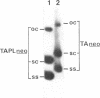
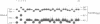Abstract
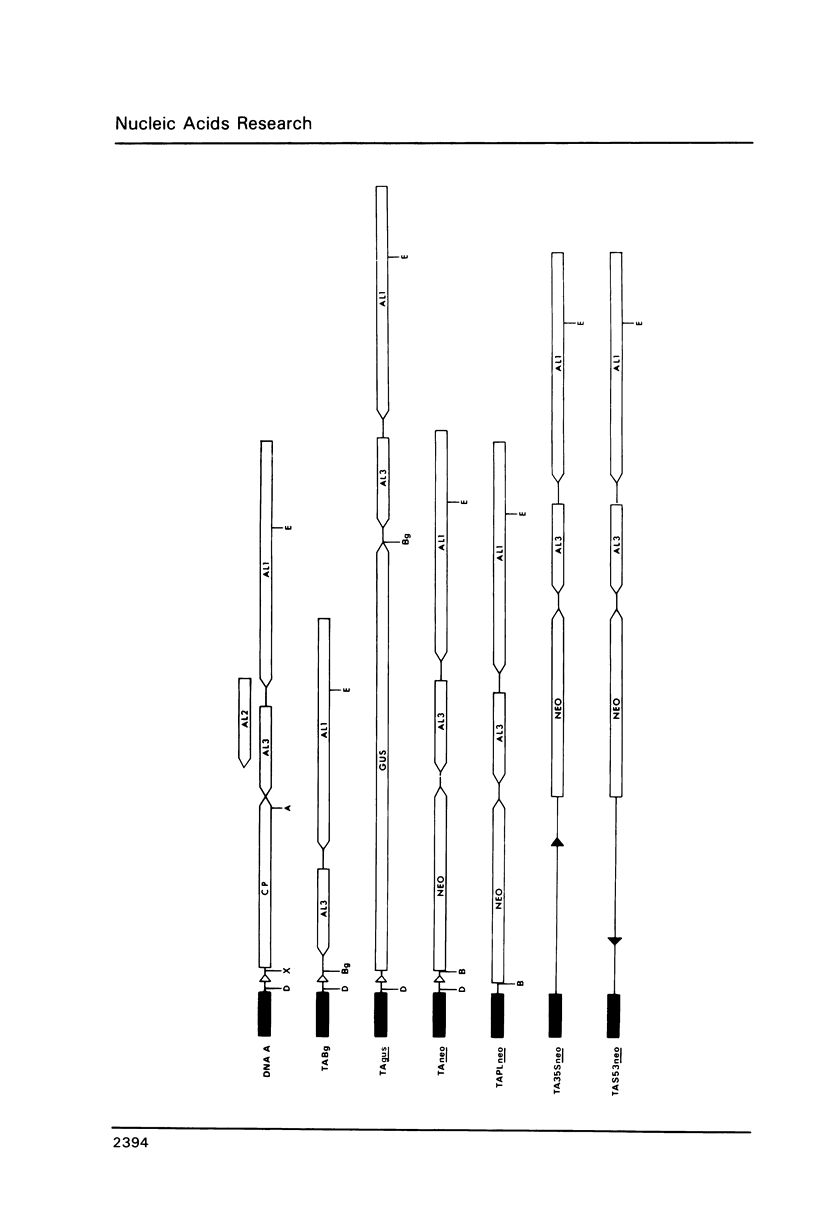
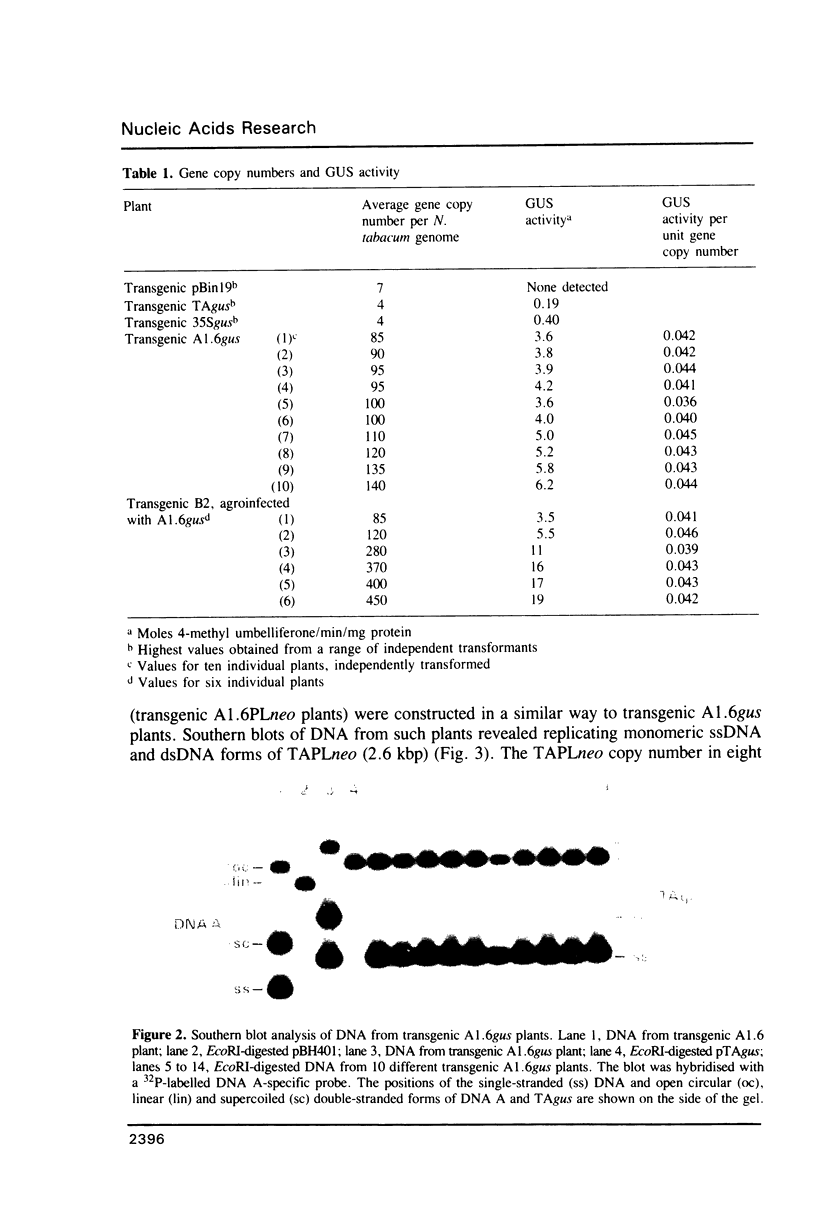
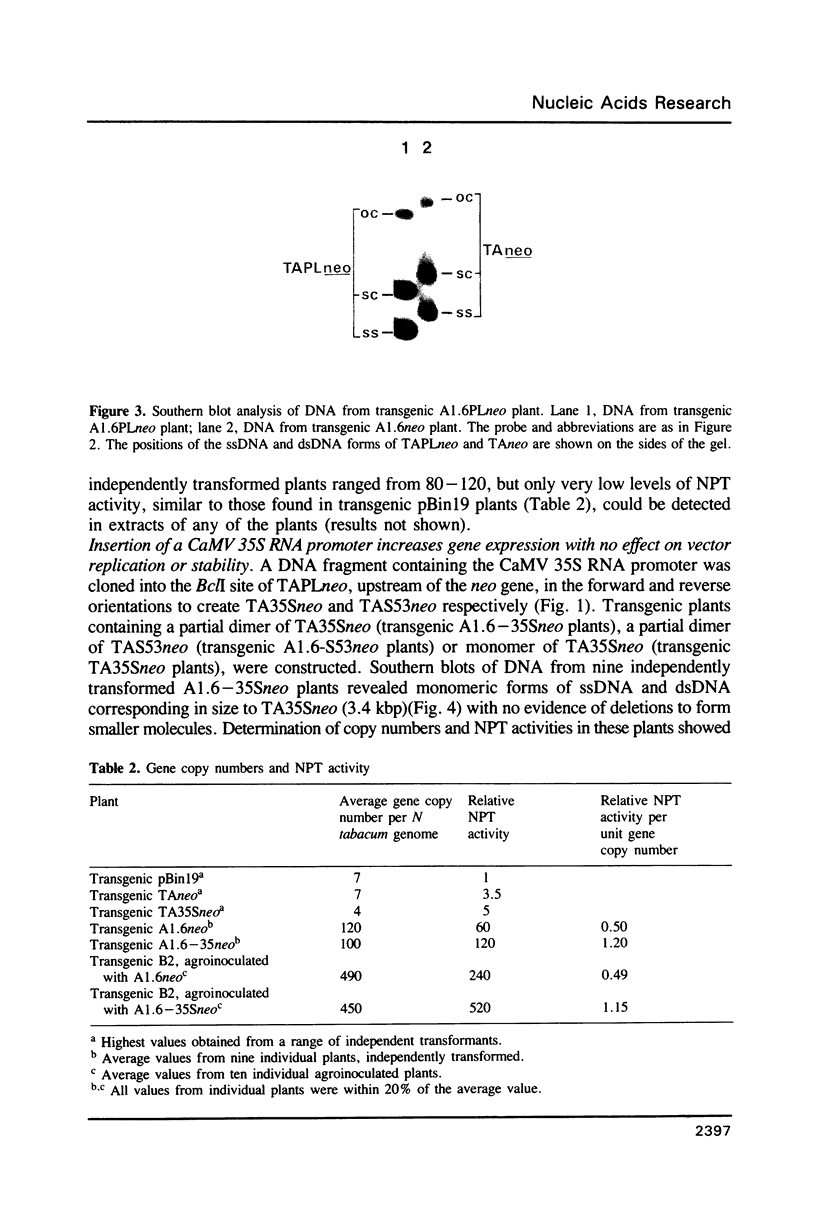
Bacterial beta-glucuronidase (gus) and neomycin phosphotransferase (neo) genes were introduced into coat protein replacement vectors based on DNA A of tomato golden mosaic virus (TGMV). Recombinant gus and neo vectors up to 1.1 kbp larger than DNA A were shown to replicate stably in transgenic plants containing partial dimers (master copies) of the vectors integrated into their chromosomal DNA in the absence of DNA B. Beta-glucuronidase and neomycin phosphotransferase activities in independently transformed plants were proportional to the copy number of the double-stranded forms of the vector. Deletion analysis has shown that an essential part of the TGMV coat protein promoter, including a TATA box, lies within 76 nt upstream of the initiation codon of the gene. An increase in expression of a neo gene was obtained by replacing this 76 nt sequence by an 800 nt sequence containing a cauliflower mosaic virus 35S RNA promoter with no effect on the ability of the vector to replicate or on its stability in transgenic plants. Systemic infection of plants by agroinoculation with TGMV vectors larger than DNA A in the presence of DNA B resulted in deletions in the vector DNA in some, but not all, plants. Possible reasons for vector instability in systemically infected plants, and vector stability in transgenic plants containing master copies of the vector, are discussed.
Full text
PDF




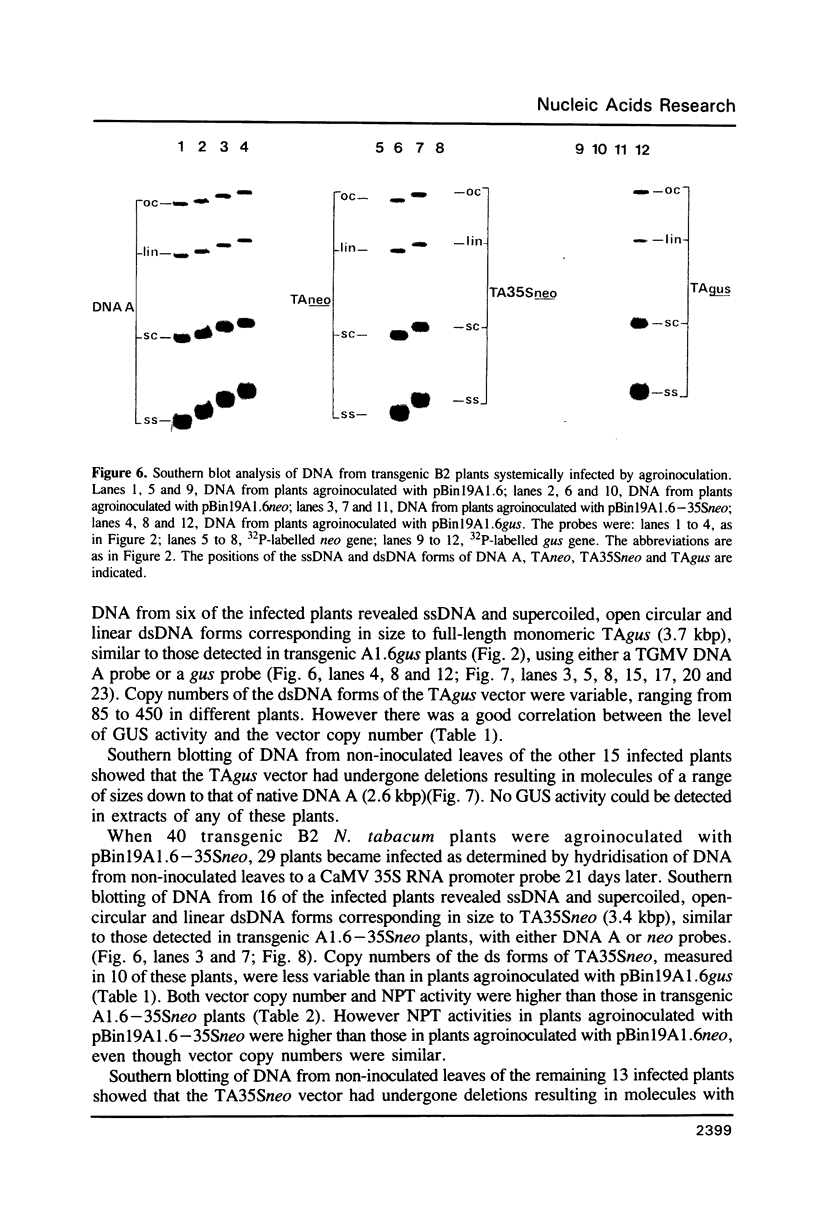
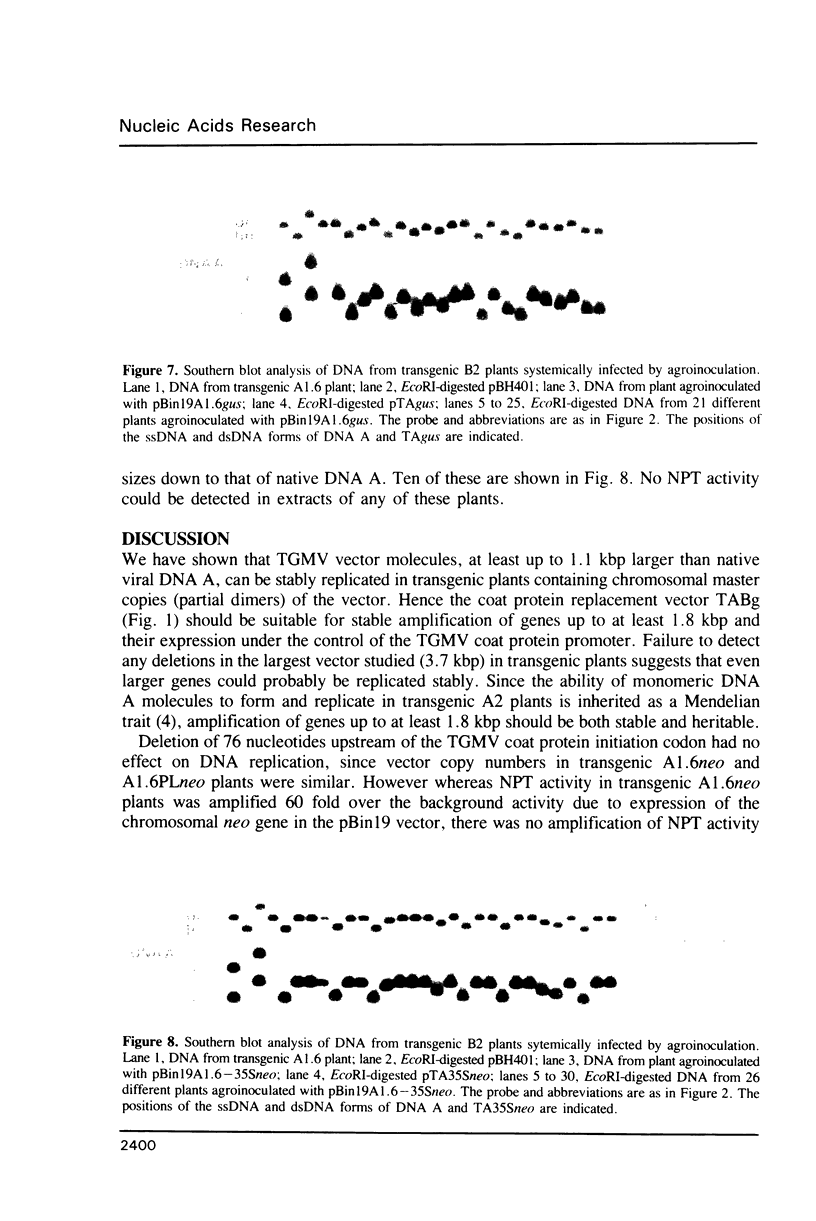



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaro D. M., Hamilton W. D., Coutts R. H., Buck K. W. Molecular cloning and characterisation of the two DNA components of tomato golden mosaic virus. Nucleic Acids Res. 1982 Aug 25;10(16):4913–4922. doi: 10.1093/nar/10.16.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Dean C., Jones J., Favreau M., Dunsmuir P., Bedbrook J. Influence of flanking sequences on variability in expression levels of an introduced gene in transgenic tobacco plants. Nucleic Acids Res. 1988 Oct 11;16(19):9267–9283. doi: 10.1093/nar/16.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer J. S., Brand L., Sunter G., Gardiner W. E., Bisaro D. M., Rogers S. G. Genetic analysis of the tomato golden mosaic virus. II. The product of the AL1 coding sequence is required for replication. Nucleic Acids Res. 1988 Jul 25;16(14B):7043–7060. doi: 10.1093/nar/16.14.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner W. E., Sunter G., Brand L., Elmer J. S., Rogers S. G., Bisaro D. M. Genetic analysis of tomato golden mosaic virus: the coat protein is not required for systemic spread or symptom development. EMBO J. 1988 Apr;7(4):899–904. doi: 10.1002/j.1460-2075.1988.tb02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Buck K. W. Identification of novel DNA forms in tomato golden mosaic virus infected tissue. Evidence for a two component viral genome. Nucleic Acids Res. 1982 Aug 25;10(16):4901–4912. doi: 10.1093/nar/10.16.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Coutts R. H., Buck K. W. Demonstration of the bipartite nature of the genome of a single-stranded DNA plant virus by infection with the cloned DNA components. Nucleic Acids Res. 1983 Nov 11;11(21):7387–7396. doi: 10.1093/nar/11.21.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Stein V. E., Coutts R. H., Buck K. W. Complete nucleotide sequence of the infectious cloned DNA components of tomato golden mosaic virus: potential coding regions and regulatory sequences. EMBO J. 1984 Sep;3(9):2197–2205. doi: 10.1002/j.1460-2075.1984.tb02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Bowdoin L., Elmer J. S., Rogers S. G. Transient expression of heterologous RNAs using tomato golden mosaic virus. Nucleic Acids Res. 1988 Nov 25;16(22):10511–10528. doi: 10.1093/nar/16.22.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDowell S. W., Coutts R. H., Buck K. W. Molecular characterisation of subgenomic single-stranded and double-stranded DNA forms isolated from plants infected with tomato golden mosaic virus. Nucleic Acids Res. 1986 Oct 24;14(20):7967–7984. doi: 10.1093/nar/14.20.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Bisaro D. M., Horsch R. B., Fraley R. T., Hoffmann N. L., Brand L., Elmer J. S., Lloyd A. M. Tomato golden mosaic virus A component DNA replicates autonomously in transgenic plants. Cell. 1986 May 23;45(4):593–600. doi: 10.1016/0092-8674(86)90291-6. [DOI] [PubMed] [Google Scholar]
- Stanley J., Townsend R. Infectious mutants of cassava latent virus generated in vivo from intact recombinant DNA clones containing single copies of the genome. Nucleic Acids Res. 1986 Aug 11;14(15):5981–5998. doi: 10.1093/nar/14.15.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A., Etessami P., Stanley J. Expression of a bacterial gene in plants mediated by infectious geminivirus DNA. EMBO J. 1988 Jun;7(6):1583–1587. doi: 10.1002/j.1460-2075.1988.tb02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]