Abstract
Introduction
Previously we reported that paracrine actions likely mediated the therapeutic effects of adipose tissue-derived stem cells (ADSC) on a rat model of cavernous nerve (CN) injury.
Aim
To identify potential neurotrophic factors in ADSC’s secretion, test the most promising one, and identify the molecular mechanism of its neurotrophic action.
Methods
Rat major pelvic ganglia (MPG) were cultured in conditioned media of ADSC and penile smooth muscle cells (PSMC). Cytokine expression in these two media was probed with a cytokine antibody array. CXCL5 cytokine was quantified in these two media by enzyme-linked immunosorbent assay (ELISA). Activation of JAK/STAT by CXCL5 was tested in neuroblastoma cell lines BE(2)C and SH-SY5Y as well as in Schwann cell line RT4-D6P2T by western blot. Involvement of CXCL5 and JAK/STAT in ADSC-conditioned medium’s neurotrophic effects was confirmed with anti-CXCL5 antibody and JAK inhibitor AG490, respectively.
Main Outcome Measures
Neurotrophic effects of ADSC and PSMC-conditioned media were quantified by measuring neurite length in MPG cultures. Secretion of CXCL5 in these two media was quantified by ELISA. Activation of JAK/STAT by CXCL5 was quantified by densitometry on western blots for STAT1 and STAT3 phosphorylation.
Results
MPG neurite length was significantly longer in ADSC than in PSMC-conditioned medium. CXCL5 was secreted 8 times higher in ADSC than in PSMC-conditioned medium. Anti-CXCL5 antibody blocked the neurotrophic effects of ADSC-conditioned medium. CXCL5 activated JAK/STAT concentration-dependently from 0 to 50 ng/ml in RT4-D6P2T Schwann cells. At 50 ng/ml, CXCL5 activated JAK/STAT time-dependently, peaking at 45 min. AG490 blocked these activities as well as the neurotrophic effects of ADSC-conditioned medium.
Conclusions
CXCL5 was secreted by ADSC at a high level, promoted MPG neurite growth, and activated JAK/STAT in Schwann cells. CXCL5 may contribute to ADSC’s therapeutic efficacy on CN injury-induced ED.
Keywords: Adipose tissue-derived stem cells, CXCL5 cytokine, cavernous nerve regeneration, erectile dysfunction, JAK/STAT
INTRODUCTION
Postoperative erectile dysfunction (ED) frequently occurs in patients who received surgical or radiation therapy for their prostate, bladder, or rectal cancers [1-3]. The cause for this medical condition is inadvertent injury to the nearby cavernous nerves (CN) that innervate the erectile tissue in the penis. While several management options exist to address this medical problem, they are not intended to and do not help CN recovery [4]. To explore potential treatment options that involve CN regeneration, we have developed a tissue culture system in which promising neurotrophic agents were tested for their ability to stimulate neurite growth from the CN region in the rat major pelvic ganglion (MPG) [5-10]. In addition, we have tested several gene and stem cell therapy strategies in various CN injury animal models [11-15]. In regard to stem cell therapy, our research with adipose tissue-derived stem cells (ADSC) led us to speculate that its therapeutic efficacy is likely through paracrine actions [16]. To test this hypothesis, we initiated the present study in which ADSC were found to outperform penile smooth muscle cells (PSMC) in our MPG culture system. We then compared these two cell systems for their secreted cytokine profile, and found that, among the 19 different cytokines examined, CXC ligand 5 (CXCL5) was secreted 8 times more abundantly by ADSC than by PSMC.
CXCL5 is known as epithelial neutrophil-activating peptide-78 (ENA-78) in humans and as lipopolysaccharide-induced chemokine (LIX) in rodents. As these names indicate, CXCL5 expression by epithelial cells has been frequently observed in conjunction with inflammatory responses [17]. For example, it has been shown that CXCL5 expression in prostate epithelial cells was associated with the presence of granulocytic inflammatory cells in the prostate coincident with benign prostatic enlargement [18]. Furthermore, CXCL5 has been shown to activate the JAK/STAT pathway in prostate epithelial cells in a concentration-dependent manner [18].
The JAK/STAT pathway is of special interest to us because we have previously shown that it mediates the neurotrophic effects of brain-derived neurotrophic factor (BDNF) in our MPG cultures [6-8]. However, we recognize that JAK/STAT is a cytokine signaling pathway [19] and therefore its activation by BDNF is probably indirect. To test this hypothesis, we have performed a series of experiments, one of which showed that BDNF activated JAK/STAT in Schwann cells but not in neuroblastoma cells. However, in other experiments aiming at the identification of the candidate cytokines, we have obtained only partially satisfactory results. As such, these data have remained unpublished. In contrast, in the present study we obtained consistent and reproducible results showing the activation of JAK/STAT in Schwann cells by CXCL5. These results, together with the MPG data, led us to conclude that CXCL5 is a neurotrophic cytokine which contributes to ADSC’s therapeutic effects in our CN injury ED models.
MATERIALS AND METHODS
Cell Culture
Rat ADSC and PSMC were isolated and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Cell Culture Facility, University of California San Francisco) supplemented with 10% fetal bovine serum (FBS) as previously described [20,21]. These cells at passage 6 were used throughout this study. Human neuroblastoma cell lines BE(2)C and SH-SY5Y as well as rat Schwann cell line RT4-D6P2T were purchased from American Type Culture Collection (ATCC, Manassas, VA). They were cultured in RPMI-1640 (Cell Culture Facility, University of California San Francisco) supplemented with 10% FBS.
MPG Culture
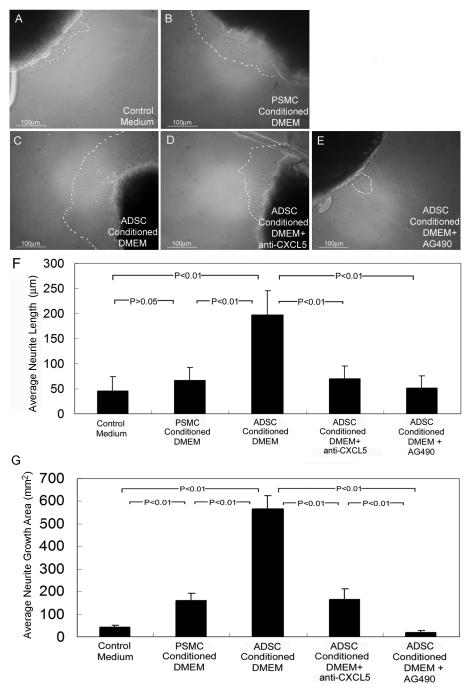
Fifteen 8-weeks-old male Sprague-Dawley rats were used in this study. All animal care, treatments and procedures were approved by our University’s Institutional Animal Care and Use Committee. The rats were killed with an i.p. injection of sodium pentobarbital (200 mg/kg) followed by bi-lateral thoracotomy. The dissection and MPG culture were as previously described [5-10]. Briefly, bilateral MPG from each rat were isolated and excised intact. After a rinse in PBS, each isolated MPG was further dissected to isolate the dorsocaudal region (DCR, from which the cavernous nerve originates) of the MPG. Each DCR was cut into three pieces of similar size; this yielded a total of 90 specimens. Each specimen was then embedded in a 40-μL drop of growth factor reduced Matrigel (01730, BD Biosciences, Bedford, MA) and treated with 3 ml of one of the following five different media: (1) control medium (DMEM without FBS), (2) ADSC-conditioned DMEM, (3) PSMC-conditioned DMEM, (4) ADSC-conditioned DMEM+4.0μg/ml anti-CXCL5 antibody (DY543, R&D Systems, Inc., Minneapolis, MN), and (5) ADSC-conditioned DMEM+100μM AG490 (PHZ1204, Biosource international, Inc., Camarillo, CA). The culture was maintained at 37°C in a humidified atmosphere with 5% CO2 for 48 h. Neurite growth from the MPG fragments was photographed at ×100 with a Nikon DXM1200 digital still camera attached to a Zeiss Axiovert microscope, using ACT-1 software (Nikon Instruments Inc., Melville, NY). The digitized images were then analyzed with ChemiImager 4000 (Alpha Innotech Corporation, San Leandro, CA) and Image-Plus 5.1 (Media Cybernetics, Bethesda, MD). Neurite growth was determined by averaging the lengths of the five longest neurites from each MPG fragment. Alternatively neurite growth was determined by averaging the growth areas in each MPG fragment. The growth areas on the digital images were traced as shown in Fig. 1; their dimensions were then quantified by Image-Plus 5.1.
Figure 1.
ADSC secretion promotes MPG neurite growth.
Rat MPG dorsocaudal region was cultured in plain DMEM (A, Control Medium) or PSMC-conditioned DMEM (B), or in ADSC-conditioned DMEM (C) plus anti-CXCL5 antibody (D) or AG490 (E). For clarity, outgrowth of neurites was outlined by dashed lines. For quantification, the lengths of the 5 longest neurites in each of 18 specimens per treatment group were measured and their average shown on the Y-axis in Panel F. Alternatively quantification was done by measuring the neurite growth areas in each of 18 specimens per treatment group, the averages of which are shown on the Y-axis in Panel G.
Cytokine Antibody Array
An antibody-based cytokine array system (RayBio Rat Cytokine Antibody Array 1, RayBiotech, Norcross, GA) was used to detect the levels of cytokines and growth factors in ADSC or PSMC-conditioned medium. Briefly, cells were cultured in 10% FBS-supplemented DMEM in 6-well dish to 80% confluence. The culture medium was then changed to serum-free DMEM medium. Twenty-four h later, the medium was collected and centrifuged at 13,000×g for 10 s. The supernatant was recovered and used in the array experiment as follows. The array membrane was placed in an eight-well tray and incubated in 4 ml of 1X blocking buffer for 45 min at room temperature (RT). Each test sample (2 ml of cell culture medium) was added to the membrane, which was then shaken for 2 h at RT. After washing the membrane three times, primary antibody cocktail was added and the reaction was allowed to proceed for 1 h at RT on shaker. After washing the membrane three times, secondary antibody was added and the reaction was allowed to proceed for 1 h at RT on shaker. Finally, the membrane was washed as above, incubated in ECL detection regents (Amersham Life Sciences Inc., Arlington Heights, IL) for 5 min, and exposed to Kodak autoradiography film for 1–15 min. The resulting images were analyzed with ChemiImager 4000 to determine the integrated density value of each protein spot.
Quantification of CXCL5 Secretion
ADSC and PSMC were seeded in DMEM with 10% FBS at 1 × 105 cells per well in 6-well culture plates. When 90% confluence was reached, the culture medium was removed and replaced with 1ml of DMEM without FBS. Twenty-four h later, the medium was harvested and centrifuged at 1200 rpm for 10 min. The supernatant was recovered and stored at −80 °C until use. To ensure unbiased assessment, all of the processed culture media (supernatants) were simultaneously subjected to ELISA for CXCL5 with a commercial kit (R&D Systems, Minneapolis, MN). To generate statistically relevant data, each cell medium was subjected to three independent ELISA experiments and each ELISA experiment included triplicates of each cell medium.
CXCL5 Treatment of Neuroblastoma and Schwann Cells
BE(2)C, SH-SY5Y, and RT4-D6P2T cells were seeded in RPMI-1640 with 10% FBS at 3 × 106 cells per well in 10-cm culture dishes in a 5% CO2 atmosphere at 37°C. When 90% confluence was reached, the medium was replaced by RPMI-1640 without FBS for 8 h. To assay the dosage effects of CXCL5 on the activation of JAK/STAT pathway, rat CXCL5 (543-RL, R&D Systems, Inc., Minneapolis, MN) was added to a final concentration of 0, 5, or 50 ng/ml for 45 minutes. To determine the time response, RT4-D6P2T cells were treated with 50 ng/ml rat CXCL5 for 0, 10, 30, 45, and 60 minutes. To demonstrate the specific involvement of the JAK/STAT pathway, 100 nM of specific inhibitor AG490 (PHZ1204, Biosource international, Inc., Camarillo, CA) was added to the RT4-D6P2T cell culture for 1 h before prior to the addition of rat CXCL5.
Western Blot
Cells were lysed in a PBS buffer containing 1% IGEPAL, 0.5% sodium deoxycholate, 0.1% SDS, aprotinin (10 μg/ml), leupeptin (10 μg/ml), 0.1 mM Na3VO4 and 0.1mM NaF. The homogenate was centrifuged at 3000 rpm for 15 min, and the supernatant was recovered as protein sample, which was measured for protein concentration by the BCA method (Pierce Chemical Company, Rockford, IL, USA). Cell lysates containing 20 μg of protein were electrophoresed in SDS-PAGE and then transferred onto PVDF membrane (Millipore Corp., Bedford, MA, USA). The membrane was incubated for 1 h with either anti-STAT1 (610115, BD Biosciences, San Diego, CA) or anti-STAT3 (610189, BD Bioscience). Detection of the reactive protein on the membrane was performed with the ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL), followed by exposure to X-ray films. Thereafter, the STAT1-probed membrane was re-probed with anti-phospho-STAT1 (612232, BD Bioscience), and the STAT3-probed membrane with anti-phospho-STAT3 (612356, BD Bioscience). Finally, all membranes were re-probed with anti-β-Actin for internal control. The resulting images were analyzed with ChemiImager 4000 to determine the integrated density value of each protein band. Before each re-probing, the membrane was stripped in 62.5 mmol/l TRIS HCl (pH 6.7), 2% SDS, 10 mmol/L 2-mercaptoethanol at 55°C for 30 min, and then washed four times in 1XTBS.
Statistical Analysis
Data was analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA, USA) and expressed as mean ± standard error of the mean for continuous variables. The continuous data was compared among the groups using one-way analysis of variance. The Tukey-Kramer test was used for post-hoc comparisons. Statistical significance was set at p < 0.05.
RESULTS
ADSC-Secreted Factors Promote Neurite Growth in Cultured MPG
To determine whether ADSC secrete neurotrophic factors, we tested ADSC-conditioned medium in a MPG culture system that has been used in several of our previous studies [5-10]. As controls and for comparison, we also tested plain culture medium and PSMC-conditioned medium, respectively. The results show that ADSC-conditioned medium was significantly more potent than control medium or PSMC-conditioned medium in promoting neurite growth from MPG (Fig. 1A-C, F, &G). As we later identified the involvement of CXCL5 and the JAK/STAT pathway (see below), we also tested and confirmed the inhibitory effects of anti-CXCL5 antibody and JAK inhibitor AG490 (Fig. 1D-G).
ADSC Secrete CXCL5 at a High Level
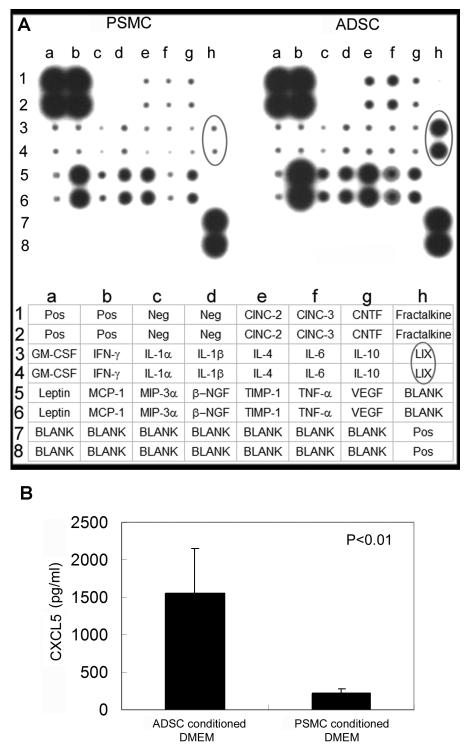
To determine which secreted factors were responsible for ADSC’s neurotrophic effects, we probed ADSC-conditioned medium with a cytokine antibody array, using PSMC-conditioned medium as control. The results show that, compared to PSMC, ADSC secreted higher levels of LIX (CXCL5), TNF-α, CINC-2, CINC-3, TIMP-1, and MCP-1 (Fig. 2A). Since CXCL5 appeared to be the most differently expressed cytokine between ADSC and PSMC, we further determined the concentration of CXCL5 in these two conditioned media. The results show that ADSC secreted approximately 8 times more CXCL5 than PSMC (Fig. 2B). Furthermore, the addition of an anti-CXCL5 antibody to ADSC-conditioned medium effectively abolished this medium’s neurotrophic effects (Fig. 1D, F, &G).
Figure 2.
ADSC secrete CXCL5 at a high level.
(A) Cytokine expression in the conditioned media of ADSC and PSMC was detected by the RayBio Rat Cytokine Antibody Array, whose key is shown at the bottom. Cytokine LIX (CXCL5) is circled in the arrays and in the key. (B) CXCL5 secretion in the conditioned media of ADSC and PSMC was quantified by ELISA. Each bar represents the average of 3 independent experiments and each measurement was done in triplicates.
CXCL5 Promotes MPG Neurite Growth through the JAK/STAT Pathway
CXCL5 has been shown to signal through the JAK/STAT pathway in prostate epithelial cells [18], and previously we have shown that brain-derived neurotrophic factor (BDNF) promotes MPG neurite growth through this pathway [6]. As such, in the present study we examined the involvement of this pathway by using JAK inhibitor AG490 in ADSC-conditioned medium. Because the MPG fragment is much larger than cells, a relatively high concentration of AG490 (100 μM) was used. The results show that AG490 effectively abolished ADSC-conditioned medium’s neurotrophic effects (Fig. 1E-G).
CXCL5 Activates the JAK/STAT Pathway in Schwann Cells
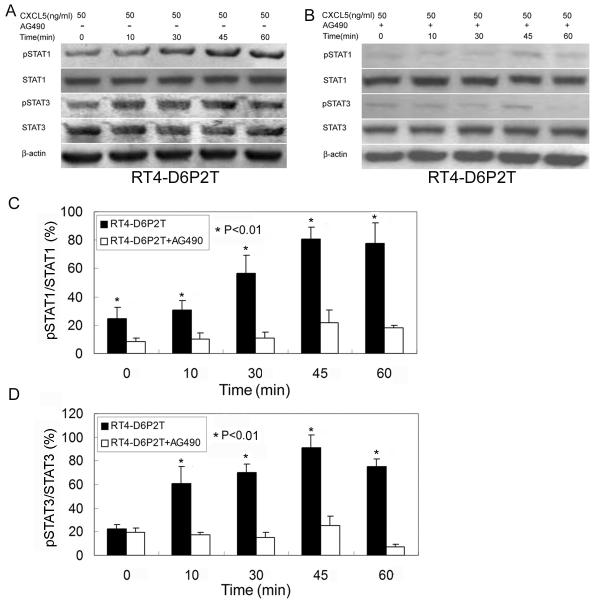
To examine CXCL5’s neurotrophic effects at the cellular and molecular levels, we tested whether it can activate the JAK/STAT pathway in neuroblastoma cells and Schwann cells. The results show that, while having no effect on neuroblastoma cells BE(2)C or SH-SY5Y, CXCL5 dose-dependently activated the JAK/STAT pathway (phosphorylation of STAT-1 and STAT-3) in Schwann cells RT4-D6P2T (Fig. 3). At the optimal dosage of 50 ng/ml, CXCL5 activated the JAK/STAT pathway in RT4-D6P2T cells in a time-dependent manner, increasingly from 0 to 45 min and dipping at 60 min (Fig. 4). Importantly, these activities were effectively suppressed by AG490.
Figure 3.
CXCL5 activates JAK/STAT in Schwann cells.
(A-C) Neuroblastoma cell lines BE(2)C (A) and SH-SY5Y (B) and Schwann cell line RT4-D6P2T (C) were treated with CXCL5 at 0, 5, and 50 ng/ml for 45 min and then analyzed by western blot for the expression of STAT1, phosphorylated STAT1 (pSTAT1), STAT3, and phosphorylated STAT3 (pSTAT3). β-actin served as control. (D) The ratio (in percentile) of pSTAT1 versus STAT1 expression for each treatment was determined by dividing the densitometric values of these two protein bands obtained from the western blots. Each bar represents the average of 3 independent experiments. (E) Same as in (D) except that the proteins are pSTAT3 versus STAT3.
Figure 4.
CXCL5 activates JAK/STAT time-dependently and with specificity.
(A) Schwann cell line RT4-D6P2T was treated with CXCL5 at 50 ng/ml for the indicate time and then analyzed by western blot for the expression of STAT1, phosphorylated STAT1 (pSTAT1), STAT3, and phosphorylated STAT3 (pSTAT3). β-actin served as control. (B) Same as in (A) except that AG490 was added to a final concentration of 100 nM prior to the addition of CXCL5. (C) The ratio (in percentile) of pSTAT1 versus STAT1 expression for each treatment was determined by dividing the densitometric values of these two protein bands obtained from the western blots. Each bar represents the average of 3 independent experiments. (E) Same as in (C) except that the proteins are pSTAT3 versus STAT3.
DISCUSSION
Postoperative, particularly postprostatectomy ED is currently an active research area in sexual medicine, and like many other medical problems, is being investigated as a potential target for stem cell therapy. In 2004 we first reported that injection of embryonic stem cells transfected with BDNF into the MPG or corpora cavernosa significantly improved erectile function in a rat CN injury model [22]. In 2008 Fall et al [23] reported the therapeutic efficacy of intracavernous injection of bone marrow stem cells. Recently we reported that intracavernous injection of either ADSC or ADSC lysate improved erectile function [16]. While this latest study was interpreted as evidence for the paracrine action of ADSC, we recognized that cell lysate cannot be equated with cell secretion. As such, we designed the present study to address this deficiency. In the first experiment using the MPG culture we confirmed our hypothesis that ADSC could secret neurotrophic factors for the potential regeneration of CN. In the next experiment using the cytokine array we, based on quantitative differences between ADSC and PSMC secreted cytokines, identified CXCL5 as a promising candidate. While there were several other cytokines that were also differentially secreted by these two cell cultures, CXCL5’s importance was affirmed by the blocking effect of anti-CXCL5 antibody on the MPG culture.
CXCL5 has been well characterized as a pro-angiogenic cytokine [24]; however, to our knowledge, it has not been shown to have neurotrophic effects. To relate CXCL5 as being neurotrophic, we first recognized that it has been shown to signal through JAK/STAT pathway in prostate epithelial cells [18]. We then connected this pathway with our previous study in which BDNF was shown to enhance MPG neurite growth by activating JAK/STAT [7]. In actual experimentation we chose two neuroblastoma cell lines and one Schwann cell line, knowing that cytokines can promote axonal regeneration through Schwann cells [25]. Indeed, activation of JAK/STAT was observed in Schwann cells but not the neuroblastoma cells, and the activation of JAK/STAT in Schwann cells was effectively blocked by JAK inhibitor AG490. Furthermore, AG490 also effectively blocked the neurite-enhancing property of ADSC’s secretion. Thus, together with anti-CXCL5 antibody’s blocking effect, these results all point to CXCL5 as being neurotrophic and which was mediated by the JAK/STAT pathway in Schwann cells.
To further confirm CXCL5’s importance in ADSC’s therapeutic potential, we have been conducting two lines of research. One is the characterization of CXCL5 and its receptor (CXCR2) in ADSC and in adipose tissue, the other is testing the possibility of suppressing CXCL5 expression or action and correlating this with a possible reduction of ADSC’s therapeutic efficacy. While the study of the CXCL5/CXCR2 system in ADSC and in adipose tissue has been submitted to a stem cell journal, that of downregulating CXCL5 is only at the beginning. Inasmuch as the present study is concerned, we provide the first demonstration of CXCL5’s neurotrophic property and an explanation for ADSC’s therapeutic effects on CN injury-related ED. However, it should be once more pointed out that our cytokine array data have identified additional candidate cytokines (e.g., MCP-1 and CINC-3), which, while not as drastically upregulated in ADSC as CXCL5, may also contribute to ADSC’s therapeutic effects. In addition, we recognize that a larger cytokine array with 34 cytokines has recently been offered, and it may thus permit the identification of other candidate cytokines as well.
CONCLUSIONS
ADSC secrete CXCL5, which promoted MPG neurite growth and activated JAK/STAT in Schwann cells. CXCL5 may contribute to ADSC’s therapeutic efficacy on CN injury-induced ED.
ACKNOWLEDGMENTS
This work was supported by Mr. Arthur Rock, the Rock Foundation, and the National Institutes of Health (DK045370).
REFERENCES
- 1.Celentano V, Fabbrocile G, Luglio G, Antonelli G, Tarquini R, Bucci L. Prospective study of sexual dysfunction in men with rectal cancer: feasibility and results of nerve sparing surgery. Int J Colorectal Dis. doi: 10.1007/s00384-010-0995-5. [DOI] [PubMed] [Google Scholar]
- 2.Hekal IA, El-Bahnasawy MS, Mosbah A, El-Assmy A, Shaaban A. Recoverability of erectile function in post-radical cystectomy patients: subjective and objective evaluations. Eur Urol. 2009;55:275–283. doi: 10.1016/j.eururo.2008.06.072. [DOI] [PubMed] [Google Scholar]
- 3.Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010;7:1687–1698. doi: 10.1111/j.1743-6109.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- 4.Miles CL, Candy B, Jones L, Williams R, Tookman A, King M. Interventions for sexual dysfunction following treatments for cancer. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005540.pub2. CD005540. [DOI] [PubMed] [Google Scholar]
- 5.Lin G, Chen KC, Hsieh PS, Yeh CH, Lue TF, Lin CS. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int. 2003;92:631–635. doi: 10.1046/j.1464-410x.2003.04439.x. [DOI] [PubMed] [Google Scholar]
- 6.Bella AJ, Lin G, Tantiwongse K, Garcia M, Lin CS, Brant W, Lue TF. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part I. J Sex Med. 2006;3:815–820. doi: 10.1111/j.1743-6109.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med. 2006;3:821–827. doi: 10.1111/j.1743-6109.2006.00292.x. discussion 828-829. [DOI] [PubMed] [Google Scholar]
- 8.Bella AJ, Lin G, Garcia MM, Tantiwongse K, Brant WO, Lin CS, Lue TF. Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signalling pathway in the major pelvic ganglion of the rat after cavernous nerve transection. Eur Urol. 2007;52:574–580. doi: 10.1016/j.eururo.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Lin G, Shindel AW, Fandel TM, Bella AJ, Lin CS, Lue TF. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 2010;105:114–120. doi: 10.1111/j.1464-410X.2009.08647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shindel AW, Xin ZC, Lin G, Fandel TM, Huang YC, Banie L, Breyer BN, Garcia MM, Lin CS, Lue TF. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J Sex Med. 2010;7:1518–1528. doi: 10.1111/j.1743-6109.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CS, Lue TF. Stem cells in urology: how far have we come? Nat Clin Pract Urol. 2008;5:521. doi: 10.1038/ncpuro1219. [DOI] [PubMed] [Google Scholar]
- 12.Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Recent advances in andrology-related stem cell research. Asian J Androl. 2008;10:171–175. doi: 10.1111/j.1745-7262.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 13.Lin G, Banie L, Ning H, Bella AJ, Lin CS, Lue TF. Potential of adipose-derived stem cells for treatment of erectile dysfunction. J Sex Med. 2009;6(Suppl 3):320–327. doi: 10.1111/j.1743-6109.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bella AJ, Lin G, Lin CS, Hickling DR, Morash C, Lue TF. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;6(Suppl 3):347–352. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CS. Advances in Stem Cell Therapy for the Lower Urinary Tract. World J. Stem Cells. 2010;2:1–4. doi: 10.4252/wjsc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of Adipose Tissue-Derived Stem Cells and Stem Cell Lysate Improve Recovery of Erectile Function in a Rat Model of Cavernous Nerve Injury. J Sex Med. doi: 10.1111/j.1743-6109.2010.01875.x. (Online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walz A, Schmutz P, Mueller C, Schnyder-Candrian S. Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol. 1997;62:604–611. doi: 10.1002/jlb.62.5.604. [DOI] [PubMed] [Google Scholar]
- 18.Begley LA, Kasina S, Mehra R, Adsule S, Admon AJ, Lonigro RJ, Chinnaiyan AM, Macoska JA. CXCL5 promotes prostate cancer progression. Neoplasia. 2008;10:244–254. doi: 10.1593/neo.07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 20.Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, Lue TF, Lin CS. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–1400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang R, Huang YC, Lin G, Wang G, Hung S, Dai YT, Sun ZY, Lue TF, Lin CS. Lack of direct androgen regulation of PDE5 expression. Biochem Biophys Res Commun. 2009;380:758–762. doi: 10.1016/j.bbrc.2009.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, Lue TF. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–909. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 23.Fall PA, Izikki M, Tu L, Swieb S, Giuliano F, Bernabe J, Souktani R, Abbou C, Adnot S, Eddahibi S, Yiou R. Apoptosis and Effects of Intracavernous Bone Marrow Cell Injection in a Rat Model of Postprostatectomy Erectile Dysfunction. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 24.Moldobaeva A, Baek A, Eldridge L, Wagner EM. Differential activity of pro-angiogenic CXC chemokines. Microvasc Res. 2010;80:18–22. doi: 10.1016/j.mvr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogata T, Yamamoto S, Nakamura K, Tanaka S. Signaling axis in schwann cell proliferation and differentiation. Mol Neurobiol. 2006;33:51–62. doi: 10.1385/mn:33:1:051. [DOI] [PubMed] [Google Scholar]