Abstract
Background
Urinary kidney injury molecule 1 is a recently discovered early biomarker for renal damage that has been proven to be correlated to urinary cadmium in rats. However, so far the association between urinary cadmium and kidney injury molecule 1 in humans after long-term, low-dose cadmium exposure has not been studied.
Methods
We collected urine and blood samples from 153 non-smoking men and women aged 60+, living in an area with moderate cadmium pollution from a non-ferrous metal plant for a significant period. Urinary cadmium and urinary kidney injury molecule 1 as well as other renal biomarkers (alpha1-microglobulin, beta2-microglobulin, blood urea nitrogen, urinary proteins and microalbumin) were assessed.
Results
Both before (r = 0.20; p = 0.01) and after (partial r = 0.32; p < 0.0001) adjustment for creatinine, age, sex, past smoking, socio-economic status and body mass index, urinary kidney injury molecule 1 correlated with urinary cadmium concentrations. No significant association was found between the other studied renal biomarkers and urinary cadmium.
Conclusions
We showed that urinary kidney injury molecule 1 levels are positively correlated with urinary cadmium concentration in an elderly population after long-term, low-dose exposure to cadmium, while other classical markers do not show an association. Therefore, urinary kidney injury molecule 1 might be considered as a biomarker for early-stage metal-induced kidney injury by cadmium.
Keywords: kidney injury molecule 1, cadmium, renal biomarkers, toxicity, chronic, kidney
Background
Cadmium (Cd) is an ever-present and global environmental pollutant [1]. Current Cd emission has been drastically reduced, but Cd continues to be a health hazard, because historically accumulated Cd cannot be degraded and its half-life in the body is long (10-30 years) [2]. Next to the bone [3], a main target for chronic, low-level Cd exposure is the kidney, leading to proximal tubule dysfunction [2,4,5]. Tubular dysfunction causes polyuria and low molecular weight proteinuria and some of these urinary proteins, e.g. α-1 microglobulin (α1M-U)[6], β-2 microglobulin (β2M-U)[7,8] and clara cell protein-16 (CC-16-U)[9] are used as urinary biomarkers of the early stages of Cd nephrotoxicity. In other studies, Cd toxicity is monitored by the Cd-binding protein metallothionein in urine [8,10,11], N-acetyl-beta-glucosaminidase (NAG) [7,8] or even urinary Cd itself [7,11-13]. Although these renal biomarkers are widely used, questions arose concerning specificity and sensitivity [6,9,11,14-17]. There clearly is a need for an early and stable biomarker for proximal tubule damage caused by Cd.
Kidney injury molecule 1 (KIM-1), originally discovered by Ichimura et al., is a type 1 membrane glycoprotein found on renal proximal tubule epithelial cells. It contains in its extracellular portion a unique 6-cysteine immunoglobulin-like domain and a mucin-domain [18]. An intracellular highly conserved tyrosine kinase phosphorylation motif is a strong indicator that KIM-1 is a cell signaling molecule [19].
KIM-1 expression is induced in a variety of renal diseases, whereas in healthy kidney tissue KIM-1 is virtually undetectable [18,20]. In the case of kidney damage, KIM-1 is expressed on the apical membrane followed by cleavage of the ectodomain (90 kDa) which is released in the urine in rodents [18,19,21-24] and in humans [25-28]. KIM-1 is upregulated in the proximal tubule during dedifferentiation of the kidney epithelium, an early manifestation in response to damage [29].
In rats, Prozialeck et al. showed that KIM-1 is a very early urinary marker for Cd-induced kidney injury [23]. They showed that KIM-1 was elevated before other urinary biomarkers of Cd nephrotoxicity, such as metallothionein, CC-16-U, proteinuria, α-glutathione-S-transferase (α-GST), NAG and Cd itself [23,30]. Moreover they showed that the Cd-induced increase in KIM-1 expression can be detected before signs of necrosis appear and when there is only a modest level of apoptosis in the proximal tubule [29].
It has been well established in humans that KIM-1 appears in the urine at an early stage in kidney damage and also, that Cd affects proximal tubule function when the Cd burden is high. Cd triggers the expression of KIM-1 at a very early stage in animal models [31]. The aim of the present pilot study is to assess the appearance of urinary KIM-1 after long-term, low-dose Cd exposure in humans, because, to our knowledge, this has not been investigated in the population.
Methods
Study population and sample collection
The total population (n = 3069) of the general practice in Genk is registered in the framework of a registration network for family practices in Flanders (INTEGO) [32]. The study area is representative of the total population. Non-smoking men and women, 60 to 80 years old, with no acute infection at enrolment and no history of malignancies, were selected in the southern region of Genk from a quarter adjacent to an industrial area where a non-ferrous metal plant, a major motor company and a power station are located and which is crossed by multiple highroads. Sampling was combined with the annual influenza vaccination at a local doctor's practice. Eligible people were notified in advance by letter. Of those that routinely are advised for influenza vaccination, approximately 86% joined the vaccination program. Of those that were eligible, 154 were recruited, and 99% agreed to participate in our study. Of the 153 persons that agreed to participate (79 women; mean age 71 yr and 74 men; mean age 70 yr), for one person no urinary sample was collected and this person was not included in the analyses. Personal information was processed anonymously in conformity with privacy policy. Informed consent was obtained from all participants and the study was approved by the ethics committee of the Ziekenhuis Oost-Limburg (ZOL), Belgium. Questionnaires were administered to assess lifestyle, profession, education, past smoking status, as well as data on age, weight and gender. Family income was given as net monthly overall family income and subdivided into low (<1500€), medium (1500€ - 3000€) and high (>3000€) family income. Education was coded as low (primary school), medium (high school) and high (university). Past smoking was quantified as pack years by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked. Finally, individual medical backgrounds were used to determine possible interference of drug administration or diseases, with kidney function. Second morning urine samples and blood samples were collected from all participants. Samples were aliquoted (6 × 2 ml), stored on ice for a maximum of four hours and subsequently frozen at -80°C.
Routine analyses and renal biomarker measurements
Routine analyses of the urine samples were performed in the clinical laboratory of the regional hospital ZOL in Genk. Using an automated analyzer (Modular® P800-ISE900 System, Roche Diagnostics; Mannheim, Germany), the following urinary analyses were performed, according to manufacturer's instructions: creatinine according to the kinetic Jaffe method (compensated, rate blanked), total protein by a colorimetric biuret test and α1M-U based on immunological agglutination. β2M-U was determined by particle-enhanced immunonephelometry using the BN ProSpec (Siemens Healthcare Diagnostics; Marburg, Germany). Microalbumin was nephelometrically determined (Immage® Immunochemistry System, Beckman Coulter; Suarlee, Belgium). Blood urea and serum creatinine were measured following the same assays as with the urinary analyses. BUN (blood urea nitrogen) was determined as blood urea times two (covering the molar mass of the two nitrogens). Urinary KIM-1 was analyzed by a commercially available sandwich ELISA: Human TIM-1/KIM-1/HAVCR Duoset (R&D Systems; Abingdon, U.K.), validated by Chaturvedi et al. [33]. The assay procedure was performed according to the prescriptions of the manufacturer. When necessary, samples were adjusted to pH 7.0 before measurement [34]. The optical density was determined with a fluorescence microplate reader (FLUOstar OPTIMA, BMG Labtechnologies; Offenburg, Germany), set to 450 nm with a wavelength correction at 540 nm. All samples were measured in duplicate.
Urinary Cd analyses
Cd concentrations in urine were analyzed by means of inductively coupled plasma mass spectrometry (ICP-MS) using the ELAN® DRC-e (Axial Field™ Technology, Perkin Elmer SCIEX; Zaventem, Belgium). Urine samples and standards were diluted 1:10 in 1% nitric acid.
Statistical analyses
For database management and statistical analyses, SAS Software version 9.1 (version 9.1, SAS Institute Inc, Cary (NC), USA) and GraphPad Prism 5.01 (GraphPad Software Inc, La Jolla (CA), USA) were used. Non-normally distributed data were log transformed. For comparison of means and proportions, we applied Student's t-test and the χ2-statistic, respectively. We investigated associations between markers of kidney function and urinary cadmium using Pearson's correlation and multiple linear regression. Estimated effect sizes and 95% CI were calculated from linear regression coefficients for a two-fold increase in urinary Cd. A priori three models were chosen: model 1 shows unadjusted data, in model 2, results are adjusted for creatinine, sex, age, past smoking, body mass index (BMI) and socio-economic status (SES; based on educational degree and monthly family income), while in model 3 data are adjusted for sex, age, past smoking, BMI and SES and given as function of creatinine. When residuals are calculated (figures), we adjusted the different parameters for creatinine, sex, age, past smoking, BMI and SES, in order to remove these potential confounding factors from the association. Correlations were considered significant when p < 0.05. All tests were two-sided.
Results
The study population consists of 153 participants (52% women) with a mean age of 71 years. Patient characteristics can be found in table 1. From all the participants, of the 54% that have ever smoked there was a significant difference between men and women (75% and 35% respectively, p < 0.0001). Those who had smoked in the past had an average of 18 pack years. The average distance between their residence and the heavy metal industrial zone was 2743 m, while the mean distance to the two main roads was 294 m and 562 m. Participants have lived at their current addresses for a mean period of 36 years (range: 3 to 75 years). Geometric mean urinary Cd level was 0.76 μg/g creatinine. Geometric mean of the urinary KIM-1 concentrations as well as the mean concentrations of other renal biomarkers (β2M-U, α1M-U, BUN, urinary proteins, microalbuminuria) and creatinine are given in table 2.
Table 1.
Participants characteristics
| Characteristics | Total group (n = 153) |
|---|---|
| Anthropometrics | |
| Sex, female | 79 (52%) |
| Age, years | 71 ± 4.5 |
| BMI, kg/m2 | 27.2 ± 4.3 |
| Socio-economic status*° | |
| Low | 62 (41%) |
| Median | 59 (39%) |
| High | 29 (19%) |
| Familial income, per month° | |
| <1500€ | 71 (47%) |
| 1500-3000€ | 77 (51%) |
| >3000€ | 2 (1%) |
| Smoking status | |
| Ex-smoker† | 81 (54%) |
| Never smoked† | 68 (46%) |
| Exposure to environmental tobacco smoke¶ | 61 (48%) |
| Use of medication§ | |
| Antiplatelet medication | 13 (9%) |
| Statins | 81 (53%) |
| ACE inhibitor | 27 (18%) |
| Insulin | 5 (3%) |
| Antidiabetic medication | 19 (13%) |
| NSAID | 23 (15%) |
| Blood analyses | |
| Hemoglobin, g/dl | 14.17 ± 1.24 |
| Red blood cells, 106/μl" | 4.75 (4.68 - 4.82) |
| White blood cells, 103/μl" | 6.58 (5.76 - 7.53) |
| Neutrophils, %" | 55.10 (53.60 - 56.66) |
| Lymphocytes, %" | 29.70 (28.35 - 31.12) |
| Monocytes, %" | 6.52 (5.76 - 7.53) |
| Eosinophils, %" | 2.79 (2.39 - 3.25) |
| Ferritin, ng/ml" | 121.9 (108.0 - 137.6) |
| CRP, mg/dl | |
| Creatinine, mg/dl" | 0.86 (0.83 - 0.89) |
| Glucose, mg/dl" | 99.95 (96.50 - 103.50) |
| Cholesterol, mg/dl | 200.7 ± 37.26 |
| HDL, mg/dl" | 56.64 (54.04 - 59.37) |
| LDL, mg/dl | 114.4 ± 34.48 |
| Triglycerides, mg/dl" | 122.1 (112.8 - 132.1) |
Data are arithmetic mean ± standard deviation or absolute number (percentage of study population);
" data which are not normally distributed and for which geometric mean (95% confidence interval) is given.
*Based on educational degree; °n = 150; †n = 149; §n = 152;¶ n = 126
Table 2.
Mean urinary cadmium and renal biomarker values
| Mean (95% CI) | |
|---|---|
| Cadmium, μg/l† | 0.80 (0.73 - 0.88) |
| Cadmium/creatinine, μg/g creat† | 0.76 (0.70 - 0.84) |
| KIM-1, pg/ml* | 569 (498 - 651) |
| KIM-1/creatinine, μg/g creat* | 0.55 (0.49 - 0.62) |
| α1-microglobulin, mg/l† | 3.19 (2.58 - 3.91) |
| α1-microglobulin/creatinine, mg/g creat† | 2.97 (2.42 - 3.64) |
| Proteins, mg/l* | 71.80 (63.81 - 80.80) |
| Proteins/creatinine, mg/g creat* | 66.61 (59.99 - 73.95) |
| Albumin, mg/dl* | 8.73 (7.46 - 10.21) |
| Albumin/creatinine, mg/g creat* | 8.43 (7.32 - 9.70) |
| β2-microglobulin, mg/l‡ | 0.12 (0.11 - 0.13) |
| β2-microglobulin/creatinine, mg/g creat‡ | 0.12 (0.11 - 0.13) |
| BUN, mg/dl† | 35.94 (34.47 - 37.46) |
| Urinary creatinine, mg/dl* | 104.9 (96.55 - 114.0) |
Geometric mean (95% confidence interval) of urinary cadmium and renal biomarkers. All parameters were measured in urine; except for blood urea nitrogen, which was measured in blood. † n = 152; * n = 153; ‡ n = 140.
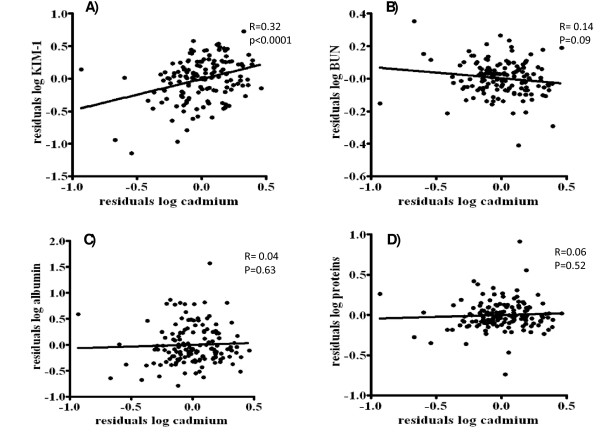
Urinary KIM-1 was not influenced by gender (p = 0.83), age (p = 0.08), distance between housing and industrial zone (p = 0.57), SES (p = 0.40), past smoking (p = 0.14) and BMI (p = 0.83). Both before (table 3 and Figure 1) and after adjustment (table 3) for sex, age, past smoking, BMI and socio-economic status (including education and income) variables, KIM-1 correlated positively and significantly with the urinary cadmium concentration. For the other biomarkers (BUN, microalbuminuria and urinary proteins) unadjusted and adjusted multiple linear regression models showed no significant correlation between these biomarkers of kidney function and urinary Cd (see Figure 1 and table 3). Both for β2M-U and α1M-U a considerable amount of urine samples that were tested (84% and 28% respectively) were below the limit of detection, suggesting the assessments that were used were not sensitive enough. Therefore, no analysis was conducted for β2M-U and α1M-U in association with Cd.
Table 3.
Estimated change (%) in urinary biomarker levels calculated for a two-fold increase in urinary cadmium concentration
| Estimated effect size (%) | 95%CI(%) | R2 | p-value | |
|---|---|---|---|---|
| Model 1 | ||||
| KIM-1 | 23.73 | 6.92 to 43.18 | 0.05 | 0.005 |
| Microalbumin | 4.40 | -13.42 to 25.89 | 0.001 | 0.65 |
| Proteins | 5.66 | -3.52 to 15.71 | 0.01 | 0.24 |
| BUN | -5.65 | -10.53 to -0.49 | 0.04 | 0.03 |
| Model 2 | ||||
| KIM-1 | 39.54 | 18.26 to 64.65 | 0.27 | 0.0001 |
| Microalbumin | 4.85 | -14.14 to 28.03 | 0.27 | 0.64 |
| Proteins | 3.15 | -6.26 to 13.52 | 0.49 | 0.53 |
| BUN | -4.66 | -9.83 to 0.80 | 0.08 | 0.10 |
| Model 3 | ||||
| KIM-1 | 26.59 | 7.82 to 48.62 | 0.13 | 0.005 |
| Microalbumin | -2.80 | -20.42 to 18.78 | 0.09 | 0.78 |
| Proteins | 0.66 | -8.65 to 10.92 | 0.11 | 0.89 |
| BUN | -3.69 | -8.99 to 1.91 | 0.07 | 0.19 |
All data were log-transformed and estimated effect size is given as % estimated effect size calculated for a two-fold increase in urinary cadmium concentrations (μg/g creatinine) with the corresponding 95% confidence interval, R2 gives the explained variance.
Abbreviations: KIM-1, kidney injury molecule 1; BUN, blood urea nitrogen; CI, confidence interval
Model 1: unadjusted but given as function of creatinine
Model 2: adjusted for creatinine (except for blood urea nitrogen), sex, age, past smoking, body mass index and socio-economic status
Model 3: adjusted for sex, age, past smoking, body mass index and socio-economic status; given as a function of creatinine.
Figure 1.
Correlations of the residuals of urinary KIM-1 and the other biomarkers with the residuals of urinary cadmium. Correlations of the residuals of A) log KIM-1, B) log blood urea nitrogen, C) log albumin and D) log proteins with the residuals of log cadmium. All parameters were measured in urine, except for blood urea nitrogen. Residuals were computed to remove the variance by age, sex, socio-economic status, body mass index, creatinine (except for blood urea nitrogen) and past smoking. Data on KIM-1, cadmium, protein, blood urea nitrogen and albumin concentrations were log-transformed to obtain a normal distribution. Abbreviations:KIM-1: kidney injury molecule 1; BUN, blood urea nitrogen.
Discussion
About a decade ago, KIM-1 was discovered in the search for molecules involved in the pathogenesis of acute kidney injury. We demonstrated among elderly a robust association between urinary KIM-1 and urinary Cd. Depending on the biomarker of nephrotoxicity thresholds of urinary Cd can range from about 2.4 μg Cd/g creatinine for the onset of early biochemical alterations (e.g. hypercalciuria) to 10 μg Cd/g creatinine for the development of the classic tubular microproteinuria [13]. Here, we showed biochemical changes at urinary Cd levels below 1 μg Cd/g creatinine.
Ichimura et al. were the first to describe KIM-1 as a type 1 membrane glycoprotein, which contains a 6-cystein immunoglobulin-like domain in its extracellular portion, and a Thr/Ser-Pro rich domain characteristic of mucin-like O-glycosylated proteins [18]. KIM-1 also has a transmembrane domain and a cytoplasmic domain, which contains a conservative tyrosine kinase phosphorylation site, indicating that KIM-1 may be a signaling molecule [19]. In healthy kidney tissue, KIM-1 is virtually undetectable whereas in the injured kidney, KIM-1 expression is rapidly upregulated at the apical side of the proximal tubule [18,21]. This process is accompanied by the shedding of the extracellular domain of KIM-1 into the urine [19]. The ectodomain is stable in urine [34] and has shown to be a sensitive biomarker of renal injury induced by a variety of agents including the heavy metal Cd: Prozialeck et al. have proven KIM-1 to be a putative early biomarker for proximal tubule damage caused by high doses of Cd in rats, outperforming the classic biomarkers such as metallothionein, CC-16, α-GST and urinary proteins [23,29,30]. In addition, KIM-1 appears before any lethal injury is detected in the proximal tubule epithelial cells [29]. Thus, using this biomarker, very early detection of cell stress may be possible, which would allow for the reversal and/or the treatment of Cd-induced kidney injury [35]. To our knowledge however, no research has ever focused on the correlation of the Cd burden and the urinary KIM-1 concentrations in humans.
Since we were mainly interested in the possible role for KIM-1 as a biomarker after long term environmental Cd intoxication, we chose a non-smoking elderly population living near a non-ferrous metal industrial zone in Genk for a longer period. In this region, levels of Cd have been reported to be higher than in other Belgian gauging regions according to the Flemish environmental agency (VMM: Mira-T indicator rapport 2008).
Both blood and urinary Cd are indicators of Cd body burden; however urinary Cd correlates better with the duration of exposure than does blood Cd [12,36,37], which makes it a better indicator for long-term Cd exposure. Therefore, we compared the urinary Cd concentrations with KIM-1 and other biomarkers of nephrotoxicity.
As shown in Figure 1, after adjustment for creatinine, sex, age, past smoking, BMI and SES, only KIM-1 was significantly correlated with Cd levels.
Urinary Cd concentration averaged below 1 μg/g creatinine among our population. This may explain why albuminuria, proteinuria and BUN did not correlate with urinary Cd. These markers mainly identify later stages of Cd-induced kidney injury [38,39].
Although α1M-U is stable across physiological pH [40], its specificity is undermined by the influence of several conditions such as liver disease [15], HIV [16], mood disorders [17] and other environmental influences, for example lead exposure [41]. In contrast to β2M-U, which is degraded rapidly in acidic urine [42], urinary KIM-1 has been proven to be stable over the physiological range of urinary pH values [34]. Moreover, it originates from proximal tubule cells [20,43], which makes it a much more specific renal biomarker than proteins originating from other parts of the body. Prozialeck et al. found in rats that urinary KIM-1 starts to increase significantly earlier and at lower doses of Cd than metallothionein, CC-16, proteins and α-GST [29,30,44]. The present study corroborates this high sensitivity for human subjects.
Since KIM-1 has a high cysteine content and Cd is known for its high binding capacity with cystein complexes [45], future research should focus on whether this influences the correlation between urinary KIM-1 and urinary Cd.
Conclusions
In conclusion, this pilot study shows that urinary KIM-1 levels are significantly correlated with urinary Cd levels in an elderly population after long-term, low-dose exposure to Cd, probably indicating beginning metal-induced kidney injury. To further elucidate the exact role KIM-1 can play as a biomarker for early cadmium-induced renal damage, future research should concentrate on the comparison of KIM-1 with other biomarkers by using the most sensitive and reliable measuring techniques, with inclusion of a paired control group, living in unpolluted areas.
List of abbreviations
Cd: cadmium; α1M-U: urinary α-1 microglobulin; α-GST: α-glutathione-S-transferase; β2M-U: urinary β-2 microglobulin; BMI: body mass index; BUN: blood urea nitrogen; CC-16-U: urinary clara cell protein-16; CI: confidence interval; ICP-MS: inductively coupled plasma mass spectrometry; KIM-1: kidney injury molecule 1; NAG: N-acetyl-beta-glucosaminidase; SES: socio-economic status; ZOL: Ziekenhuis Oost-Limburg.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
VP participated in the design of the study, the sample collection, KIM-1 measurements, the analyses of the data and the writing of the manuscript. LMD was involved in the sample collection and the KIM-1 measurements. EM participated in the design of the study and the sample collection. TN was involved in the design of the study and conducted the statistical analyses. EV and JR participated in the design of the study. CR carried out the routine analyses. HDW was involved in the design of the study and the sample collection. RC carried out the urinary cadmium analyses. JP and QS were both involved in the design of the study and the data analyses. All authors read and approved the final manuscript.
Contributor Information
Valérie Pennemans, Email: Valerie.pennemans@uhasselt.be.
Liesbeth M De Winter, Email: Liesbeth.dewinter@uhasselt.be.
Elke Munters, Email: elke.munters@uhasselt.be.
Tim S Nawrot, Email: tim.nawrot@uhasselt.be.
Emmy Van Kerkhove, Email: emmy.vankerkhove@uhasselt.be.
Jean-Michel Rigo, Email: jeanmichel.rigo@uhasselt.be.
Carmen Reynders, Email: carmen.reynders@ZOL.be.
Harrie Dewitte, Email: harrie.dewitte@gvhv.be.
Robert Carleer, Email: Robert.carleer@uhasselt.be.
Joris Penders, Email: joris.pnders@ZOL.be.
Quirine Swennen, Email: quirine.swennen@uhasselt.be.
Acknowledgements
The Environmental Health Research at Hasselt University is supported by a grant from the Flemish Scientific Fund (Krediet aan navorsers) [1.5.158.09.N.00], and transnational University Fund (tUL-impulse).
References
- Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, Ruttens A, Smeets K, Clijsters H, Vangronsveld J. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals. 2010;23:769–82. doi: 10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- Bernard A. Cadmium & its adverse effects on human health. Indian J Med Res. 2008;128:557–564. [PubMed] [Google Scholar]
- Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Lancet. 1999;353:1140–1144. doi: 10.1016/S0140-6736(98)09356-8. [DOI] [PubMed] [Google Scholar]
- Sabolic I. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol. 2006;104:107–114. doi: 10.1159/000095539. [DOI] [PubMed] [Google Scholar]
- Jarup L. Cadmium overload and toxicity. Nephrol Dial Transplant. 2002;17(Suppl 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, Ukai SH, Sakurai H. Critical evaluation of alpha1- and beta2-microglobulins in urine as markers of cadmium-induced tubular dysfunction. Biometals. 2004;17:539–541. doi: 10.1023/b:biom.0000045735.39613.52. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Lauwerys RR, Ide G, Roels HA, Vyncke G, Amery A. Renal function and historical environmental cadmium pollution from zinc smelters. Lancet. 1994;343:1523–1527. doi: 10.1016/S0140-6736(94)92936-X. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Jin T, Wu X, Lu J, Chen L, Lei L, Hong F, Nordberg M. Prevalence of kidney dysfunction in humans - relationship to cadmium dose, metallothionein, immunological and metabolic factors. Biochimie. 2009;91:1282–1285. doi: 10.1016/j.biochi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Bernard AM, Thielemans NO, Lauwerys RR. Urinary protein 1 or Clara cell protein: a new sensitive marker of proximal tubular dysfunction. Kidney Int Suppl. 1994;47:S34–37. [PubMed] [Google Scholar]
- Shaikh ZA, Ellis KJ, Subramanian KS, Greenberg A. Biological monitoring for occupational cadmium exposure: the urinary metallothionein. Toxicology. 1990;63:53–62. doi: 10.1016/0300-483X(90)90068-R. [DOI] [PubMed] [Google Scholar]
- Chen L, Jin T, Huang B, Nordberg G, Nordberg M. Critical exposure level of cadmium for elevated urinary metallothionein--an occupational population study in China. Toxicol Appl Pharmacol. 2006;215:93–99. doi: 10.1016/j.taap.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Bernard A, Roels H, Buchet JP, Cardenas A, Lauwerys R. Cadmium and health: the Belgian experience. IARC Sci Publ. 1992. pp. 15–33. [PubMed]
- Roels HA, Hoet P, Lison D. Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risks of nephrotoxicity. Ren Fail. 1999;21:251–262. doi: 10.3109/08860229909085087. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Kobayashi E, Suwazono Y, Uetani M, Oishi M, Inaba T, Kido T, Shaikh ZA, Nogawa K. Excretion of urinary cadmium, copper, and zinc in cadmium-exposed and nonexposed subjects, with special reference to urinary excretion of beta2-microglobulin and metallothionein. Biol Trace Elem Res. 2005;108:17–31. doi: 10.1385/BTER:108:1-3:017. [DOI] [PubMed] [Google Scholar]
- Vincent C, Kew MC, Bouic P, Flacher M, Revillard JP. Alpha 1-microglobulin (HC protein) in human hepatocellular carcinoma. Br J Cancer. 1989;59:415–416. doi: 10.1038/bjc.1989.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T, Schmechta H, Hentschel C, Doepel H, Pas P, Becker J, Pergande M, Jung K, Nugel E. Development of an immunoenzymometric assay for alpha 1-microglobulin and measurement of its serum concentration in normal and HIV-infected persons. J Clin Chem Clin Biochem. 1990;28:669–675. [PubMed] [Google Scholar]
- Shikimi T, Kaku K, Uegaki J, Inagaki T, Seno H, Ishino H, Takaori S. Serum contents of the free forms of alpha(1)-microglobulin and ulinastatin: relation to diseased states in patients with mood disorders. Neuropsychobiology. 2001;43:145–149. doi: 10.1159/000054883. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- Amin RP, Vickers AE, Sistare F, Thompson KL, Roman RJ, Lawton M, Kramer J, Hamadeh HK, Collins J, Grissom S, Bennett L, Tucker CJ, Wild S, Kind C, Oreffo V, Davis JW, Curtiss S, Naciff JM, Cunningham M, Tennant R, Stevens J, Car B, Bertram TA, Afshari CA. Identification of putative gene based markers of renal toxicity. Environ Health Perspect. 2004;112:465–479. doi: 10.1289/ehp.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, Miller TJ, Bonventre JV, Goering PL. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–170. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, Bonventre JV, Jaber BL. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Boventre JV. Urinary Biomarkers for Sensitive and Specific Detection of Acute Kidney Injury in Humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries AP, Gans RO, van Goor H, Stegeman CA, Bonventre JV, Bakker SJ. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation. 2007;84:1625–1630. doi: 10.1097/01.tp.0000295982.78039.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol. 2009. pp. 306–314. [DOI] [PMC free article] [PubMed]
- Prozialeck WC, Edwards JR, Vaidya VS, Bonventre JV. Preclinical Evaluation of Novel Urinary Biomarkers of Cadmium Nephrotoxicity. Toxicol Appl Pharmacol. 2009;238:301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR. Early biomarkers of cadmium exposure and nephrotoxicity. Biometals. 2010;23:793–809. doi: 10.1007/s10534-010-9288-2. [DOI] [PubMed] [Google Scholar]
- Bartholomeeusen S, Kim CY, Mertens R, Faes C, Buntinx F. The denominator in general practice, a new approach from the Intego database. Fam Pract. 2005;22:442–447. doi: 10.1093/fampra/cmi054. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennemans V, De Winter LM, Faes C, Van Kerkhove E, Reynders C, Rigo JM, Swennen Q, Penders J. Effect of pH on the stability of kidney injury molecule 1 (KIM-1) and on the accuracy of its measurement in human urine. Clin Chim Acta. 2010;411:2083–2086. doi: 10.1016/j.cca.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Wu I, Parikh CR. Screening for kidney diseases: older measures versus novel biomarkers. Clin J Am Soc Nephrol. 2008;3:1895–1901. doi: 10.2215/CJN.02030408. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Ohashi F, Fukui Y, Sakuragi S, Moriguchi J. Closer correlation of cadmium in urine than that of cadmium in blood with tubular dysfunction markers in urine among general women populations in Japan. Int Arch Occup Environ Health. 2010;84:121–9. doi: 10.1007/s00420-010-0527-1. [DOI] [PubMed] [Google Scholar]
- WHO. Air quality guidelines for Europe. WHO Reg Publ Eur Ser. 2000;V-X:1–273. [PubMed] [Google Scholar]
- Kobayashi E, Suwazono Y, Uetani M, Inaba T, Oishi M, Kido T, Nishijo M, Nakagawa H, Nogawa K. Estimation of benchmark dose as the threshold levels of urinary cadmium, based on excretion of total protein, beta2-microglobulin, and N-acetyl-beta-D-glucosaminidase in cadmium nonpolluted regions in Japan. Environ Res. 2006;101:401–406. doi: 10.1016/j.envres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28(5):478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payn MM, Webb MC, Lawrence D, Lamb EJ. Alpha1-microglobulin is stable in human urine ex vivo. Clin Chem. 2002;48:1136–1138. [PubMed] [Google Scholar]
- Endo G, Konishi Y, Kiyota A, Horiguchi S. Urinary alpha 1 microglobulin in lead workers. Bull Environ Contam Toxicol. 1993;50:744–749. doi: 10.1007/BF00194671. [DOI] [PubMed] [Google Scholar]
- Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals. 2004;17:519–523. doi: 10.1023/b:biom.0000045731.75602.b9. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR. Cell adhesion molecules in chemically-induced renal injury. Pharmacol Ther. 2007;114:74–93. doi: 10.1016/j.pharmthera.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottari E, Festa MR. On the behaviour of cysteine as ligand of cadmium(II) Talanta. 1997;44:1705–1718. doi: 10.1016/S0039-9140(97)00015-5. [DOI] [PubMed] [Google Scholar]