Abstract
The efficacy of current cancer vaccines is limited by the functional heterogeneity and poor availability and expansion of professional antigen-presenting cells (APCs). Besides their potent innate effector properties, γδ T cells have been suggested to be involved in the initiation and maintenance of adaptive immune responses. Here, we investigated the capacity of human γδ T cells to induce expansion of virus-specific T cells to Epstein Barr virus (EBV) antigens. Aminobisphosphonate-stimulated human peripheral blood-derived γδ T cells (Vγ9+Vδ2+) acquired a dual phenotype characteristic for both APCs and effector memory T cells. Coincubation of activated γδ T cells pulsed with HLA-restricted epitopes of either the highly stimulatory EBV lytic cycle antigen BZLF-1 or the tumor-associated latent EBV antigen LMP2a with autologous peripheral blood lymphocytes induced selective expansion of peptide-specific, fully functional CD3+CD8+ cytolytic effector memory T cells. Furthermore, γδ T-APCs efficiently processed and presented endogenous antigen, as demonstrated by the capacity of LMP2a gene-transduced γδ T cells to induce expansion of T cells with broad specificity for various LMP2a peptides. The capacity of autologous γδ T cells to induce LMP2a-specific autologous CTLs was confirmed in two patients with Hodgkin lymphoma. In summary, bisphosphonate-activated human γδ T cells stimulate expansion of cytotoxic effector T cells specific for both subdominant and dominant viral epitopes and thus show promise as a novel source of efficient APCs for immunotherapy of viral and malignant disease.
Keywords: Human, T cells, Immunotherapy, Antigen Presentation
INTRODUCTION
Clinical trials of cellular immunotherapy have shown promise for the treatment of viral and malignant disease and revealed the importance of efficient antigen presentation for the induction of therapeutic T cell immunity1–3. Since most tumor cells fail to present antigen, the development of cellular immunotherapies relies on the availability of surrogate antigen-presenting cells (APCs)4. The standard approach to generating tumor-specific cytotoxic T cells (CTLs) is based on antigen presentation by dendritic cells (DCs)5. While DCs are the most efficient APCs so far, serious drawbacks to their use in adoptive immunotherapy exist, including their scarcity in the peripheral blood, their limited expansion, and their functional heterogeneity. Furthermore, DCs were found to be functionally deficient in cancer patients6. These limitations have motivated an intense search for alternative sources of APCs7–9.
Recently, T lymphocytes bearing Vγ2Vδ2+ T cell receptors (referred to as γδ T cells) have emerged as a promising candidate cell population for the stimulation of antigen-specific immune responses10.γδ T cells constitute a small proportion (1–5%) of the lymphocytes that circulate in human blood. In a TCR-dependent manner, they recognize a restricted set of phosphorylated compounds and can be pharmacologically activated by nitrogen-containing bisphosphonates (pamidronate, zoledronate)11. Activated γδ T cells exert potent and antigen-specific effector functions, including both cytolysis and secretion of cytokines12. Their physiological significance is a matter of intensive investigation. Besides their contribution to the first line defense against microbial pathogens13,γδ T cells have been attributed an important role in autoimmunity14 and a surveillance function against tumors15–17. An antigen-presenting function of γδ T cells was suggested by the recent observation that upon activation, these cells acquire phenotypic and functional characteristics of professional APCs, concomitant with the capacity of inducing primary CD4+ and CD8+ αβ T cell responses to allogeneic and microbial antigens10.
We hypothesized that the immunostimulatory properties of activated γδ T cells can be exploited for the induction of therapeutic antiviral and antitumor immunity. We chose T cell immunity to Epstein Barr virus (EBV) as a model system, since EBV antigens are important therapeutic targets in pediatric and adult hematology and oncology1–3. While EBV antigens raise potent and protective T cell responses in healthy individuals18, EBV infections or reactivations in immunocompromised patients can cause uncontrolled lymphoproliferation2. Furthermore, the virus is linked to a range of malignancies, including Hodgkin disease19, non-Hodgkin lymphomas20 and nasopharyngeal carcinoma21. Most EBV-associated tumor cells express a set of latency antigens restricted to LMP1 and LMP2 in the absence of lytic cycle and EBNA3 antigens22. Unfortunately, the generation of LMP-specific T cells is complicated by their limited immunogenicity and is highly dependent on the immunostimulatory quality of the APC3;23.
Here, we addressed whether antigen presentation by γδ T cells has the capacity for inducing functional effector T cell responses to both highly and weakly immunogenic EBV antigens in an epitope-specific manner. We found that aminobisphosphonate stimulation of human Vδ2+Vγ9+ T cells induces upregulation of multiple costimulatory molecules as well as secretion of high amounts of TNF-α. After pulsing with model peptides derived from both BZLF-1 and LMP2a or genetic modification with the LMP2a gene, activated γδ T cells induced selective expansion of peptide-specific and fully functional CD8+ CTLs from both EBV-seropositive healthy donors and cancer patients. Their effector functions and pattern and breadth of epitope recognition are comparable to CTLs generated by presentation of the same peptides on DCs or by stimulation with EBV-transformed lymphoblastoid cell lines. These observations pave the way for the use of γδ T cells as cellular adjuvants for immunotherapy of viral infection and cancer.
METHODS
Cell lines
EBV-immortalized lymphoblastoid cell lines (LCLs) were generated as described previously24. 293T cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The ecotropic and amphotropic packaging cell lines Phoenix-eco and Phoenix-ampho25 were provided by Gary P. Nolan (Stanford, CA). PG-13 (ATCC) is a retrovirus packaging cell line that produces virus pseudotyped with the gibbon-ape leukemia virus (GALV). The amphotropic FLYRD18 (provided by E. Vanin, Houston, TX) cell line provides viral recombinants with the RD114 envelope.
Plasmids and production of recombinant retrovirus
The LMP2a sequence was cloned from LCLs into AgeI and XhoI sites of the retroviral vector SFG-GFP26 (provided by Martin Pule, London, UK). For control experiments, SFG-14.G2aζ27-GFP or SGF-CD19ζ28-GFP was used. Stable retroviral producer cell lines were generated as described28;29. Transient production of retroviral supernatant by 293T cells was achieved by cotransfection with the RD114 env expression plasmid RDF, the MoMLV gag/pol expression plasmid PeqPam3(-env), the SFG-LMP2a-GFP vector or control vectors respectively, at a ratio of 2:3:3. The supernatant was harvested at days 2 and 3 after transfection.
Expansion and transduction of γδ T cell cultures
Peripheral blood mononuclear cells (PBMCs) (2×106/well) were stimulated with zoledronate (1 μg/ml; kindly provided by Novartis Deutschland, Nürnberg, Germany) in RPMI 1640 medium, supplemented with recombinant human interleukin-2 (rhIL-2; Proleukin, Chiron, Emeryville, CA) at 100 IU/ml, 10% FCS and 2 mM L-glutamine, in a 24-well plate. Two weekly doses of rhIL-2 (100 IU/ml) were added. Positive selection of γδ T cells for coincubation experiments was performed on day 9 using TCR γδ-specific magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). For retroviral transductions, γδ T cells were prestimulated as above, then transferred to 24-well non-tissue culture-treated plates (Becton Dickinson, Franklin Lakes, New Jersey) coated with recombinant fibronectin fragment FN CH-296 (Retronectin, Takara Shuzo, Otsu, Japan) at 4 μg/cm2, and coincubated with viral supernatant for 48 hours.
Generation of DCs
CD14+ cells were selected from freshly isolated PBMCs by magnetic cell selection using CD14-specific microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), seeded at 106/well into a 24-well plate in RPMI medium supplemented with 10% FCS, 2 mM L-glutamine, 800 U/mL GM-CSF and 1,000 U/mL IL-4 (both cytokines from ImmunoTools, Friesoythe, Germany), and incubated at 37°C and 5% CO2. Medium supplemented with IL-4 and GM-CSF was replaced on day 3. For maturation, DCs were harvested on day 6 and replated in medium supplemented with IL-6 (100 ng/ml), IL-1β (10 ng/ml), PGE2 (1 μg/ml) and TNF-α (10 ng/ml). Mature DCs were harvested on day 9 and their phenotype was assessed by flow cytometry.
Peptides and pentamers
For BZLF-1, the HLA-B8 binding epitope RAKFKQLL30, hereafter named RAK, and for LMP2a, the HLA-A2 epitope FLYALALLL31 (FLY) were used. All peptides and pentamers were synthesized by ProImmune (Oxford, UK). A peptide library consisting of 122 15-mer peptides with 11-aa overlap covering the complete sequence of LMP2a (B95-8 strain; Swiss prot access P13285) was purchased from D. Stoll (Natural and Medical Sciences Institute, University of Tuebingen, Germany) and pooled as described previously3. To determine the minimal recognized LMP2 epitope sequence, additional peptides, varying in length from 9 to 14 aa, were obtained from Genemed Synthesis and reconstituted at 10 mg/ml in DMSO. For their use as stimulator cells, DCs or γδ T cells were pulsed with 5 μg/ml of peptide for 1 hour at 37°C, followed by three washing steps with phosphate-buffered saline (PBS).
Flow cytometry
For immunophenotyping, cells were stained with fluorescein-conjugated monoclonal antibodies (Becton Dickinson, San Jose, CA) directed against CD3, CD4, CD8, TCRαβ, TCRγδ, HLA-DR, CD11c, CD40, CD54, CD80, CD83, CD86, CDw137L (4-1BBL), CD45RA, CD45RO, CCR7, CD27, anti-IL-12 p40/p70 (clone C11.5), anti-IFN-γ and anti-TNF-α proteins. Anti-Vγ2Vδ2 (clone IMMU 389) was purchased from Beckman Coulter (Karlsruhe, Germany). For each sample, 20,000 cells were analyzed with FACSCalibur and Cell Quest Software or FACSCanto and Diva Software (Becton Dickinson, San Jose, CA). For pentamer staining, T cells (1×106) were incubated with 1 μl of unlabeled pentameric complex at room temperature for 15 minutes, followed by staining with 4 μl PE-labeled fluorotag, anti-CD8 FITC and anti-CD3 PerCP.
Activation and expansion of antigen-specific T cells
5×104/well irradiated (30 Gy) peptide-pulsed APCs were seeded into 24-well plates and coincubated with 1×106 autologous PBMCs per well (S:R ratio of 1:20) in RPMI 1640 medium supplemented with 10% FCS and 2 mM L-glutamine without the addition of cytokines for 10–12 days. When CD8+ T cells were used as responder cells, magnetic cell selection from freshly isolated PBMCs was performed by using CD8-specific microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), and rhIL-2 (25 IU/ml) and rhIL-7 (20 ng/ml) were added to the cocultures on day 1 and 7. SFG-LMP2a-GFP or SFG-14.G2aζ-GFP (control) transduced γδ T cells were sorted for TCRαβ−/GFP+ cells (>95% purity) before coincubation with autologous CD8+ T cells at a S:R ratio of 1:20.
Cytotoxicity assays
Cytotoxic specificity was determined in standard 51Cr release assays. Various numbers of T effector cells were coincubated in triplicate with 2500 target cells labeled with 100 μCi 51Cr/0.5×106 cells (PE Applied Biosystems) in a total volume of 200 μl in a V-bottomed 96-well plate. At the end of a 4-hour incubation period at 37°C and 5% CO2, supernatants were harvested, and radioactivity was counted in a gamma counter. Maximum release was determined by lysis of target cells with Triton X.
Measurement of cytokine production
Triplicate samples of effector T cells (5×104/well) were cocultured with peptide-pulsed γδ T cells or LCLs at a stimulator-to-effector ratio of 3:1 in the presence of rhIL-2 (25 IU/ml) in 96-well round bottomed plates. After 72 hours, the supernatants were harvested and analyzed for human IFN-γ by enzyme-linked immunosorbent assay (Hölzel Diagnostika, Köln). For intracellular cytokine detection, cytokine secretion was blocked with 10 μg/ml brefeldin A (BFA; Sigma, Deisenhofen, Germany) for 4 hours, and the cells were permeabilized using a proprietary solution (Becton Dickinson, San Jose, CA), then stained according to the manufacturer's recommendations.
ELISPOT assay
ELISPOT analysis was used to determine the epitope specificities of expanded CTLs in response to LMP2 pepmix, which contains all 15-mer peptides of LMP2 in one pool, and overlapping peptide pools for LMP2, as previously described1;3. IFN-γ ELISPOT assay was also used for the detection of LMP2a-specific T cells in a patient with Hodgkin lymphoma. Irradiated (30 Gy) SFG-LMP2a-GFP transduced and GFP-positive selected γδ T cells were used as stimulator cells in 11 day cocultures with positive-selected autologous CD8+ T cells in RPMI medium containing 8% autologous serum. For restimulation, LMP2a-transduced CD8-negative T cells were used. For this purpose, the negative cell fraction after sorting of CD8+ T cells was prestimulated at 1×106/ml for 48 hours in 24-well plates precoated with OKT-3 (1μg/ml; Ortho Pharmaceuticals, Raritan, NJ) and anti-CD28 antibody (1 μg/ml; Pharmingen, San Diego, CA), then transduced with either SFG-LMP2a-GFP or an irrelevant control vector (SFG-LMP2a-CD19ζ), and GFP-positive cells were selected by FACS. To determine antigen-specific cytokine secretion, LMP2a-prestimulated cells (2×104cells/well) were restimulated with 8×104/well autologous transduced CD8-negative T cells in triplicate wells of 96-well filtration plates (Millipore, Schwalbach, Germany) precoated with IFN-γ-specific antibody clone 1-D1K (Mabtech, Hamburg, Germany; 15 μg/ml). Control wells contained LMP2a-prestimulated CD8+ T cells coincubated with autologous non-transduced or control vector-transduced CD8-negative T cells, or with medium alone. After 20 hours, the plates were washed and incubated with detector mAb 7-B6-1 biotin (Mabtech) followed by Strepatavidin-AP (Mabtech). Spots were detected by adding AP substrate BCIP/NBT (Moss, Pasadena, U.S.A.) for 15 min and enumerated with a BIOSYS BIOREADER 3000 PRO.
Statistical analysis
The student T test was used to test for significance in each set of values, assuming equal variance. Mean values ± SD are given unless otherwise stated.
RESULTS
Zoledronate-activated γδ T cells acquire a combined phenotype characteristic for both APCs and effector memory cells and secrete TNF-α
In all seven healthy donors, in vitro stimulation of PBMCs with a single dose of zoledronate (1 μg/ml) induced significant expansion of γδ T cells, resulting in a relative increase of γδ TCR (Vγ2Vδ2)-expressing T cells. On day 8, the majority (87.1± 6.8%) of the cultured cells coexpressed CD3 and Vγ2Vδ2, corresponding to an overall γδ T cell expansion of 150.6 ± 54.5-fold. In absolute numbers, a mean of 39.8 ± 5.7×106γδ T cells (range 32.3 – 47.2×106) were obtained from 10×106 PBMCs. These numbers compare favorably to the numbers of immature DCs obtained from the same amount of blood in 5 donors (0.6×106±0.3×106, range 0.5×106–1.1×106). γδ T cells were also efficiently expanded from the peripheral blood of four patients with Hodgkin lymphoma, with 10.8 to 103.1-fold (43.5 ± 41.6-fold) overall γδ T cell expansion within 8 days, yielding cultures containing 28.5 to 93.5% (64.5±27.5%) γδ TCR+ T cells on day 8. By positive selection using TCR γδ-specific magnetic beads, >95% γδ T cell purity was routinely obtained.
To evaluate the potential of bisphosphonate-activated γδ T cells to serve as APCs, we assessed the pattern and kinetics of surface expression of immunostimulatory molecules, which can be used as a morphological readout for APC function32. During zoledronate-induced expansion, γδ T cells acquired a phenotype characteristic for APCs, including upregulation of HLA-DR as well as the costimulatory ligands CD80, CD86, CD83, CD11c, CD54, CD40, and CDw137L (4-1BBL) (Figure 1A). Whereas expression of most markers decreased after peak levels were reached on day 5, CD86 persisted for >10 days, and HLA-DR remained upregulated on >70% of the cells for prolonged culture periods of >2 to 3 weeks (Figure 1B).
Figure 1. Zoledronate-activated γδ T cells acquire a phenotype characteristic for APCs.
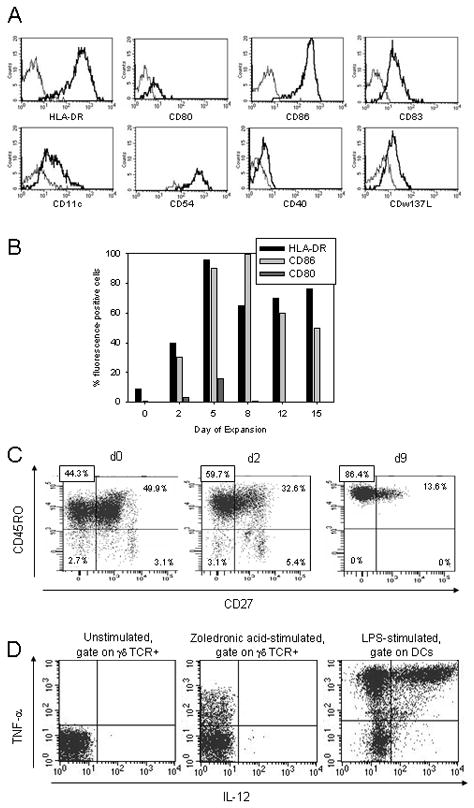
(A) Expression of various surface molecules was assessed by flow cytometry on gated CD3+ TCRγδ+ T cells on days 0 and 5 after stimulation with zoledronate. (B) Kinetics of expression of HLA-DR, CD80 and CD86 on gated CD3+TCRγδ+ T cells were determined by flow cytometry on days 0, 2, 5, 8, 12 and 15 after stimulation. (C) To discriminate between distinct effector cell populations within zoledronate-stimulated γδ T cells, gated CD3+ TCRγδ+ T cells were costained with fluorescence-labeled CD45RO- and CD27-specific antibodies on days 0, 2 and 9 after stimulation. Shown are representative experiments of three. (D) Intracellular IL-12 and TNF-α secretion by gated CD3+ TCRγδ+ T cells within cultures of unstimulated or zoledronic-acid (22 hrs) stimulated PBMCs. Gated DCs within cultures of PBMCs stimulated with LPS (1 μg/ml) for 22 hrs served as positive controls. Shown are representative experiments of three.
On the basis of expression of the surface markers CD45RA, CD45RO and CD27, γδ T cells can be further subclassified into distinct effector cell populations with varying functional properties33. We found that during zoledronate-induced cell culture, the proportion of effector memory γδ T cells (TEM; CD27−/CD45R0+/CD45RA−) increased while central memory T cells (TCM; CD27+/CD45R0+/CD45RA−) were found at decreasing percentages (Figure 1C). Neither naïve nor fully differentiated, RA+ effector memory T cells (TEMRA) were found after 9 days of in vitro culture. CCR7 remained downregulated on the majority of γδ T cells after stimulation with zoledronic acid and during prolonged in vitro culture (not shown). The T-cell stimulatory function of professional APCs is largely determined by secretion of TNF-α and IL-12. Whereas IL-12 was not detected in activated γδ T cells, an intracellular cytokine detection assay revealed production of TNF-α at median intensities about 10-fold inferior to lipopolysaccharide (LPS)-stimulated mature DCs (Figure 1D).
Thus, in response to aminobisphosphonate stimulation, γδ T cells differentiate towards potent effector T cells and at the same time acquire some of the typical phenotypic and functional properties of antigen-presenting cells.
Zoledronate-activated γδ T cells induce epitope-specific CTL expansion to EBV lytic cycle antigen BZLF-1
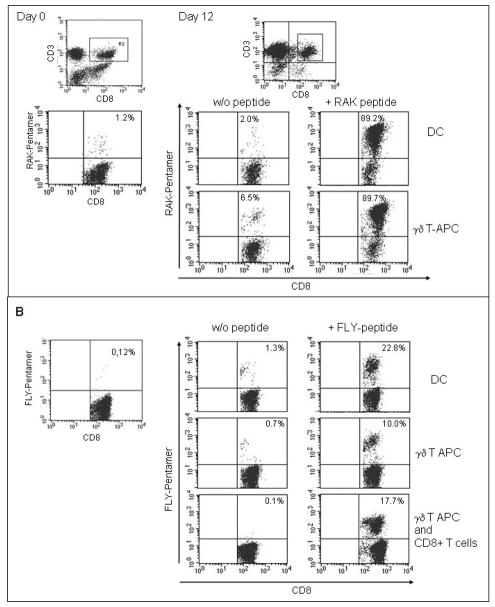
To investigate the stimulatory properties of bisphosphonate-activated γδ T cells, we analyzed their ability to induce expansion of αβ effector T cells specific for an HLA-B8-restricted peptide derived from the EBV lytic cycle antigen BZLF-1 (RAK). Purified populations of γδ T cells were obtained from the peripheral blood of four HLA-B8 positive healthy donors by zoledronate stimulation and subsequent positive selection using γδ TCR-specific beads. The γδ T cells were pulsed with RAK peptide at 5 μg/ml, irradiated with 30 Gy and coincubated with autologous PBMCs at a 1:20 stimulator-to-responder ratio. In parallel cocultures, peptide-pulsed autologous DCs were used as stimulators cells. 10–12 days of coculture with peptide-pulsed γδ T cells resulted in selective expansion of RAK-pentamer-positive CD3+ CD8+ T cells from <1% to a mean of 51.1%±26.2% (range 8.3%–89.7%) (Figure 2A, Table I). This increase in the percentage of RAK peptide-specific T cells was accompanied by numerical expansion by a mean of 40.8-fold (range 15.9 to 80.1) within 12 days of stimulation. The percentages of pentamer-positive CD8+ T cells in cocultures with peptide-pulsed DCs were equal or superior in a donor-dependent manner (mean of 64.5%±35.4%, range 10.6–96.0%) (Figure 2A, Table I). Peptide-specific T cell expansion in response to γδ T cell or DC stimulation in the absence of peptide was minimal (mean of 3.3%±2.3%, range 0.9%–7.0% for γδ T-APCs and 2.2%±0.9%, range 0.9–3.2% for DCs).
Figure 2.
(A) Activated γδ T cells induce epitope-specific CTL expansion to EBV lytic cycle antigen BZLF-1. PBMCs from healthy donors were coincubated with autologous stimulator cells (upper panel, DCs; lower panel, γδ T-APCs), either unpulsed or pulsed with the BZLF-1 epitope RAK. The percentages of RAK-specific CTLs prior to (day 0) and on day 12 after stimulation were quantified by pentamer staining followed by flow cytometry analysis within a gate set on CD3+/CD8+ T cells. Shown is one representative experiment of seven. (B) Activated γδ T cells propagate expansion of epitope-specific CTLs to EBV latency antigen LMP2a. Healthy donor PBMCs were coincubated with autologous stimulator cells (upper panel, DCs; middle panel, γδ T cells), either unpulsed or pulsed with the LMP2a epitope FLY. In further experiments, CD8+ sorted T cells were used as responder cells and coincubated with γδ T-APCs in the presence or absence of FLY peptide (lower panel). The percentages of FLY-specific CTLs prior to (day 0) and on day 12 after stimulation were quantified by pentamer staining followed by flow cytometry analysis within a gate set on CD3+/CD8+ T cells. Shown is one representative experiment of six.
Table I.
Activated γδ T cells induce epitope-specific CTL expansion to EBV antigens BZLF-1 (RAK) and LMP2a (FLY). The table summarizes the percentages of pentamer-positive T cells in response to stimulation with peptide-pulsed or unpulsed γδ T cells and DCs, respectively, from multiple experiments using effector cells obtained from eight individual donors.
| Donor-Cell line | peptide | γδ w/o peptide | γδ + peptide | DCs w/o peptide | DCs + peptide |
|---|---|---|---|---|---|
| A-1 | RAK | 7.0% | 60.0% | n.d. | 82.0% |
| A-2 | RAK | n.d. | 67.7% | n.d. | 83.0% |
| A-3 | RAK | 6.5% | 89.7% | 2.0% | 89.2% |
| B-1 | RAK | 6.0% | 80.0% | 2.0% | 96.0% |
| B-2 | RAK | 3.1% | 63.0% | 3.2% | 93.0% |
| B-3 | RAK | 2.8% | 55.5% | n.d. | n.d. |
| C-1 | RAK | 2.4% | 53.0% | n.d. | n.d. |
| D-1 | RAK | 2.0% | 41.9% | n.d. | 10.6% |
| E-1 | RAK | 1.3% | 8.3% | 3.0% | 13.4% |
| E-2 | RAK | 0.9% | 8.5% | n.d. | n.d. |
| E-3 | RAK | 0.9% | 35.0% | 0.9% | 49.0% |
| mean | 3.3% | 51.1% | 2.2% | 64.5% |
| C-1 | FLY | 0.7% | 10.0% | 1.3% | 22.8% |
| C-2 | FLY | 0.2% | 15.2% | n.d. | n.d. |
| C-3 | FLY | 1.4% | 13.3% | 1.3% | 21.6% |
| C-4 | FLY | 1.0% | 30.2% | n.d. | 29.0% |
| C-5 | FLY | 1.6% | 14.6% | n.d. | n.d. |
| F-1 | FLY | 1.0% | 4.3% | n.d. | n.d. |
| F-2 | FLY | 1.1% | 4.9% | n.d. | n.d. |
| F-3 | FLY | 0.4% | 8.1% | n.d. | 9.0% |
| G-1 | FLY | 0.8% | 33.2% | n.d. | n.d. |
| H-1 | FLY | 1.0% | 5.8% | n.d. | n.d. |
| H-2 | FLY | 0.7% | 2.1% | n.d. | n.d. |
| H-3 | FLY | 0.7% | 1.6% | 0.5% | 1.2% |
| mean | 0.9% | 12.6% | 1.0% | 16.7% |
Zoledronate-activated γδ T cells efficiently propagate expansion of specific CTLs to EBV latency antigen LMP2a
Due to the immunogenicity of BZLF-1, the APC requirements for inducing CTLs specific for this antigen are limited34. To obtain further evidence for the antigen-presenting qualities of activated γδ T cells, we assessed their ability to support T cell expansion to a less immunogenic antigen. The choice of the latency antigen LMP2a was based on its low T-cell stimulatory properties as well as its importance as a target for tumor immunotherapy of Hodgkin′s lymphoma and nasopharyngeal carcinoma1;3. Starting from <0.2% pentamer-positive T cells at the onset of the cultures, stimulation of autologous PBMCs with γδ T cells pulsed with the HLA-A2-restricted LMP2a epitope FLY resulted in a substantial increase to a mean of 12.6%±10.6% (range: 1.6–33.2%) in 6 donors, corresponding to 36.6-fold (range 27.7 to 60.0) numerical expansion, and comparable to stimulation by peptide-pulsed autologous DCs (mean of 16.7±11.3% pentamer-positive cells, range 1.2–29.0%) (Figure 2B, Table I). Again, γδ T cell stimulation in the absence of peptide failed to induce substantial FLY-specific T cell proliferation (mean of 0.9±0.4%; 0.2–1.6%).
Within cocultures relying on unselected PBMCs as responder cells, alternative cells with APC function, including CD4+ T cells, B cells and monocytes, may be partially responsible for peptide-specific CTL stimulation. To separate the antigen-presenting role of γδ T cells from this potential background, we performed additional experiments using purified CD8+ T cells as responder cells. Cocultures with purified γδ T cells and purified CD8+ T cells consistently yielded high proportions of FLY-specific T cell populations (mean of 6.6±7,7%, range 1.3–17.7%) comparable to those obtained with unselected responder cells (Figure 2B). Taken together, γδ T cells efficiently stimulate peptide-specific CD8+ CTL expansion in response to both high and moderately immunogenic antigens.
CTLs expanded in response to antigen presented by γδ T cells have an effector memory phenotype and exert potent and peptide-specific effector functions
Suboptimal antigen presentation may result in the selective expansion of T cell populations with inappropriate phenotypic and functional properties35. Therefore, we compared the immunophenotypes of pentamer-positive CTLs on day 15 after initial stimulation with γδ T-APCs with those generated in the presence of mature DCs. Among gated CD8+ pentamer-positive T cells, mature effector memory T cells (TEM), defined by expression of CD45RO in the absence of CCR7, dominated under both culture conditions (Figure 3A), while a minority of the T cells were TCM (CD45RO+, CCR7+). No significant difference was found between the proportions of TCM cells in γδ T cell-induced versus DC-induced cultures (2.3 ± 0.9% versus 2.6 ± 1.6% cultures.
Figure 3. CTLs expanded in response to antigen presentation by γδ T cells have an effector memory phenotype and exert potent and peptide-specific effector functions.
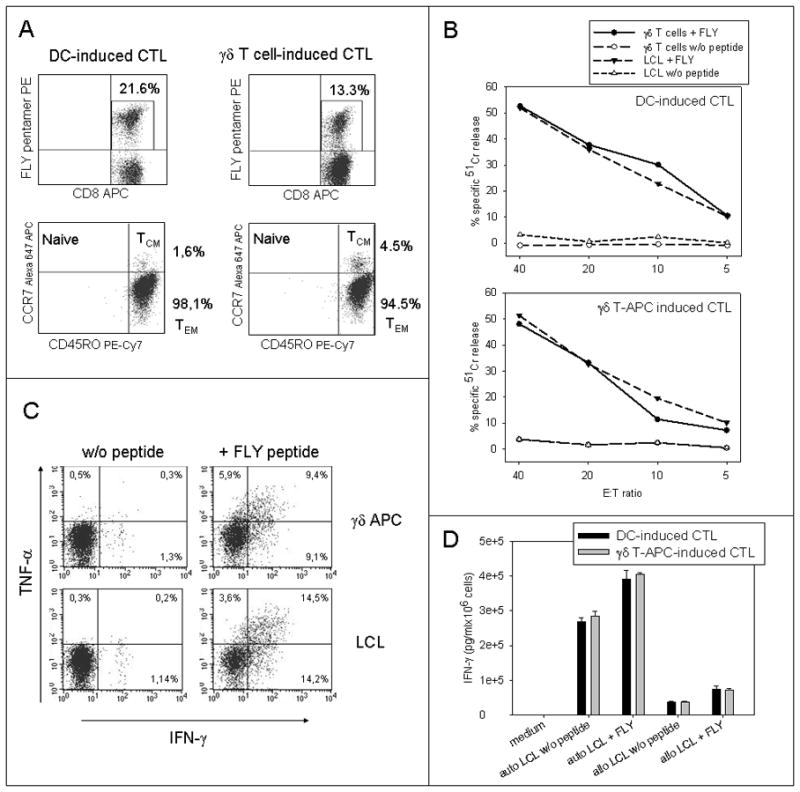
(A) The effector phenotypes of FLY-specific CTLs generated by stimulation with peptide-pulsed autologous γδ T-APCs were compared with CTLs expanded in response to peptide presentation by mature autologous DCs by staining of gated CD8+ FLY pentamer-positive cells with CD45RO- and CCR7-specific antibodies (representative experiment of four). (B) Cytolysis of FLY peptide-pulsed and unpulsed targets by autologous CTLs induced by coincubation with peptide-pulsed autologous DCs or γδ T cells (representative experiment of three). Unpulsed γδ T cells provide the negative control in this experiment. (C) Intracellular IFN-γ and TNF-α secretion by CD8+ pentamer+ CTLs induced by coincubation with peptide-pulsed autologous γδ T cells in response to stimulation with either unpulsed or peptide-pulsed LCLs (lower panel) or autologous γδ T cells (upper panel), as assessed by flow cytometry (representative experiment of two). (D) IFN-γ secretion by CTLs induced by coincubation with FLY peptide-pulsed autologous DCs or γδ T-APCs in response to either unpulsed or pulsed autologous (auto) or allogeneic mismatched (allo; negative control) LCLs, as assessed by ELISA (representative experiment of three, means and standard deviations of triplicate samples).
To assess the functional properties of the expanded T cells, we determined their specific cytokine secretion and target cytolysis. CD8+ T cells expanded in the presence of FLY peptide-pulsed activated γδ T cells efficiently and specifically lysed peptide-pulsed LCLs or autologous γδ T cells (Figure 3B). and secreted IFN-γ and TNF-α in response to peptide-pulsed targets (Figure 3C, D). While the capacity of CTLs expanded in the presence of peptide-pulsed autologous DCs versus γδ T cells to perform peptide-specific cytolysis and cytokine secretion slightly varied within individual donors, no consistent superiority of either of the two CTL populations was apparent (not shown). The peptide specificity and MHC restriction of target recognition were confirmed in control experiments using HLA-mismatched allogeneic LCLs or unpulsed APCs as stimulator cells of cytokine release (Figure 3D), as well as in MHC class I blocking assays (not shown). Lysis of autologous LCLs expressing wild-type LMP2 by CTLs expanded in the presence of either kind of antigen-presenting cells was above the background of unpulsed γδ T cells, but in a range not exceeding 15% in any of the donors. Likewise, the flow cytometry based IFN-γ secretion assay failed to demonstrate significant functional responses of CD8+ T cells to unpulsed LCLs. By contrast, assessment of IFN-γ secretion by the more sensitive ELISA revealed efficient interaction of FLY-specific CTLs with autologous but not allogeneic LCLs, likely representing endogenous presentation of the LMP2a antigen at low densities (Figure 3D). These data demonstrate that peptide presentation by γδ T cells induces robust expansion of fully functional and antigen-specific effector memory CTLs.
γδ T cells efficiently present endogenous full-length antigen and induce expansion of CTLs with broad epitope specificity
To investigate whether γδ T cells are capable of endogenously processing full-length antigens, followed by transport to the surface and presentation on class I MHC molecules, we introduced the full sequence of the LMP2a gene into activated γδ T cells from 4 individual EBV-seropositive donors by retroviral gene transfer. The transduction efficiency as assessed by GFP expression was 13.8±6.4% (range 4.7–20.0%). For coincubation experiments, LMP2a-expressing γδ T APCs were selected by flow cytometry cell sorting of GFP-positive cells (purity >95%). In the presence of LMP2a-gene-transduced autologous γδ T cells, T cells specific for the LMP2a peptide FLY were successfully expanded from polyclonal T cells (1.1± 0,7%, range 0.4–2.2%) (Figure 4A). Thus, activated γδ T cells efficiently process and present endogenous antigen.
Figure 4. LMP2a gene-transduced γδ T APCs induce peptide-specific CTLs within a population of CD8+ autologous responder T cells.
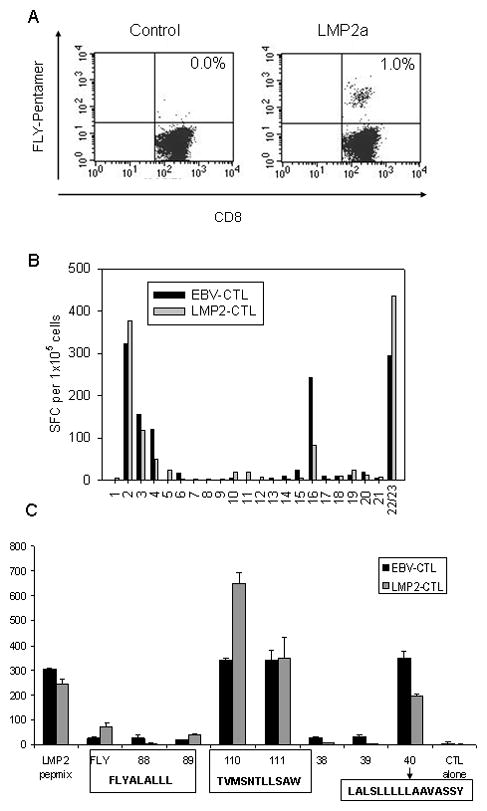
(A) Purified CD8+ T cells from a normal donor (HLA type A2;25/B15(62)) were coincubated with autologous γδ T cells retrovirally transduced with either control (14.G2aζ) or LMP2a gene for 12 days, followed by quantification of HLA A2-restricted FLY pentamer-positive cells by flow cytometry. (B) The breadth of the LMP2 epitope specificity of a CTL line initiated using γδ T cells transduced with retrovirus LMP2 (LMP2-CTL) was compared to a CTL line initiated with an autologous lymphoblastoid cell line (EBV-CTL). CTLs (1×105/well) (HLA-A2;25/B15(62)) were stimulated with an LMP2-peptide library pooled into 20 pools. Responses were measured in an 18-hour IFN-γ ELISPOT assay. All peptides were divided into 20 pools in such a way that each peptide is present in 2 pools. Shown is one of two experiments with two donors. (C) Responses to these peptide pools could subsequently be mapped to 3 different epitopes (HLA-A2- restricted FLYALALLL, HLA-A25- restricted TVMSNTLLSAW and a further epitope the restriction of which is yet to be determined LALSLLLLLAAVASSY) demonstrating that the breadth of the LMP2-specific T cell response is similar in both EBV-CTL and LMP-CTL. Shown is mean and standard deviation of duplicate wells.
In additional experiments, the epitope specificities of LMP2a-specific CTLs generated from HLA-A2-positive donors by stimulation with autologous LCLs versus LMP2a-transduced autologous γδ T cells were compared by screening against a variety of known and unknown LMP2 peptides. CTLs expanded in response to LMP2a full-length antigen presentation secreted IFN-γ in response to several different LMP2 peptides, representing discrete epitopes on the LMP2 antigen. For each of the two donors tested, the pattern of epitope reactivity was comparable to that obtained by stimulation with autologous LCL (Figure 4B). Thus, the breadth of the antigen response induced by γδ T cells is not inferior to natural antigen presentation.
Autologous γδ T cells induce LMP2a-specific T cell expansion in EBV-seropositive cancer patients
To investigate the feasibility of the approach in cancer patients, we analysed the specific stimulatory capacity of γδ T cells from two pediatric patients undergoing chemotherapy for Hodgkin lymphoma. In the Hodgkin lymphoma patient who was HLA-A2-positive, FLY pentamer-positive T cells were efficiently induced in cocultures of non-selected PBMCs with FLY peptide pulsed autologous γδ T cells (Figure 5A). In the second patient who was HLA-A2-negative, SFG-LMP2a-GFP-transduced γδ T cells were used as stimulator cells. As a read-out for the induction of LMP2a-specific T cell responses, we assessed the number of spot-forming cells in an IFN-γ ELISPOT assay. Comparable to the healthy donors, retroviral transduction of aminobisphosphonate-activated T cells resulted in 14% GFP-expressing γδ T cells. Stimulation of autologous CD8+ T cells with positively selected SFG-LMP2a-GFP γδ T cells induced a strong increase of IFN-γ producing cells compared to those exposed to non-transduced γδ T cells or γδ T cells transduced with SFG-GFP containing an irrelevant gene insert (Figure 5B).
Figure 5. Autologous γδ T cells induce LMP2a-specific CTL expansion in cancer patients.
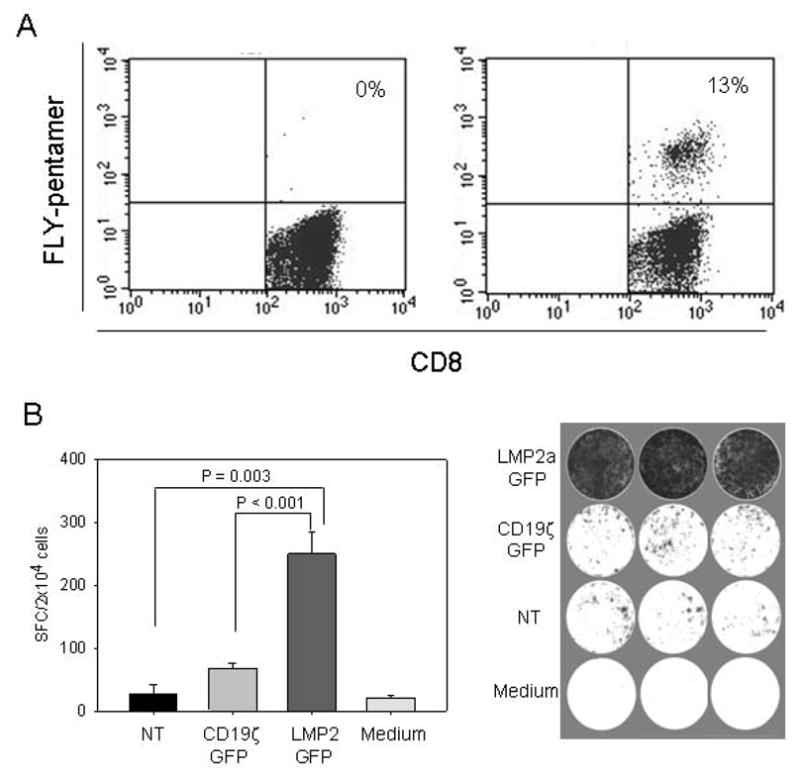
(A) PBMCs from an HLA-A2-positive patient with Hodgkin lymphoma were coincubated with autologous γδ T cells as stimulator cells, either unpulsed (left panel) or pulsed with the LMP2a epitope FLY (right panel). The percentage of FLY-specific CTLs 10 days after stimulation was assessed by pentamer staining followed by flow cytometry analysis within a gate set on CD3+/CD8+ T cells. (B) γδ T cells from an HLA-A2-negative patient with Hodgkin lymphoma were retrovirally transduced with full-length LMP2a (SFG-LMP2a-GFP), and GFP-expressing cells were positively selected by FACS. Autologous CD8+ T cells purified from peripheral blood lymphocytes by FACS were prestimulated with LMP2a-transduced γδ T cells for 11 days. The negative cell fraction after sorting of CD8+ T cells was prestimulated with CD3- and CD28-specific antibodies, then transduced with either SFG-LMP2a-GFP or an irrelevant control vector (SFG-CD19ζ-GFP). To determine antigen-specific cytokine secretion, triplicate samples of LMP2a-prestimulated cells (2×104cells/well) were restimulated with 8×104/well autologous transduced CD8-negative T cells, and IFN-γ secretion was quantified by ELISPOT analysis. Control wells contained LMP2a-prestimulated CD8+ T cells coincubated with autologous non-transduced or control vector-transduced CD8-negative T cells, or with medium alone.
DISCUSSION
In this paper, we propose to exploit the immunostimulatory qualities of human γδ T cells for establishing more effective immunotherapeutic strategies in viral and malignant disease. Such strategies rely on the potent ex vivo or in vivo expansion of effector memory T cells with defined specificities for subdominant antigens and adequate functional properties. We show that aminobisphosphonate-activated human peripheral blood γδ (Vγ9Vδ2) T cells acquire surface expression of various T-cell-stimulatory molecules and secrete substantial amounts of TNF-α as major prerequisites for efficient T cell stimulation. In an EBV model, γδ T cells stimulate potent and specific CD8+ T cell responses to even subdominant, tumor-associated antigens. Importantly, they efficiently process and present endogenous antigen, demonstrated by induction of T cell responses to a broad spectrum of LMP2 peptides following transduction with the gene encoding full-length LMP2 antigen. Thus, γδ T cells emerge as another cell population to induce powerful cytolytic T cell responses to weak viral and tumor-associated antigens and may thus provide useful tools for generating effector cells for immunotherapy.
Immunity to EBV was previously shown to provide an excellent model for the induction of therapeutic T cell memory responses in humans2. Due to the expression of EBV antigens in virus-associated tumor cells19–21, their significance extends beyond viral disease towards cancer. EBV antigens with varying T cell stimulatory capacities have been identified. The immediate early protein BZLF-1 has a high capacity for inducing potent T cell responses, resulting in efficient immune control of viral reactivation36. Here, it was used as a prototype for a highly immunogenic antigen with marginal requirements for professional antigen presentation. The viral latency antigen LMP2a, by contrast, is a weak stimulator of the immune response and thus more representative of the majority of tumor-associated antigens. Its consistent expression on the malignant cells in EBV-positive Hodgkin′s lymphoma and other EBV-associated diseases has stimulated ongoing efforts in therapeutic targeting of this protein, and the first clinical studies have now demonstrated the feasibility and promise of this approach1;3. The dependence of LMP2a-specific CTL expansion on the potency of antigen presentation is well defined and thus allowed us to evaluate the immunostimulatory quality of γδ T cells.
The ideal APC for clinical adoptive immunotherapy will permit selective expansion of potent T cells with a cytolytic effector phenotype and exclusive specificity for a given tumor antigen. We show here that bisphosphonate-activated γδ T cells fulfill this requirement. While the stimulation of T cells with suboptimal APCs was shown to induce anergy or apoptosis35, presentation of viral epitopes to memory CD8+ T cells by γδ T cells induced robust expansion of peptide-specific, functionally competent CTLs. Importantly, potent CD8+ T cell stimulation was achieved both by short-term presentation of exogenous peptides and after retroviral delivery of full-length antigen, which more closely mimics the physiological presentation of viral and tumor antigens. In direct comparison with DCs, neither the epitope specificity nor the effector functions of γδ T-cell induced CTLs were compromised. LMP2 epitope-specific CD8 cells did not kill wild-type LCL targets in short-term cytotoxicity assays, however, specific and functional interactions with autologous LCLs became apparent in a high sensitivity IFN-γ ELISA, confirming previous observations with EBV-latent cycle antigen-specific T cells1;37. Importantly, γδ T cells expressing full-length antigen elicited an equally broad epitope-specific T cell response as natural EBV antigen presentation provided by autologous LCLs.
From a practical point of view, a therapeutic APC should be available in large numbers and easily expandable. This is an important disadvantage of DCs, since their expansion is a time-consuming process that involves expensive cytokines which are often unavailable as clinical-grade reagents. Another significant obstacle to the therapeutic use of DCs is their functional heterogeneity and unpredictable characteristics after ex vivo manipulation, as well as their lack of adequate function in many cancer patients6. To facilitate clinical immunotherapy, various sources of alternative APCs have been investigated. Schultze et al. were the first to propose the use of B cells activated via their CD40 receptor as alternative APCs for inducing tumor antigen-specific T cell responses9. More recently, contact-activated monocytes were shown to function as potent APCs for the stimulation of recall responses against subdominant viral antigens38. Furthermore, artificial APCs (AAPCs), generated by modification of a scaffold cell with the desired costimulatory molecules, were effective stimulators of CTLs specific for tumor-associated peptides7;8. Antigen-presenting function was also found in activated CD4+ T cells39–41, though comparative experiments with DCs have revealed a substantially inferior capacity to induce CTL proliferation10.
Exploiting γδ T cells to generate antigen-specific CTLs for adoptive immunotherapy has a number of potential advantages over existing strategies. An important point is the reported ease of γδ T cell expansion and ex vivo manipulation. As we confirm in this study, high numbers of γδ T cells, superior to iDCs, can be obtained by ex vivo culture of peripheral blood lymphocytes from both normal donors and immunocompromised cancer patients in the presence of nitrogen-containing bisphosphonates11. The APC properties of γδ T cells are induced by stimulation with pharmacologically relevant doses of bisphosphonates, which activate γδ T cells by interference with the mevalonate pathway and subsequent intracellular enrichment of their natural TCR ligand isopentenyl pyrophosphate (IPP)16. Thus, by drug-induced restimulation in vivo, γδ T cells may become a persistent and renewable source of APCs. In contrast to “off the shelf” artificial APCs, which may trigger allogeneic immune responses, autologous γδ T-APCs are immunologically inert, which allows to focus the immune activation on the desired, generally weakly immunogenic tumor antigens. As shown here, the susceptibility of γδ T cells to genetic modification allows for stable expression of full-length antigen, enabling autologous presentation of the entire spectrum of relevant peptides for a certain virus or tumor protein. This is an important advantage over DCs which, due to their failure to divide and proliferate, do not allow for stable integration of foreign genes. Stable gene transfer into γδ T cells may further be a useful tool to manipulate their natural characteristics and improve their APC function, homing and survival by artifical expression of costimulatory, cytokine or chemokine genes. For instance, transgenic expression of IL-2 and IL-15 in T-cell based APCs was recently shown to increase the expansion of CTLs both in vitro and in vivo in a strictly antigen-dependent manner42 and may thus be useful to prolong the in vivo survival of therapeutic γδ T cells.
A strong argument supporting the use of γδ T cells in cancer therapy is their effector function and native antitumor activity15;16, which are thought to contribute to tumor immune surveillance in vivo and provide the rationale for current concepts of using bisphosphonate-activated γδ T cells for immunotherapy of cancer28;43. Indeed, two independent studies have shown a correlation between increased numbers of γδ T cells and antitumor control in patients with advanced cancer treated with aminobisphosphonates and IL-211;43. As demonstrated in Figure 1C, we confirm that aminobisphosphonate activation of γδ T cells induces not only upregulation of immunostimulatory molecules, but also selective expansion of CD45RO+CD27- TEM cells, which have previously been shown to exert potent cytolytic effector functions44.
Combined effector and stimulatory properties have recently been identified in individual immune cell populations in mice and humans45–47. γδ T cells apparently represent a further example for a multifunctional cell receiving integrated signals that lead to both APC and immediate effector activities. An unresolved question regards the extent to which γδ T cells can mimic the superb qualities of DCs in lymph node homing, processing of phagocytosed external antigen, and subsequent priming of CD4+ T cell responses. While first evidence was provided that γδ T cells can stimulate primary T-cell responses to allogeneic and microbial antigens10, the detailed role of γδ T cells in inducing adaptive immune responses will have to be subject of further investigations. The most relevant question from a therapeutic point of view is whether T-cell priming is an absolute requirement for obtaining clinical anti-cancer immune responses. Numerous reports illustrate that antigen-experienced memory CTLs to tumor-associated antigens preexist in the bone marrow and peripheral blood of many cancer patients48–50. Their selective in vitro expansion for subsequent adoptive transfer or in vivo stimulation by effective immunization strategies are primary goals of current immunotherapy trials. As demonstrated here, γδ T cells are attractive candidates for inducing superior and sustained effector CTL responses to subdominant antigens in vivo and may thus serve as potent cellular vaccines in viral disease and malignancy.
Acknowledgments
This work was supported by a grant (#107844) from the Dr. Mildred-Scheel-Stiftung der Deutschen Krebshilfe (to C.R.).
Reference List
- 1.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heslop HE, Ng CYC, Li CF, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Medicine. 1996;2:551–5. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 3.Straathof KC, Leen AM, Buza EL, et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. Journal of Immunology. 2005;175:4137–47. doi: 10.4049/jimmunol.175.6.4137. [DOI] [PubMed] [Google Scholar]
- 4.Huang AYC, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of Bone-Marrow-Derived Cells in Presenting Mhc Class I-Restricted Tumor-Antigens. Science. 1994;264:961–5. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nature Reviews Immunology. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 6.Maecker B, Mougiakakos D, Zimmermann M, et al. Dendritic cell deficiencies in pediatric acute lymphoblastic leukemia patients. Leukemia. 2006;20:645–9. doi: 10.1038/sj.leu.2404146. [DOI] [PubMed] [Google Scholar]
- 7.Maus MV, Thomas AK, Leonard DGB, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nature Biotechnology. 2002;20:143–8. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 8.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nature Medicine. 2003;9:619–24. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 9.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: An alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. Journal of Clinical Investigation. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gamma delta T cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 11.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–8. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 13.Boismenu R, Havran WL. Gammadelta T cells in host defense and epithelial cell biology. Clin Immunol Immunopathol. 1998;86:121–33. doi: 10.1006/clin.1997.4468. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino T, Ohta A, Nakao M, et al. TCR gamma delta + T cells in peripheral blood of patients with adult Still's disease. J Rheumatol. 1996;23:124–9. [PubMed] [Google Scholar]
- 15.Fisch P, Malkovsky M, Kovats S, et al. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990;250:1269–73. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 16.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gamma delta cells recognize endogenous mevalonate metabolites in tumor cells. Journal of Experimental Medicine. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanno M, Seki H, Mathioudakis G, et al. Gamma/delta T cell antigen receptors expressed on tumor- infiltrating lymphocytes from patients with solid tumors. Eur J Immunol. 1992:679–87. doi: 10.1002/eji.1830220310. [DOI] [PubMed] [Google Scholar]
- 18.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annual Review of Immunology. 1997;15:405–31. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 19.Herbst H, Dallenbach F, Hummel M, et al. Epstein-Barr-Virus Latent Membrane-Protein Expression in Hodgkin and Reed-Sternberg Cells. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4766–70. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuppers R. B cells under influence: Transformation of B cells by Epstein-Barr virus. Nature Reviews Immunology. 2003;3:801–12. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- 21.Niedobitek G. Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Journal of Clinical Pathology-Molecular Pathology. 2000;53:248–54. doi: 10.1136/mp.53.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedobitek G, Kremmer E, Herbst H, et al. Immunohistochemical detection of the Epstein-Barr virus-encoded latent membrane protein 2A in Hodgkin's disease and infectious mononucleosis. Blood. 1997;90:1664–72. [PubMed] [Google Scholar]
- 23.Gahn B, Siller-Lopez F, Pirooz AD, et al. Adenoviral gene transfer into dendritic cells efficiently amplifies the immune response to LMP2A antigen: A potential treatment strategy for Epstein-Barr virus-positive Hodgkin's lymphoma. International Journal of Cancer. 2001;93:706–13. doi: 10.1002/ijc.1396. [DOI] [PubMed] [Google Scholar]
- 24.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein- Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 25.Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–13. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 26.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A. 1995;92:6733–7. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer. 2001;94:228–36. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- 28.Rischer M, Pscherer S, Duwe S, Vormoor J, Jurgens H, Rossig C. Human gamma delta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. British Journal of Haematology. 2004;126:583–92. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 29.Landmeier S, Altvater B, Pscherer S, et al. Gene-engineered varicella-zoster virus-reactive CD4+ cytotoxic T cells exert tumor-specific effector function. Cancer Research. 2007;67:8335–43. doi: 10.1158/0008-5472.CAN-06-4426. [DOI] [PubMed] [Google Scholar]
- 30.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SP, Thomas WA, Murray RJ, et al. HLA A2. 1-restricted cytotoxic T cells recognizing a range of Epstein-Barr virus isolates through a defined epitope in latent membrane protein LMP2. J Virol. 1993;67:7428–35. doi: 10.1128/jvi.67.12.7428-7435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croft M, Dubey C. Accessory molecule and costimulation requirements for CD4 T cell response. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 33.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human gamma delta T cells with zoledronate and interleukin-2 for immunotherapy of hormohe-refractory prostate cancer. Cancer Research. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pudney VA, Leese AM, Rickinson AB, Hislop AD. CD8(+) immunodominance among Epstein-Barr virus lytic cycle antigens directly reflects the efficiency of antigen presentation in lytically infected cells. Journal of Experimental Medicine. 2005;201:349–60. doi: 10.1084/jem.20041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang JYS, Chai JG, Lechler R. Antigen presentation by mouse CD4(+) T cells involving acquired MHC class II : peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101:2704–10. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 36.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. Journal of Virology. 2005;79:1296–307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SP, Brooks JM, Al Jarrah H, et al. CD8 T cell recognition of endogenously expressed Epstein-Barr virus nuclear antigen 1. Journal of Experimental Medicine. 2004;199:1409–20. doi: 10.1084/jem.20040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leen A, Ratnayake M, Foster A, et al. Contact-activated monocytes: efficient antigen presenting cells for the stimulation of antigen-specific T cells. J Immunother. 2007;30:96–107. doi: 10.1097/01.cji.0000211325.30525.84. [DOI] [PubMed] [Google Scholar]
- 39.Adamopoulou E, Diekmann J, Tolosa E, et al. Human CD4(+) T cells displaying viral epitopes elicit a functional virus-specific memory CD8(+) T cell response. Journal of Immunology. 2007;178:5465–72. doi: 10.4049/jimmunol.178.9.5465. [DOI] [PubMed] [Google Scholar]
- 40.Atanackovic D, Matsuo M, Ritter E, et al. Monitoring CD4(+) T cell responses against viral and tumor antigens using T cells as novel target APC. Journal of Immunological Methods. 2003;278:57–66. doi: 10.1016/s0022-1759(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 41.Foster AE, Leen AM, Lee T, et al. Autologous designer antigen-presenting cells by gene modification of T lymphocyte blasts with IL-7 and IL-12. Journal of Immunotherapy. 2007;30:506–16. doi: 10.1097/CJI.0b013e318046f3b1. [DOI] [PubMed] [Google Scholar]
- 42.Quintarelli C, Vera JF, Savoldo B, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilhelm M, Kunzmann V, Eckstein S, et al. gamma delta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 44.Angelini DF, Borsellino G, Poupot M, et al. Fc gamma RIII discriminates between 2 subsets of V gamma 9V delta 2 effector cells with different responses and activation pathways. Blood. 2004;104:1801–7. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- 45.Chan CW, Crafton E, Fan HN, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nature Medicine. 2006;12:207–13. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 46.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. Journal of Experimental Medicine. 2007;204:1441–51. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taieb J, Chaput N, Menard C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nature Medicine. 2006;12:214–9. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 48.Feuerer M, Beckhove P, Bai L, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–8. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 49.Greiner J, Schmitt M, Li L, et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood. 2006;108:4109–17. doi: 10.1182/blood-2006-01-023127. [DOI] [PubMed] [Google Scholar]
- 50.Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–23. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]