Abstract
A research strategy which integrates known biological aspects of schizophrenia is proposed. The strategy includes genotype and phenotype components and emphasizes interactions. Its central feature is the comprehensive diagnostic assessment of patients with schizophrenia. Clinical and laboratory based methodologies are applied within the genotype and phenotype components of the strategy. Examples of research from each area and the potential interactions with other aspects of the strategy are presented. The expectation is that a greater understanding of the pathophysiology of schizophrenia will result from the application of the genotype-phenotype strategy and that consequently more efficacious treatments will ultimately be developed.
Understanding the pathophysiology of schizophrenia continues to be one of the major challenges in psychiatric medicine. Revolutionary advances have been made in biomedical science over the past 15 years, and these are now available for application to schizophrenia research. However, with limited financial resources available, the development of a strategy to apply these findings in a coherent, thoughtful manner is a fundamental requirement for success. A genotype-phenotype strategy is proposed, based on integration of current knowledge about schizophrenia and progress in other fields using new approaches. Implicit in our proposal is belief that major advances in the treatment of schizophrenia will be made through increased understanding of the mechanism of the illness. However, the principles of the genotype-phenotype strategy could also be applied directly to research on therapeutics.
An outline of the genotype-phenotype strategy is illustrated in Figure 1. The common central axis consists of pathophysiology, the understanding of which leads to improvements in treatment and preventive measures applicable to patients. Brain pathophysiology is the process of faulty interaction between brain genes, their protein products, experience and the environment, which can lead to illness.
Figure 1.
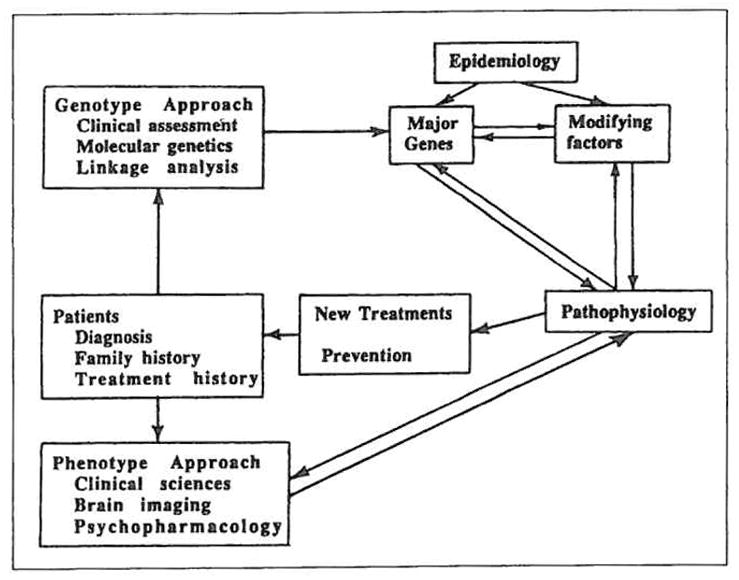
Schematic of the genotype-phenotype strategy. Patients from the central core. The goal of research is to improve treatment and prevent illness. Both genotype and phenotype approaches interact through the understanding of pathophysiology to result in such improvements.
The proposed strategy includes genotype and phenotype components. In this context, the genotype component refers to study of elements of an individual’s genetic composition which predispose to illness. These may include genetic abnormalities as well as normal genes which may interact with genes predisposing him to disease. Investigations included in this component range from clinical studies of inheritance patterns and the genetic epidemiology of schizophrenia to laboratory studies involving high resolution mapping and sequencing of genes. The phenotype component of the strategy refers to studying the manifestations of the illness in a patient. These elements represent gene products and the interactive effects of experience and environment. Phenotype component studies range from clinical studies designed to define subgroups of patients based on particular signs and symptoms to laboratory investigations of the protein products of genes in postmortem brain tissues.
The genotype-phenotype strategy is essentially an interactional model into which various research findings can be fit. The strategy provides a framework for interpreting research results and guidance for the development of future studies. Following a brief discussion of methodological aspects, examples from the authors research will be presented, with discussion of the utility of the genotype-phenotype strategy in the study of schizophrenia.
Methodology
Comprehensive Diagnostic Assessment
Accurate diagnosis of mental disorders is fundamental to the success of any research strategy for schizophrenia (1). However, there is no consensus on the elements to be included in the assesment of schizophrenic patients (see Figure 2). Clearly, the evalutation should include psychiatric and medical diagnostic assessments, family history, treatment history, and an account of the course of the illness. The assessment should include interviews of patients performed by a psychiatrist. While this may seem obvious, this essential component of the research design involves a significant cost, both of professional time and of financial resources. Studies of patients with schizophrenia often do not include a face-to-face psychiatric examination. Bertelsen (1) has emphasized the importance of such a clinical diagnostic assessment and notes the limitations of relying exclusively on lay interviewers even when highly structured interviews are used. A psychiatric interview provides an opportunity to observe the patient, conduct a structured diagnostic assessment (2,3) and perform a formal examination of the patient’s mental status. This examination may include assessment of cognitive function (4) and movement disorders (5). It is important to use a consistent method for recording information.
Figure 2.
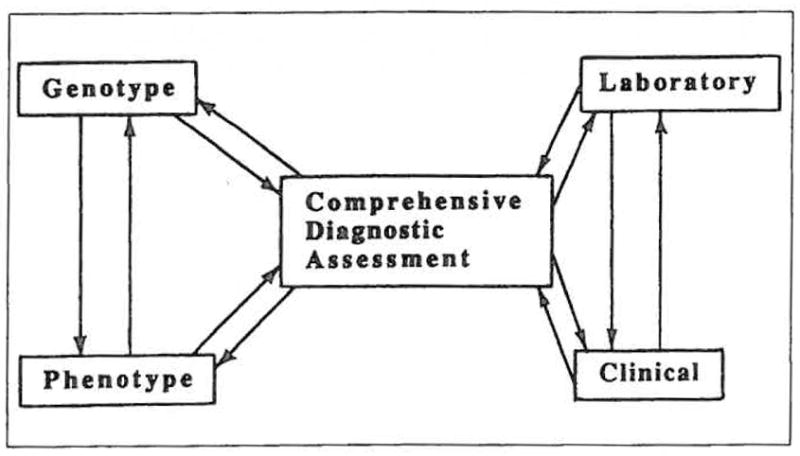
Schematic of methodologies used with the genotype-phenotype strategy. Comprehensive diagnostic assessment is the core. The clinical and laboratory components of both the genotype and phenotype approaches are interactive, as are the approaches themselves.
Clinical Approaches
The origins of progress in medical research often differ from those in the more experimentally based sciences. Medical research is characterized by having a significant basis in astute observations made by clinicians experienced in the care of patients. Such observations may be directly related to pathophysiology; however, they more frequently contribute to characterizing subgroups of patients in which pathophysiology may be more homogeneous. In the framework of the genotype-phenotype strategy, an example of the application of genotype-clinical methods was the demonstration of pedigrees where father to son transmission of bipolar illness does not occur, rather the illness is inherited in an X-linked pattern (6). The application of phenotype-clinical methods by Kraepelin formed the very basis for the differentiation of schizophrenia from bipolar illness (7).
Laboratory Approaches
Laboratory based approaches can now be used to study the structure and function of genes and their products in the human brain. The study of the genes themselves forms the basis of the genotype-laboratory approach. The example of research into cystic fibrosis illustrates the power of the application of gene mapping and sequencing techniques to the study of human disease (8). The phenotype-laboratory approach involves study of the products of genes and their effects on brain structure and function. The use of this approach in the study of Alzheimer’s disease, through immunological techniques, has helped define the molecular basis for the characteristic pathological lesions of this disease (9).
Results and Comments
In this section, examples of work from the authors will be used to illustrate the application of the genotype-phenotype strategy. For details of our previous studies the reader is referred to the original publication of our findings, since our goal here is to emphasize the interactive possibilities of the genotype-phenotype approach.
1. Genotype Component
Genetic studies provided some of the earliest data establishing the biological basis of schizophrenia. The classical approaches included studies of adopted offspring and of twins. Studies of adopted offspring revealed the rate of schizophrenia to be higher in the biological relatives of schizophrenics raised in other families than in the biological relatives of non schizophrenic adoptees (10, 11). A different format of adoption study examined the adopted offspring of schizophrenic parents and, again, despite a different social environment, the biological children of schizophrenic parents were significantly more likely to suffer from schizophrenia than expected (12–14). Studies of schizophrenia in identical and fraternal twins clearly point to genetic factors. There is a concordance rate of approximately 50% for schizophrenia in monozygotic twin pairs. Kendler (15) recently summarized familial aggregation studies. He concluded that genetic factors play a major etiological role in schizophrenia and that nongenetic familial factors play perhaps a minor role. However, the genes involved in schizophrenia remain unknown. The new molecular genetic techniques of mapping DNA markers in families with schizophrenia may permit us to identify these genes.
Genotype-Clinical Approach
The primary role of clinical investigations in genotype studies is to accurately diagnose family members participating in genetic studies. However, clinical investigation has played another important role. The human genome is very large, and a random search for genes involved in schizophrenia is a daunting task. Clues to the possible location of the genes involved in a particular disorder can come from clinical investigations. As an example, the frequency of Alzheimer’s disease in patients with trisomy 21 (Down’s syndrome) led to the study of chromosome 21 in familial Alzheimer’s disease (16). In certain of these families, a gene defect on chromosome 21 appears to be linked to Alzheimer’s disease.
While there are no chromosomal abnormalities as common as trisomy 21 involved in schizophrenia, case reports of such abnormalities do provide important clues for genetic investigations. Bassett et al (17) reported a family in which an unbalanced translocation involving a partial trisomy of chromosome 5 was linked to schizophrenia. This observation grew from an apparently routine first psychiatric admission of a 20 year old man. Documentation of mild physical anomalies in the patient, and an account of the the same pattern of abnormalities in an uncle with schizophrenia led to a more detailed genetic investigation. Each patient had a constellation of musculoskeletal and left renal abnormalities, and were found by cytogenetic analysis to be trisomic for the 5q11.2 to 5q 13.3 segment of chromosome 5.
This segment of chromosome 5 was therefore implicated in schizophrenia providing an opportunity for genotype-laboratory studies. Accordingly, several research groups studied this segment in schizophrenia using genetic linkage approaches, illustrating the interactions between clinical and laboratory approaches. The results of these studies are described below (see Genotype-Laboratory Approach).
The trisomy chromosome 5 patients also provided a unique opportunity evaluate the phenotype of the illness. Clinically, the symptoms and course of illness in the affected individuals were in no way atypical, nor was the good response to low dose antipsychotic medication. The affected family members exhibited a pattern of abnormalities in smooth pursuit eye movements seen in schizophrenia (18). Of interest, both family members with schizophrenia had impaired performance in olfactory functioning, a new finding in a subgroup of male patients with schizophrenia (19). Further studies of the phenotypic manifestations of trisomy 5 schizophrenia are in progress. More extensive description at the phenotype level in this special family may help guide molecular genetic investigations.
Genotype-Laboratory Approach
The objective of this approach is to define at a molecular level the abnormalities in genes involved in schizophrenia. The goal must be to localize and identify the genes involved, using one or more of the approaches reviewed by Gurling (20, 21), and by Kennedy et al (22). Essentially, a subject’s DNA is probed for the occurrence of polymorphic patterns of markers which cosegregate with the illness, using either a “random” or “candidate” approach. With enough random markers, the entire genome can be first combed for regions then for the genes contributing to schizophrenia. One alternative “candidate” strategy involves choosing probes known to map to a particular location in the genome. This is the “candidate region” approach that was used to study the chromosome 5q 11.2-13.3 segment in research into schizophrenia. A third strategy is to use brain genes of known function as probes; this “candidate gene” strategy is also being used in the study of families with schizophrenia.
The random search strategy most accurately represents the genotype-laboratory approach. Several groups are actively pursuing this arduous project, and positive results may be some time in coming.
The candidate region approach relies on clinical identification of regions of interest, primarily through cytogenetic analysis of patients with rare chromosomal abnormalities associated with schizophrenia. The identification of a family with partial trisomy of chromosome 5 and schizophrenia was important in this regard (17). Sherrington et al (23) successfully localized a gene in this region predisposing to schizophrenia in five Icelandic and two British families. However, at least six studies have now attempted to replicate the positive findings (24–29), and none has found any significant evidence of linkage of chromosome 5 markers to schizophrenia. A few families among the many tested showed some weakly positive evidence for linkage of 5q 11.2-q 13.3 markers, but the overall results were strongly negative. Thus, including the report of Bassett et al, there are two positive reports of the involvement of the 5q 11.2-q 13.3 chromosomal region in schizophrenia, and at least six negative reports. In all likelihood, the genetic factors in schizophrenia are heterogeneous; the gene or genes leading to the development of schizophrenia in one family may be different from those underlying the disease in another family. Additional cases of schizophrenia may have no genetic component. In any case, the two positive results and several negative ones likely indicate that predisposing genes in the 5q 11.2-13.3 region apply only to a minority of cases of schizophrenia. Further clinical observations, such as those of the association of schizophrenia and abnormalities of the sex chromosomes, may help identify regions of interest in the genome.
The candidate gene approach demonstrates how the genotype and phenotype strategies interact. For example, both dopamine and neurodevelopmental factors are thought to play important roles in the mechanism of illness in schizophrenia. Gene loci such as that coding for tyrosine hydroxylase (the rate-limiting enzyme in dopamine synthesis) and for HOX-2 (a homeobox gene whose product helps orchestrate brain development) were therefore used as probes in the case of a large Swedish family with many schizophrenic members, unfortunately without a positive result (30). The dopamine D2 receptor gene is another candidate gene, due to the involvement of this receptor in the mechanism of action of antipsychotic drugs. This gene does not appear to be linked to schizophrenia in the Swedish family (31). Defining the mechanisn illness in schizophrenia more clearly would provide more useful gene probes.
Once the genes involved in schizophrenia are identified and characterized, phenotype-laboratory studies on the mechanism of the illness will have a clearly defined focus — the products of these genes. From a clinical perspective, the role of experiential and environmental factors in altering the expression or regulation of these gene products could also be studied. New therapies will undoubtedly be developed from this research. For example, medications could be designed to block the effects of deleterious genes or replace the products of deficient genes.
2. Phenotype Component
While descriptions of the clinical phenomenology of schizophrenia have a long history, Kraepelin’s discussion (7) of the course of illness as a fundamental characteristic of the phenotype was the advance which enabled research into the disorder to proceed in a coherent fashion. Many elaborate classification schemes have followed; however, their heuristic value for understanding the mechanism of the illness is limited. Clinical studies remain the foundation for defining more homogeneous subgroups of patients. Important in this regard is increasing evidence on sex differences in the age of onset, severity and course of illness (32). Laboratory phenotype studies have focused primarily on the role of abnormalities of the dopamine system in schizophrenia. New developments in neurobiology and neuropathology hold further promise for investigation into the pathophysiology of schizophrenia. Positron emission tomography now allows clinical and laboratory studies of brain metabolism, neurotransmitters and receptors to be integrated. New immunological techniques permit study of molecular abnormalities in postmortem brain tissues from patients with schizophrenia.
Phenotype-Clinical Approach
As with the trisomy chromosome 5 findings, a new finding concerning a phenotypic marker of a group of cases of schizophrenia has emerged from a clinical observation (33). A patient with schizophrenia complained of problems with his sense of smell. Specifically, he complained that he was unable to smell, and this was investigated with recently developed tests for olfaction. This patient and others with schizophrenia were found to have impaired olfactory function when compared with both normal controls and a group of patients with affective disorder who were receiving antipsychotic medications.
Like other perceptual systems, the olfactory system consists of peripheral sensory receptors, a central processing mechanism, and numerous feedback loops which may modify the impulse traffic along the input path. The nature of the receptor mechanisms subserving the ability of humans to discriminate several thousand different odorants remains unclear (34). The olfactory system includes primary projections to the anterior olfactory nucleus, the prepiriform cortex, and the amygdala as well as secondary projections to the orbitofrontal cortex (35). Afferent projections to the olfactory bulb include those originating from the olfactory tubercle and anterior olfactory nucleus, the preoptic nucleus, the cholinergic substantia innominata, the serotonergic raphe nucleus, the noradrenergic locus coeruleus and the dopaminergic ventral tegmental area (35,36).
The etiology of the abnormalities in olfactory identification in some patients with schizophrenia remains unclear, but can be conceptualized from the genotype-phenotype perspective. Discrete anosmias can be inherited (34). As noted previously, smell identification was abnormal only in the affected patients with trisomy chromosome 5, and not in the family members without the genetic abnormality. Smell identification testing could be evaluated in the family members of patients with schizophrenia, with a similar design as that used to assess eye tracking abnormalities in such families (37). Further laboratory work detailing the nature of proteins and genes involved in olfactory functions may ultimately provide additional candidate genes for molecular genetic studies of the illness.
As in patients with schizophrenia, patients with Korsakoff’s syndrome and those having lesions to the orbital frontal cortex display abnormalities of olfactory identification with normal olfactory acuity (38,39). The abnormalities observed in schizophrenics could be an indicator of one or more brain lesions. The exploration of this possibility illustrates application of the phenotype component of the strategy. At a clinical level, the finding of impaired olfactory identification appears to apply only to a subgroup of male patients with schizophrenia (19). Possible reasons for this sex difference are discussed by Kopala and Clark (40). The lesions could be in one of several regions involved in perceptual processing, or could be related to an abnormality in the modulatory afferent systems projecting to the olfactory system. Investigations using structural and functional brain imaging techniques may help clarify the etiology of these abnormalities in schizophrenia. The olfactory findings encourage further study of the relevant brain regions using laboratory postmortem approaches, as discussed below.
Phenotype-Laboratory Approach
Neuropathologic studies have been productive in defining aspects of the phenotype and pathophysiology of a number of neurological disorders, such as Alzheimer’s disease. At the turn of the century Kraepelin declared schizophrenia to be a disorder in which degeneration of neurons and gliosis was present (7). These conclusions, based the work of Alzheimer and Nissl, were challenged by the results of controlled studies such as those of Dunlap (41). Numerous studies were subsequently presented at the 1952 Congress on Neuropathology. However, the consensus was that there were no consistent neuropathological changes in schizophrenia (42). No further research was conducted until the 1970s, when the demonstration of structural brain abnormalities in schizophrenia with computed tomography revitalized interest in the neuropathology of the disorder (43). Carefully controlled studies have since demonstrated structural and histologic abnormalities in the brain in schizophrenia (44–46). Although no specific consistent abnormality distinguishes the brain tissue of subjects with and without schizophrenia, on a regional basis the large majority of studies report one or more abnormalities of the limbic system.
A major discovery in immunology was made also in the past 15 years — the development of techniques to make monoclonal antibodies (47). This technology was soon applied in neurobiology, and essentially permits “molecular dissection” of tissues of interest. This is accomplished by immunizing mice with tissue homogenates which contain numerous antigens. The monoclonal antibody producing cells generated are collected and immortalized. Each cell line thus produced secretes an antibody directed against a single antigen from the tissue of interest. Since many thousands of monoclonal antibody producing cells can be created, screening strategies can be implemented to select for antibodies of particular interest. This strategy was applied to the study of Alzheimer’s disease by Davies’ group. They developed an antibody called Alz-50 which binds to an epitope uniquely found in the brain tissue of patients with Alzheimer’s disease (48,49).
We have now applied a similar approach to the investigation of schizophrenia (50). Twelve monoclonal antibodies have been developed, which were shown to discriminate between postmortem brain tissue in normal and schizophrenic subjects in a preliminary study. Schizophrenia limbic brain regions were used as the immunogens, and the antibodies developed were screened against schizophrenia and normal brain tissue. Certain antibodies bind relatively more to schizophrenia brain homogenates than to controls, while others exhibit relatively more binding to control tissues. Using histological methods, the selective antibodies appear to bind to different elements of the nervous system, including antibodies specific for synapses, for neuronal cell bodies and dendrites, and for axons (51).
We are hopeful that using these monoclonal antibodies to purify and characterize molecules in the brain will provide us with new clues to the pathophysiology of schizophrenia. Characterization of the molecules involved could lead to a direct approach at the genome level. These molecules, like the dopamine D2 receptor, have become candidate genes for genetic linkage studies. Depending on the nature of the molecules, important information for structural and functional brain imaging studies may also come from this approach. Independent of the results of this strategy using monoclonal antibodies, immunological techniques will be of great importance if the genotype strategy leads to cloning of a gene involved in schizophrenia. For example, in the case of cloning of the Duschenne muscular dystrophy gene, monoclonal antibodies to the gene product were successfully used to help determine the cellular distribution and function of the protein (52). Antibodies against a putative schizophrenia gene product are expected to be similarly useful. A final illustration of interactive possibilities is with the phenotype-clinical approach. Antibodies selective for the olfactory system could be developed, and these could be used to study postmortem schizophrenia tissues.
3. Example Application
Drug treatment in schizophrenia potentially confounds studies investigating the illness. A traditional method for controlling the effects of antipsychotic drugs is to compare findings in treated cases of schizophrenia to findings in cases of bipolar disorder or neurological illness treated with similar medications. However, the genotype-phenotype strategy may offer useful alternatives.
The focus on a comprehensive diagnostic assessment is important in this regard. Obtaining information about the patient from family members provides a description of symptoms prior to any exposure to medications, and also may allow an impression to be made of the patient’s symptoms on and off medication. The genotype perspective encourages enlisting the cooperation of patients and their families for genetic and other studies. In our experience, through studying family members of individuals with schizophrenia, cases of untreated, longstanding schizophrenia may be identified. Once family members become aware of the importance of studying cases of schizophrenia that have never been treated with drugs, affected family members may be directed towards research protocols for assessment and treatment when they first become ill. Groups such as the Friends of Schizophrenics in Canada, and the National Alliance for the Mentally Ill and others in the USA are particularly helpful, and clinicians located near centres of schizophrenia research may be of similar assistance. A final genotype related approach is the high risk strategy, in which the offspring of an affected parent are enlisted in research investigations prior to the normal age of onset of. the illness. Valuable information about the drug-naive state may be obtained prior to the onset of illness in these studies. From the phenoptype perspective, an alternative is to identify drug response or non response as an overall phenotypic characteristic, then study similarities and differences between the groups of patients. This could be incorporated into a study of high-risk individuals designed to identify premorbid characteristics predicting drug responsiveness. At the laboratory level, identification of molecules which change in response to medication could provide important clues to genetic or neurobiological aspects of the etiology of schizophrenia.
Conclusion
Studying the mechanism of schizophrenia as a complex and difficult undertaking. Historically, premier investigators in related fields have been less than enthusiastic about the studying schizophrenia. However, major new developments in science encourage us that significant progress in this endeavor is within reach. The comprehensive diagnostic assessment of patients will be vital to the success of any research study. Approaches based on clinical and laboratory efforts are synergistic, and developing strengths in each is important. We propose an interdisciplinary, genotype-phenotype strategy as one approach to organizing research efforts. With this strategy, we may improve our understanding of the pathophysiology of the illness as a means to improved treatment and care of patients.
Acknowledgments
This work was supported by NARSAD (Drs. Honer and Kennedy), MRC Canada (Dr. Honer), the Keck Foundation, Scottish Rite Schizophrenia Research Program and Eli Lilly (Dr. Basset), the Canadian Psychiatric Research Foundation and the British Columbia Health Care Research Foundation (Dr. Kopala). The authors thank Drs. Peter Davies, Donald Klein and Elizabeth Squires-Wheeler for their comments.
References
- 1.Bertelsen A. Diagnosis and classification of mental disorders in relation molecular research. In: Bulyzhenkov V, Christen Y, Prilipko L, editors. Genetic approaches in the prevention of mental disorders. Berlin: Springer-Verlag; 1990. pp. 1–11. [Google Scholar]
- 2.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer RL, et al. Structured clinical interview for DSM-III-R. New York: New York State Psychiatric Institute; 1987. [Google Scholar]
- 4.Cleghorn JM. A neurodiagnostic approach to schizophrenia. Can J Psychiatry. 1988;33(6):555–561. doi: 10.1177/070674378803300620. [DOI] [PubMed] [Google Scholar]
- 5.National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS) Washington: US Department of Health, Education and Welfare; 1974. [Google Scholar]
- 6.Winokur G, Tanna V. Possible role of X-linked dominant factors in mani depressive disease. Dis Nerv Syst 1969; 30: 89–94. eghorn JM. A neurodiagnostic approach to schizophrenia. Can J Psychiatry. 1988;33(6):555–561. [PubMed] [Google Scholar]
- 7.Kraepelin E. Dementia praecox and paraphrenia. New York: Robert E. Krieger; 1919. [Google Scholar]
- 8.Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 9.Davies P. Protein abnormalities in the Alzheimer brain. In: Henderson AS, Henderson JH, editors. Etiology of dementia of the Alzheimer type. Chichester: John Wiley and Sons; 1988. pp. 135–147. [Google Scholar]
- 10.Kety SS, Rosenthal D, Wender PM, et al. Mental illness in the biological and adoptive families of adopted individuals who have become schizophrenic: a preliminary report based on psychiatric interviews. In: Fieve RR, Rosenthal D, Brill H, editors. Genetic research in psychiatry. Baltimore MD: Johns Hopkins University Press; 1975. pp. 147–165. [PubMed] [Google Scholar]
- 11.Kendler KS, Gruenberg AM. An independent analysis of the Danish adoption study of schizophrenia: VI. The relationship between psychiatric disorders as defined by DSM-III in the relatives and adoptees. Arch Gen Psychiatry. 1984;41:555–564. doi: 10.1001/archpsyc.1984.01790170029004. [DOI] [PubMed] [Google Scholar]
- 12.Heston LL. Psychiatric disorders in foster home reared children of schizophrenic mothers. Br J Psychiatry. 1966;112:819–825. doi: 10.1192/bjp.112.489.819. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal D, Wender PH, Kety SS, et al. The adopted-away offspring of schizophrenics. Am J Psychiatry. 1971;128:307–311. doi: 10.1176/ajp.128.3.307. [DOI] [PubMed] [Google Scholar]
- 14.Lowing PA, Mirsky AF, Pereira R. The inheritance of schizophrenia spectrum disorders: a reanalysis of the Danish adoptee study data. Am J Psychiatry. 1983;140:1167–1171. doi: 10.1176/ajp.140.9.1167. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS. The genetics of schizophrenia and related disorders: a review. In: Dunner DL, Gershon ES, Barrett JE, editors. Relatives at risk for mental disorder. New York: Raven Press; 1988. pp. 247–263. [Google Scholar]
- 16.St George-Hyslop PH, Tanzi RH, Polinsky RJ, et al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science. 1987;235:885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- 17.Bassett AS, Jones BD, McGillivray BC, et al. Partial trisomy chromosome 5 cosegregating with schizophrenia. Lancet. 1988;i:799–801. doi: 10.1016/s0140-6736(88)91660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacono WG, Bassett AS, Jones BD. Eye tracking dysfunction is associated with partial trisomy of chromosome 5 and schizophrenia. Arch Gen Psychiatry. 1988;45:1140–1141. doi: 10.1001/archpsyc.1988.01800360088014. [DOI] [PubMed] [Google Scholar]
- 19.Kopala L, Clark C, Hurwitz T. Sex differences in olfactory function in schizophrenia. Am J Psychiatry. 1989;146:1320–1322. doi: 10.1176/ajp.146.10.1320. [DOI] [PubMed] [Google Scholar]
- 20.Gurling H. Application of molecular biology to mental illness. Analysis of genomic DNA and brain mRNA. Psychiatr Dev. 1985;3:257–273. [PubMed] [Google Scholar]
- 21.Gurling H. Candidate genes and favoured loci: strategies for molecular genetic research into schizophrenia, manic depression, autism, alcoholism and Alzheimer’s disease. Psychiatr Dev. 1986;4:289–309. [PubMed] [Google Scholar]
- 22.Kennedy JL, Giuffra LA, Moises HW, et al. Searching for genes predisposing to neuropsychiatric disorders. In: Berg K, editor. From phenotype to gene in common disorders. Copenhagen: Munksgard A/S Press; (in press) [Google Scholar]
- 23.Sherrington RS, Brynjolfsson J, Petursson H, et al. Localization of a susceptibility locus for schizophrenia on chromosome 5. Nature (Lond) 1988;336:164–167. doi: 10.1038/336164a0. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy JL, Giuffra LA, Moises HW, et al. Evidence against linkage of schizophrenia to markers on chromosome 5 in a northern Swedish pedigree. Nature (Lond) 1988;336:167–170. doi: 10.1038/336167a0. [DOI] [PubMed] [Google Scholar]
- 25.St Clair D, Blackwood D, Muir W, et al. No linkage of chromosome 5q11- 113 markers to schizophrenia in Scottish families. Nature (Lond) 1989;339:305–309. doi: 10.1038/339305a0. [DOI] [PubMed] [Google Scholar]
- 26.Detera-Wadleigh SD, Goldin LR, Sherrington R, et al. Exclusion of linkage 5q11-13 in families with schizophrenia and other psychiatric disorders. Nature (Lond) 1989;340:391–393. doi: 10.1038/340391a0. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann CA, DeLisi LE, Lehner T, et al. Physical mapping, linkage analysis of a putative schizophrenia locus on chromosome 5q. Schizophrenia Bull. 1989;15:441–452. doi: 10.1093/schbul/15.3.441. [DOI] [PubMed] [Google Scholar]
- 28.Aschauer HN, Aschauer-Treiber G, Isenberg KE, et al. No evidence for linkage between chromosome 5 markers and schizophrenia. Hum Herd. doi: 10.1159/000153915. (in press) [DOI] [PubMed] [Google Scholar]
- 29.Diehl S, Su Y, Aman M, et al. Linkage studies of schizophrenia in Irish pedigrees. Cytogenet Cell Genet. 1989;51:989. [Google Scholar]
- 30.Kennedy IL, Giuffra LA, Moises HW, et al. Molecular genetic studies in schizophrenia. Schizophrenia Bull. 1989;15:383–391. doi: 10.1093/schbul/15.3.383. [DOI] [PubMed] [Google Scholar]
- 31.Moises HW, Gelernter J, Grandy DK, et al. Exclusion of the D2-dopamine receptor gene as a candidate gene for schizophrenia in a large pedigree from Sweden. Proceedings of the First World Congress on Psychiatric Genetics; Canbridge. 1989. p. 43. [Google Scholar]
- 32.Seeman MV. Gender differences in schizophrenia. Can J Psychiatry. 1982;27(2):107–112. doi: 10.1177/070674378202700204. [DOI] [PubMed] [Google Scholar]
- 33.Hurwitz T, Kopala L, Clark C, et al. Olfactory deficits in schizophrenia E iol. Psychiatry. 1988;23:123–128. doi: 10.1016/0006-3223(88)90081-9. [DOI] [PubMed] [Google Scholar]
- 34.Snyder SH, Sklar PB, Hwang PM, et al. Molecular mechanisms of olfaction. Trends Neurosci. 1989;12:35–38. doi: 10.1016/0166-2236(89)90154-9. [DOI] [PubMed] [Google Scholar]
- 35.Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system. 3. Berlin: Springer-Verlag; 1988. [Google Scholar]
- 36.Nieuwenhuys R. Chemoarchitecture of the brain. Berlin: Springer-Verlag; 1988. [Google Scholar]
- 37.Holzman PS, Kringlen E, Matthysse S, et al. A single dominant gene can account for eye tracking dysfunctions and schizophrenia in offspring of discordant twins. Arch Gen Psychiatry. 1988;45:641–647. doi: 10.1001/archpsyc.1988.01800310049006. [DOI] [PubMed] [Google Scholar]
- 38.Potter H, Butters N. An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia. 1980;18:621–628. doi: 10.1016/0028-3932(80)90101-3. [DOI] [PubMed] [Google Scholar]
- 39.Mair RG, Doty RL, Kelly KM, et al. Multimodal sensory discrimination deficits in Korsakoff’s psychosis. Neuropsychologia. 1986;24:831–839. doi: 10.1016/0028-3932(86)90082-5. [DOI] [PubMed] [Google Scholar]
- 40.Kopala L, Clark C. Implications of olfactory agnosia for understanding sex differences in schizophrenia. Schizophrenia Bull. 1990;16:255–262. doi: 10.1093/schbul/16.2.255. [DOI] [PubMed] [Google Scholar]
- 41.Dunlap CB. Dementia praecox: some preliminary observations on brair s from carefully selected cases, and a consideration of certain sources of error. Am J Psychiatry. 1924;3:403–421. [Google Scholar]
- 42.Proceedings of tle First International Congress of Neuropathology; Turin: Rosenberg and Sellier; 1952. pp. 465–653. [Google Scholar]
- 43.Johnstone EC, Crow TJ, Frith CD, et al. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;ii:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- 44.Benes FM, Davidson J, Bird E. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry. 1986;43:31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- 45.Bogerts B, Meertz E, Schonfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia. Arch Gen Psychiatry. 1985;42:784–796. doi: 10.1001/archpsyc.1985.01790310046006. [DOI] [PubMed] [Google Scholar]
- 46.Brown R, Colter N, Corsellis JAN, et al. Postmortem evidence of structural brain changes in schizophrenia. Arch Gen Psychiatry. 1986;43:36–42. doi: 10.1001/archpsyc.1986.01800010038005. [DOI] [PubMed] [Google Scholar]
- 47.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature (Lond) 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 48.Wolozin BL, Pruchnicki A, Dickson DW, et al. A neuronal antigen in the brains of Alzheimer patients. Science. 1986;232:648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- 49.Wolozin B, Davies P. Alzheimer-related neuronal protein A68: specificih and distribution. Ann Neurol. 1987;22:521–526. doi: 10.1002/ana.410220412. [DOI] [PubMed] [Google Scholar]
- 50.Honer WC, Kaufmann CA, Kleinman JE, et al. Monoclonal antibodies to study the brain in schizophrenia. Brain Res. 1989;500:379–383. doi: 10.1016/0006-8993(89)90335-1. [DOI] [PubMed] [Google Scholar]
- 51.Honer WG, Kaufmann CA, Kleinman JE, et al. New antibodies to study schizophrenia. Soc Neurosci Abstr. 1989;15:283.6. [Google Scholar]
- 52.Watkins SC, Hoffman EP, Slayter HS, et al. Immunoelectron microscopic localization of dystrophin in myofibres. Nature (Lond) 1988;333:863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]


