [PMC free article] [PubMed] [Google Scholar]