Abstract
Significance
Fatigue is one of the most common symptoms of cancer and its treatment, manifested in the clinic through weakness and exercise intolerance. These side effects not only compromise patient's quality of life (QOL), but also diminish physical activity, resulting in limited treatment and increased morbidity.
Recent Advances
Oxidative stress, mediated by cancer or chemotherapeutic agents, is an underlying mechanism of the drug-induced toxicity. Nontargeted tissues, such as striated muscle, are severely affected by oxidative stress during chemotherapy, leading to toxicity and dysfunction.
Critical Issues
These findings highlight the importance of investigating clinically applicable interventions to alleviate the debilitating side effects. This article discusses the clinically available chemotherapy drugs that cause fatigue and oxidative stress in cancer patients, with an in-depth focus on the anthracycline doxorubicin. Doxorubicin, an effective anticancer drug, is a primary example of how chemotherapeutic agents disrupt striated muscle function through oxidative stress.
Future Directions
Further research investigating antioxidants could provide relief for cancer patients from debilitating muscle weakness, leading to improved quality of life. Antioxid. Redox Signal. 15, 2543–2563.
Introduction
By the end of 2010, the American Cancer Society expects 1.5 million new cancer cases will be diagnosed (220). Cancer and its treatment compromises quality of life (QOL), an important indicator of patient outcome and survival in numerous cases (e.g., breast, colorectal, melanoma) (163). A component of QOL is patients' perceived fatigue, one of the most common symptoms of cancer and its treatment (182). Fatigue in cancer patients is a multifactorial condition defined by the National Comprehensive Cancer Network as “a common persistent, and subjective sense of tiredness related to cancer or to the treatment for cancer that interferes with usual functioning” (157). This type of fatigue is not relieved by rest, is exhibited by cancer patients through QOL self-assessments (31, 193, 233, 270) and intensified with the aggressiveness of chemotherapy (58, 108, 182, 193).
Pater and Loeb (181) were among the first to show that perceived fatigue is an independent predictor of QOL and survival in cancer patients. Numerous other studies have followed confirming their results (14, 20, 38, 202). A component of perceived fatigue could be a decline in cognitive function. Evaluation of cognitive impairment involves functionality of multiple domains, which include visuospatial skill, memory, language, and motor function (258).
Over half of patients undergoing chemotherapy exhibit cognitive impairment (109), which is associated with patients perceived fatigue (18). This sense of tiredness can persist from 6 months to 2 years following remission, providing insight into the debilitating, and sometimes long-term side effects of cancer and its treatment (120, 132, 149).
While documenting perceived fatigue is useful clinically, it is difficult to discriminate between a sense of tiredness (i.e., perceived fatigue) and physiological fatigue. Physiological fatigue can be divided into two components, central and peripheral fatigue. Central fatigue involves the central nervous system and the inhibition of neurological reflexes. Central fatigue is a factor with cancer (275), however the interaction of central fatigue and chemotherapy is unknown, limiting the discussion of this component further. Peripheral fatigue is muscle specific and involves the loss of muscle function, divided into two components: muscle fatigue and muscle weakness. Muscle fatigue is defined as the loss of force that is reversible by rest, while muscle weakness is an impaired ability to generate force and is not relieved by rest (177a). Based on available data, this review will discuss the effects of chemotherapy on muscle weakness.
Previous studies have used performance assessments to document muscle weakness in cancer patients that have received chemotherapy. Compared to healthy controls, patients show a slower chair-rise time, indicating a decrease in muscle strength (25, 85). Hand-grip force, another measurement of muscle weakness, is decreased in cancer patients (25, 96, 230, 231, 269). These studies point to a prominent clinical problem of debilitating muscle weakness with cancer treatment.
One underlying mechanism of the muscle weakness experienced by chemotherapy-treated patients is a developed state of oxidative stress, defined in this review as a disruption of redox signaling and control (112). Numerous chemotherapeutic agents directly or indirectly produce a state of oxidative stress. Drugs that include a quinone moiety in their chemical structure can directly produce a state of oxidative stress by interacting with molecular oxygen and undergoing redox cycling, leading to the generation of reactive oxygen species (ROS) (40). Other chemotherapeutic agents can indirectly produce a state of oxidative stress by decreasing antioxidant levels, crippling the cell's defenses against elevated oxidants (7, 158).
Circulating biomarkers are a nonspecific systemic index of oxidative stress in the body. In cancer patients under going treatment, circulating markers of oxidative stress, in the form of lipid peroxidation and protein carbonyl content, are elevated (44, 91, 105). These markers reflect events of oxidative stress that may exist in various tissues, including skeletal muscle. No current data exist to describe the level of muscle-derived oxidants in cancer patients. However, circulating biomarkers serve as an index about the level of oxidative stress in the body and could signify an elevation in muscle-derived oxidants (201).
In skeletal muscle, exposure to elevated oxidants are known to cause muscle weakness and accelerate the rate of fatigue (191, 235). Antioxidant exposure delays the rate of fatigue, supporting this connection (118, 136, 165). Chemotherapy-induced oxidative stress in cancer patients could be a reflection of the elevated muscle-derived oxidants, an underlying mechanism for the muscle weakness experienced by patients. This article reviews how chemotherapy can affect striated muscle, increasing muscle-derived oxidants and leading to muscle weakness in patients.
Anthracycline Therapy
Numerous chemotherapy drugs have been approved by the Food and Drug Administration to treat patients in the clinic (97). For the purpose of this review we focused on a class of chemotherapy drugs called anthracyclines. For over 50 years, anthracyclines have been used widely in the clinic to treat multiple types of cancers (e.g., leukemia, lymphoma, breast, prostate, ovarian, lung) (see Table 1; (86, 209)). Due to the extensive literature available, the effects of chemotherapy on muscle function are best defined in this class and accessible for evaluation. The mechanisms by which anthracyclines kill tumor cells are various, including: inhibition of DNA replication and RNA transcription, free radical generation leading to DNA damage or lipid peroxidation, DNA alkylation, interference with DNA unwinding or DNA strand separation and helicase activity, and inhibition of topoisomerase II (209). Some or all of these effects are responsible for inhibiting tumor cell growth, preventing division and metastasis. Anthracyclines can also negatively affect noncancerous tissues, including striated muscle, which contributes to the fatigue and muscle weakness in patients treated with anthracycline-based chemotherapy. A comprehensive literature search over the past 5 years (2005–2010) was performed to document fatigue reports associated with each drug in the anthracycline group (Table 1). Two anthracycline drugs (teniposide and valrubicin) were not included in the table due to the absence of current data documenting associated fatigue with drug administration.
Table 1.
Fatigue Severity Associated with Anthracycline Chemotherapy over the Last Five Years (2005–2010)
| |
NCI Common Toxicity Criteria for Adverse Events |
|
|||
|---|---|---|---|---|---|
| Anthracyclines | 1 | 2 | 3 | 4 | QOL Questionnaire |
| Daunorubicin | 11 myeloid leukemia patient (232) | ||||
| Doxorubicin | 41 esophageal cancer patients (101) | ||||
| 1,801 breast cancer patients (131) | |||||
| 38 ovarian cancer patients (183) | |||||
| 30 heterogeneous cancer patients (247) | |||||
| 23 multiple myeloma patients (36) | |||||
| 32 heterogeneous cancer patients (170) | |||||
| 162 breast cancer patients (278) | |||||
| 67 heterogeneous cancer patients (99) | |||||
| 25 breast cancer patients (279) | |||||
| 20 heterogeneous cancer patients (211) | |||||
| 38 heterogeneous cancer patients (214) | |||||
| 37 heterogeneous cancer patients (53) | |||||
| 26 heterogeneous cancer patients (130) | |||||
| 17 thyroid cancer patients (12) | |||||
| 48 ovarian cancer patients (185) | |||||
| 28 breast cancer patients (203) | |||||
| 317 endometrial cancer patients (26) | |||||
| 646 multiple myeloma patients (178) | |||||
| 271 breast cancer patients (128) | |||||
| 151 breast cancer patients (51) | |||||
| 46 prostate cancer patients (22) | |||||
| 24 biliary tract cancer patients (84) | |||||
| 106 lymphoma patients (68) | |||||
| 12 prostate cancer patients (9) | |||||
| 19 heterogeneous cancer patients (65) | |||||
| 25 breast cancer patients (27) | |||||
| 49 ovarian cancer patients (251) | |||||
| 32 ovarian cancer patients (92) | |||||
| 44 breast cancer patients (104) | |||||
| Epirubicin | 37 biliary tract cancer patients (32) | ||||
| 24 heterogeneous cancer patients (133) | |||||
| 6 breast cancer patients (262) | |||||
| 43 NSCLC patients (126) | |||||
| 63 breast cancer patients (79) | |||||
| 46 pancreatic cancer patients (200) | |||||
| 200 breast cancer patients (81) | |||||
| 60 breast cancer patients (207) | |||||
| 37 gastric cancer patients (171) | |||||
| 16 breast cancer patients (121) | |||||
| 50 breast cancer patients (212) | |||||
| 40 bladder cancer patients (246) | |||||
| 19 hepatic cancer patient (280) | |||||
| 31 prostate cancer patients (176) | |||||
| 39 SCL cancer patients (80) | |||||
| Etoposide | 22 lymphoma patients (204) | ||||
| 33 SCL cancer patients (266) | |||||
| 81 NSCL cancer patients (168) | |||||
| 447 NSCL cancer patients (221) | |||||
| 56 SCL cancer patients (184) | |||||
| 32 heterogeneous cancer patients (277) | |||||
| 63 SCL cancer patients (153) | |||||
| 36 heterogeneous cancer patients (186) | |||||
| Idarubicin | 41 leukemia patients (114) | ||||
| Irinotecan | 22 heterogeneous cancer patients (77) | ||||
| 15 cervical cancer patients (72) | |||||
| 21 pancreatic cancer patients (127) | |||||
| 51 SCL cancer patients (196) | |||||
| 11 NSCL cancer patients (179) | |||||
| 39 heterogeneous cancer patients (64) | |||||
| 51 small cell lung cancer patients (225) | |||||
| 52 head and neck cancer patients (13) | |||||
| 28 gastrointestinal cancer patients (239) | |||||
| 15 heterogeneous cancer patients (210) | |||||
| 39 esophagogastric cancer patients (70) | |||||
| 32 pancreatic cancer patients (177) | |||||
| 209 colorectal cancer patients (219) | |||||
| 32 glioblastoma patients (194) | |||||
| 16 heterogeneous cancer patients (111) | |||||
| 57 colorectal cancer patients (116) | |||||
| 56 SCL cancer patients (184) | |||||
| 68 SCL cancer patients (227) | |||||
| 48 NSCL cancer patients (83) | |||||
| 84 SCL cancer patients (8) | |||||
| 33 SCL cancer patients (222) | |||||
| 31 pancreatic cancer patients (90) | |||||
| 54 SCL cancer patients (102) | |||||
| 51 heterogeneous cancer patients (205) | |||||
| 43 NSCL cancer patients (152) | |||||
| 46 NSCL cancer patients (161) | |||||
| 55 colorectal patients (257) | |||||
| 46 NSCL cancer patients (42) | |||||
| 20 colorectal cancer patients (156) | |||||
| 142 colorectal cancer patients (56) | |||||
| 43 esophagogastric cancer patients (69) | |||||
| 48 breast cancer patients (229) | |||||
| 39 NSCL cancer patients (281) | |||||
| 45 heterogeneous cancer patients (216) | |||||
| 28 colorectal cancer patients (100) | |||||
| 50 breast cancer patients (82) | |||||
| Mixtoxantrone | 19 prostate cancer patients (35) | ||||
| Topotecan | 16 heterogeneous cancer patients (47) | ||||
| 61 SCL cancer patients (226) | |||||
| 25 heterogeneous cancer patients (265) | |||||
| 37 heterogeneous cancer patients (160) | |||||
| 17 SCL cancer patients (180) | |||||
| 41 heterogeneous cancer patients (166) | |||||
| 22 SCL cancer patients (215) | |||||
| 309 SCL cancer patients (66) | |||||
| 63 heterogeneous cancer patients (206) | |||||
| 38 SCL cancer patients (159) | |||||
| 36 heterogeneous cancer patients (186) | |||||
| 21 ovarian cancer patients (23) | |||||
The grade refers to the severity of the adverse event with a numerical scale: (1) Mild, (2) Moderate, (3) Severe, and (4) Disabling. Abbreviations: NCI, National Cancer Institute; NSCL, non-small cell lung; QOL, quality of life; SCL, small cell lung.
Most studies observing fatigue with anthracycline chemotherapy use the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) to grade the severity of patients perceived fatigue (173a). This method allows reporting of adverse events with descriptive terminology. The grade refers to the severity of the adverse event with a numerical scale: (1) Mild, (2) Moderate, (3) Severe, and (4) Disabling. The adverse event fatigue has specific descriptions associated with the numerical scale: (1) Mild fatigue relieved by rest, (2) Fatigue not relieved by rest—difficulty performing activities of daily life, (3) Fatigue not relieved by rest—interfering with activities of daily life, and (4) Disabling fatigue. In Table 1, each study lists the cancer population and the range of the grades of fatigue associated with the chemotherapy drug. A few studies used a standard QOL questionnaire the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) to report chemotherapy-associated fatigue. This questionnaire has three multi-item symptom scales measuring fatigue and global health status (3, 228). Studies that assessed fatigue using a QOL questionnaire are also included. Table 1 documents the grades of fatigue in cancer patients treated with a specific anthracycline. In studies assessing patient fatigue with anthracycline-based chemotherapy, 47% document grade 4 fatigue, categorized as disabling and effecting physical capabilities of the patients. This table also illustrates that fatigue is a common problem with anthracycline chemotherapy occurring independent of the drug type.
Aside from the antitumor effects, anthracyclines are known to have toxic side effects in normal tissue, including oxidative stress (86). Anthracyclines can generate oxidants through two mechanisms: interaction with the mitochondrial respiratory chain and through a nonenzymatic reaction with ferric iron (103). Numerous drugs in the anthracycline class cause oxidative stress in both humans and rodents.
Elevated oxidants in circulation have been reported in cancer patients following administration of the anthracycline epirubicin (28, 134, 150). Following epirubicin administration in rodents, oxidants are elevated and antioxidants are decreased in both cardiac (54) and hepatic tissue (117). Irinotecan is another example of an anthracycline that causes oxidative stress. Markers of lipid peroxidation are elevated in both plasma and intestines of rodents following irinotecan administration (259, 264). In human breast cancer cells, topotecan exposure causes a decrease in glutathione, along with an increase in lipid peroxidation (7, 243). This drug-induced oxidative stress is a potential mechanism underlying the documented fatigue experienced by cancer patients.
Chemotherapy is generally a combination of drugs administered in a standardized treatment regimen, specific for the cancer type. Various interactive effects of different drugs could occur, leading to collateral damage of nontargeted tissues. The current reductionist approach in the literature provides some clarity on the negative effects of a single chemotherapeutic agent on nontargeted tissues. The available studies offer valuable information on the drug's mechanism of action and potential interventions that could offset the negative side effects. The next step for the field is to move toward a more clinically relevant approach, in order to directly apply the findings to patients. The existing data focuses on a single chemotherapeutic agent, which provides foundational knowledge about the individual drug and lays the groundwork for future studies in patients. Based on available data involving chemotherapy effects on striated muscle, this review will focus on a single chemotherapy agent, doxorubicin, a representative anthracycline.
Doxorubicin as the Prototype
Doxorubicin is an antibiotic that exerts its antitumor activity by inhibiting DNA Topoisomerase II, thus preventing DNA replication (240). Other antitumor activities of doxorubicin include: generation of ROS leading to DNA damage and apoptotic cell death, stimulation of p53-DNA binding, activation of the caspase cascade, and DNA cross-linking (76, 155). The Farmitalia Research Laboratories of Milan discovered the drug in the early 1960s (11). Since its discovery, doxorubicin has been used widely in the clinic, seen as one of the most effective anticancer drugs (43, 263). Early on in the clinic, reports of doxorubicin causing severe fatigue (174, 175) and affecting cardiac muscle function (124) were documented. Further investigation into the mechanism of the drug revealed elevated oxidants in noncancerous tissues were a likely cause of the cardiotoxicity (40, 49, 60).
Cardiac muscle
Doxorubicin-induced cardiotoxicity is well known in the field of chemotherapy drug research, and has been reviewed widely in the literature (39, 40, 155, 162, 237, 244). The first observations of doxorubicin cardiotoxicity were by Lefrak and colleagues (124). They documented the deterioration of cardiac muscle function by echocardiography, and assessed postmortem cardiac muscle tissue of two patients after doxorubicin administration. Since that first report, numerous studies over the past 30 years have investigated the mechanisms behind doxorubicin-induced cardiotoxicity (237).
The principal mechanism of doxorubicin cardiotoxicity is an increase in oxidative stress, manifested through elevated oxidants, markers of protein oxidation, and decreased antioxidant activity (39). Elevated oxidant activity is observed in cardiomyocytes following doxorubicin exposure (52, 208, 224, 242, 274). A similar response is observed in rodent cardiac tissue, localized to the mitochondria and sarcoplasmic reticulum (59). This elevation in oxidants comes from multiple sources in the cell. The mitochondria are thought to be the primary source of doxorubicin-induced oxidants (34, 37, 48, 60, 261). Complex I (NADH dehydrogenase) of the electron transport chain is the specific site of doxorubicin reduction, forming an unstable semiquinone (49). The doxorubicin semiquinone is then oxidized to the stable quinone form, transferring an electron to oxygen to produce the superoxide anion (O2-) (162).
The elevation of oxidants caused by doxorubicin is protected with the overexpression of mitochondrial specific antioxidants. Figure 1 illustrates how overexpression of manganese superoxide dismutase protects against mitochondrial and myofilament damage caused by doxorubicin (276). Overexpression of other antioxidants such as glutaredoxin 2 (57) and glutathione peroxidase (GpX) (271) also protect against doxorubicin-induced cardiotoxicity, pointing to the involvement of mitochondria.
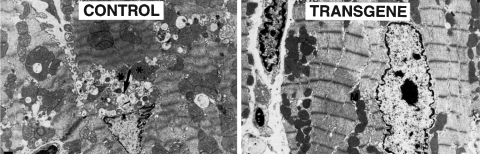
FIG. 1.
Overexpression of the mitochondrial-specific antioxidant MnSOD protects against doxorubicin-induced cardiotoxicity. Electron micrographs of mouse heart 5 days after a single injection of doxorubicin (25 mg/kg). The nontransgenic mouse treated with doxorubicin (control, left) shows variations in mitochondria shape and size, loss of cristae, and exhibits focal swelling. Myofilaments show disarray with loss of Z-bands. The transgenic mouse (transgene, right) that overexpresses human manganese superoxide dismutase (MnSOD) shows uniform mitochondria and intact cardiac myofilaments. Asterisks indicate damaged mitochondria, and M denotes intact mitochondria. The arrow points to intracytoplasmic vacuoles, a nonspecific indicator of cell injury. [From Yen et al. (276); reprinted with permission from the American Society of Clinical Investigation].
Other secondary sources of oxidants include NADPH oxidase and altered Fenton chemistry. Doxorubicin-induced oxidant activity was blunted in cardiomyocytes treated with an inhibitor of NADPH oxidase (223). Mice deficient in gp91, a required subunit for NADPH oxidase activity (123), are protected from doxorubicin-induced cardiotoxicity (268). Altered Fenton chemistry occurs during the redox cycling of doxorubicin. During this process aglycone metabolites are produced that alter iron homeostasis, leading to elevated oxidants (155).
Based on its chemical structure, doxorubicin can directly stimulate an increase in oxidants by undergoing redox cycling. However, a secondary, indirect method for doxorubicin to stimulate oxidants is via an inflammatory response. One potential secondary mediator of the inflammatory response is tumor necrosis factor alpha (TNF), a pro-inflammatory cytokine produced by many cell types, including cardiac and skeletal myocytes. Circulating levels of TNF are elevated in cancer, with chemotherapy exacerbating this response (24). Doxorubicin-treated animals and patients exhibit a stress response, characterized by an increase in serum levels of inflammatory cytokines, especially TNF (87, 167, 238, 250). Doxorubicin not only stimulates an increase in circulating TNF, but also increases the production of TNF by cardiac muscle (169) and upregulates the TNF receptor subtype 1 (TNFR1) in cardiomyocytes (41). Inhibition of TNF prevents doxorubicin-induced cardiotoxicty and diminishes glutathione depletion and lipid peroxidation (158). TNF is known to elevate ROS in striated muscle (95, 135), mediated through TNFR1 signaling (95). Elevated levels of TNF caused by doxorubicin are an indirect method to increase oxidants and lead to cardiac muscle dysfunction.
This increase in oxidants can modify vital proteins, leading to cardiac muscle dysfunction. Markers of protein oxidation, such as nitrotyrosine and protein carbonylation, are increased in cardiac muscle with doxorubicin exposure (34, 129, 217). Alterations of vital proteins can alter cardiac structure, leading to impaired contractile function. Cardiac tissue exposed to hydrogen peroxide results in altered myofibrillar proteins: troponin I, tropomyosin, actin, and myosin-binding protein-C (16, 30). The post-translational modifications of myofibrillar proteins leads to a decrease in maximal force and compromises cardiac function (16). Oxidative modifications of myofibrillar proteins can alter myofilament structure, resulting in dysfunction of striated muscle.
Doxorubicin can also cause oxidative stress by altering cellular antioxidant expression and activity. In the literature, discrepancies exist between whether doxorubicin inhibits inherent antioxidants, or stimulates an increase in antioxidant activity. In rodent cardiac muscle, data exist in both groups. In vivo doxorubicin administration decreases the content of antioxidants: superoxide dismutase (SOD) (93, 158), catalase (CAT) (158), and glutathione (GSH) (106, 125, 158). Activity of SOD is also diminished with doxorubicin (67, 125, 154). These studies hypothesize one mechanism doxorubicin stimulates oxidants is by diminishing cellular antioxidants. However, other studies show an increase in SOD (4, 6, 73), CAT (6, 73, 93, 106, 158), and GpX (4) activities with doxorubicin treatment. These data support the hypothesis of a cellular adaptive response to doxorubicin, elevating antioxidant activity to combat the doxorubicin-induced increase in oxidants.
Studies involving the administration of antioxidants in combination with doxorubicin support oxidant involvement. Rodents administered N-acetylcysteine (NAC) before doxorubicin exposure were protected from doxorubicin-induced cardiotoxicity and myocardial lesions (61). The same results were observed in a canine model of doxorubicin treatment (97). NAC is a nonspecific reduced thiol donor that increases muscle cysteine and GSH availability (78). Doxorubicin is known to decrease GSH content (106, 125, 158), a vital antioxidant in striated muscle. Infusion of glutathione prevented the cardiac contractile impairment caused by doxorubicin (256). Other antioxidants also protect cardiac muscle function in doxorubicin-induced cardiotoxicity. Vitamin E, a lipid soluble antioxidant, protected against doxorubicin-induced left ventricular dysfunction (195) and prevented elevated oxidant activity (19).
Cardiotoxicity limits the amount of doxorubicin given in the clinic (43, 236). However, on this limited dose, numerous reports show patients receiving doxorubicin-based chemotherapy experience debilitating fatigue, often in the moderate to severe category (Table 1). This persistence of weakness indicates that other striated muscles, involved in exercise and daily activity, may be affected.
Skeletal muscle
Individual case reports document physician-observed lower extremity muscle weakness in patients undergoing doxorubicin-based chemotherapy (94, 110). Schwartz documented weakness and fatigue in breast cancer patients through four cycles of doxorubicin chemotherapy (213). Following doxorubicin exposure, patients exhibited a decline in functional ability (12 min walk test) and a rapid increase in fatigue (Visual analogue scale). Weakness and fatigue are evident 1–5 years after doxorubicin exposure in lymphoma and leukemia patients (68, 248, 255). These studies suggest skeletal muscle weakness caused by doxorubicin exposure.
Existing studies in both rodents and humans show the negative effects of doxorubicin on skeletal muscle. The toxicity of doxorubicin is used in the clinic for the treatment of blepharospasm and cervical dystonia, causing permanent muscle necrosis in patients. Direct injection of doxorubicin into skeletal muscle causes loss of muscle mass, altered myofilament structure and depressed force in rodents (45, 46, 74, 75, 139–143, 145), nonhuman primates (138, 139, 144, 146), and patients (147, 267). Doxorubicin is also administered through isolated limb perfusion in patients with limb sarcoma tumors. This therapy leads to loss of limb muscle function and reduction in size of both Type 1 and Type 2 muscle fibers (21). Pfieffer and associates used a canine model of isolated limb perfusion with doxorubicin, observing a significant increase in doxorubicin concentrations in the quadriceps, along with muscle atrophy and weakness (187).
Rodent models of systemic doxorubicin treatment consistently reveal a negative affect of doxorubicin on skeletal muscle function. The few studies in the field use an intraperitoneal route of administration for doxorubicin, adapted from the cardiac literature and related to clinical treatment of advanced ovarian cancer or peritoneal carcinomatosis (234, 252). Doroshow and colleagues observed skeletal muscle loss of myofibrillar organization and interstitial edema following a single intraperitoneal injection of doxorubicin (62). This method results in skeletal muscle toxicity exhibited by nucleolar segregation and altered distribution of the perinucleolar chromatin in hindlimb skeletal muscle (151, 254).
Doxorubicin also causes a catabolic response leading to the loss of muscle mass. Patients undergoing doxorubicin-based chemotherapy show loss of muscle mass (21, 245). The skeletal muscle atrophy induced by doxorubicin is thought to occur through the upregulation of the E3 ubiquitin-ligase atrogin1/MAFbx, suggesting catabolism through the proteasome pathway (273). Doxorubicin is a known stimulator of apoptosis in cardiac myocytes (237), with possible similar effects on skeletal muscle that could contribute to catabolism. Apoptosis is induced in C2C12 myotubes following exposure to doxorubicin in vitro (98). Oxidant-mediated apoptosis is a common signaling pathway in skeletal muscle atrophy, leading to caspase activation and proteolysis (15). In striated muscle, doxorubicin stimulates both the formation of ROS (242) and activation of caspases (261), which could be linked to apoptotic signaling pathways. We have documented significant loss of muscle mass in both hindlimb (87) and respiratory muscle (88) following doxorubicin treatment.
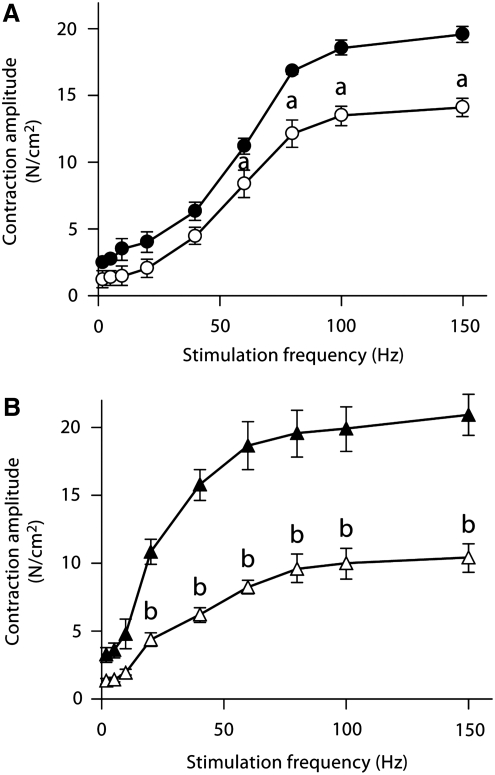
In vitro contractile function preparations have been used to determine the negative effects of doxorubicin on skeletal muscle function. Daily injections of a low dose of doxorubicin (1.15 mg/kg) depressed hindlimb muscle force, both fast-twitch and slow-twitch muscles, 4 weeks following exposure (71) (Fig. 2). The authors hypothesized the loss of force was associated with a decrease in the muscle-specific isoform of SERCA, an effect on calcium homeostasis (71). We have shown a single injection of doxorubicin depresses force of hindlimb and respiratory muscles (Fig. 3) (87). Most recently, we established a model of doxorubicin-chemotherapy using an intravenous injection, a method commonly used in the clinic (33, 43). Our studies conclude that doxorubicin causes respiratory muscle dysfunction, despite the route of administration (88).
FIG. 2.
Doxorubicin depresses skeletal muscle force. Hindlimb muscles were obtained from rats 15 days after daily injections of doxorubicin (1.15 mg/kg/day). Muscles were placed in a tissue bath and isometric contraction measurements were made with a force transducer. Doxorubicin depressed force (N/cm2) in both the EDL (A) and soleus (B) muscles. Open symbols represent the doxorubicin group, closed symbols represent the saline injected controls (n=6–9 muscles for each group). Data are mean±SEM. ap<0.05, bp<0.01 vs. control. [From Ertunc et al. (71); reprinted with permission from S. Karger AG, Basel].
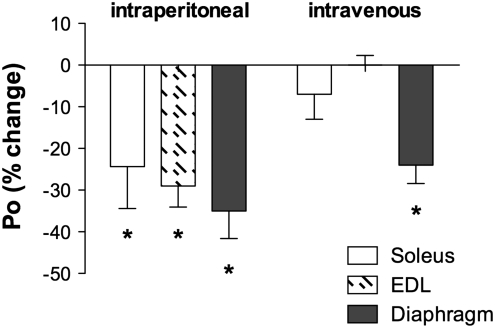
FIG. 3.
A single injection of doxorubicin depresses force in hindlimb and respiratory muscles. Figure depicts data replotted from recent reports (87, 88) and unpublished observations (L.A. Gilliam). Maximal specific force (N/cm2, Po) of soleus (open bars), EDL (hatched bars), and diaphragm (solid bars) was measured 72 h following a single injection of doxorubicin (20 mg/kg) via an intraperitoneal (left) or intravenous (right) injection (n=3–11 per muscle). Data expressed as a percent change of experimental control. Mean values shown±SEM; *p<0.01.
Studies have shown doxorubicin accumulates in skeletal muscle up to 24 h following administration (62, 187). This accumulation of doxorubicin in the muscle suggests a direct effect of doxorubicin on skeletal muscle function, however the data are divided. In vitro doxorubicin experiments have been conducted using varying concentrations of the drug (1–175 μM). van Norren and colleagues observed a depression in absolute force and an accelerated rate of fatigue in intact hindlimb muscles exposed to doxorubicin (253). They observed a dose-dependent depression in force, with impaired relaxation. Parallel experiments using lower doxorubicin bath concentrations (2 μM), similar to serum levels found in patients (55, 188), found no change in skeletal muscle force (87, 89).
Studies utilizing permeabilized skeletal muscle fibers have observed how direct doxorubicin exposure alters calcium homeostasis. Doxorubicin increases the rate of tension development in calcium-activated fibers (50, 282). The doxorubicin-induced tension development is blunted by ruthenium red, an inhibitor of the ryanodine receptor, suggesting doxorubicin alters calcium availability. Isolated skeletal muscle sarcoplasmic reticulums exposed to doxorubicin show increased calcium release (241, 282). This is in agreement with another study showing increased calcium influx in C2C12 myotubes exposed to doxorubicin (253). The data suggests doxorubicin acts similar to caffeine, sensitizing the ryanodine receptor to activation calcium, and stimulating calcium release from the SR (5, 282). Intact single fibers exposed to doxorubicin show an increase in tetanic intracellular calcium that does not alter tetanic force (87). These studies investigating doxorubicin-induced skeletal muscle dysfunction establish a negative effect at the molecular level that could be related to weakness in patients undergoing chemotherapy.
One potential mechanism of doxorubicin-induced skeletal muscle dysfunction is an induced state of oxidative stress, similar to cardiac muscle. Few studies exist that analyze doxorubicin-induced oxidative stress in skeletal muscle. We have shown doxorubicin increases oxidant activity in skeletal muscle, along with markers of protein oxidation (88). Multiple sources where oxidants are produced exist in skeletal muscle, including the mitochondrial electron transport chain, NADPH oxidase, and phospholipase A2 (78, 107, 191). Yamada and colleagues showed a decrease in complex I activity in isolated mitochondria from skeletal muscle following multiple low dose doxorubicin injections (272).
Similar to cardiac muscle, elevated TNF levels caused by doxorubicin can lead to increased oxidants and muscle weakness in skeletal muscle. Exposure to TNF depresses both respiratory and hindlimb skeletal muscle force (95, 198), shown to be mediated through TNFR1 (95). The accepted mechanism by which TNF mediates contractile dysfunction is through elevated ROS (95). Pretreating with Trolox, a hydrophilic antioxidant, can prevent TNF-induced contractile dysfunction (95). Taken together, these results suggest that elevated levels of TNF caused by doxorubicin could exert an additive oxidant effect, leading to skeletal muscle dysfunction. We have shown TNFR1 signaling is required for doxorubicin-induced muscle weakness in both respiratory and hindlimb muscle (87, 89). TNF mediates the majority of signaling through TNFR1, leading to the activation of cytotoxic cascades and elevated ROS (95, 260). Increased levels of TNF following doxorubicin exposure lead to an additive oxidant effect, contributing to doxorubicin-induced muscle weakness.
In skeletal muscle, elevated oxidants are known to cause muscle weakness and accelerate fatigue, reviewed previously (191, 218, 235). Exposure to high concentrations of exogenous oxidants results in muscle weakness (10, 29, 122, 173, 189, 190). The rate of fatigue is slowed with antioxidant exposure, suggesting a prominent role for oxidants (119, 164, 165, 197). In a state of oxidative stress, redox-sensitive proteins can be modified, altering signaling and contractile function. Exposing myofibrillar proteins, such as myosin and actin, to oxidants can result in modifications that alter protein structure and formation, and decrease force generation (17, 115, 192).
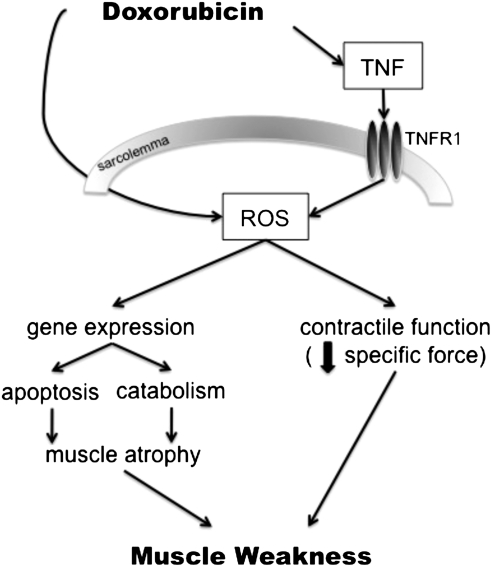
How doxorubicin effects skeletal muscle function is an emerging field. Based on the cardiac literature and current pool of data, the expected underlying mechanism is oxidative stress, occurring via a two-fold pathway (Fig. 4). Doxorubicin can directly stimulate ROS production through redox cycling, or indirectly via TNF-signaling. These two mediators, ROS and TNF, can then lead to the negative effects of doxorubicin on skeletal muscle function. The documented weakness in cancer patients undergoing chemotherapy is a significant clinical problem, welcoming studies for interventions to alleviate these severe side effects.
FIG. 4.
Hypothesized pathways for doxorubicin-induced weakness in skeletal muscle. Illustration depicts the two proposed mediators, ROS and TNF, along with hypothesized downstream signaling involved in mediating muscle weakness caused by doxorubicin. ROS, reactive oxygen species; TNF, tumor necrosis factor-alpha; TNFR1, TNF receptor subtype 1.
Future Studies
The emerging field of skeletal muscle dysfunction with chemotherapy warrants further investigation. The majority of reports in the literature document perceived fatigue through patient self-report or physician observations. Only a few studies use quantitative measures to show a decrease in muscle strength (21, 142, 230, 267). The process by which chemotherapy drugs elevate oxidants and cause weakness is also undefined. Documentation of skeletal muscle weakness, along with the mechanism involved, are required.
Translational studies are needed to investigate interventions that would prevent the debilitating weakness and fatigue with chemotherapy. The majority of chemotherapy drugs administered cause oxidative stress in noncancerous tissues, making antioxidant interventions attractive. A recent study published in this journal showed that a cysteine-rich protein diet increased muscle strength (hand-grip force) and quality of life in patients undergoing chemotherapy (245). Control comparisons were made with patients receiving an alternative protein diet that was equal in protein content with only minimal quantities of cysteine, indicating a possible antioxidant effect. Cysteine availability is vital in maintaining adequate glutathione levels, an important cellular antioxidant. Cysteine is the rate-limiting step in glutathione synthesis, an amino acid that can replenish loss of glutathione caused by doxorubicin (4, 106, 113). Rodent studies utilizing the antioxidant NAC also support antioxidant therapy. Pretreatment with NAC prevented loss of body weight and cardiotoxicity caused by doxorubicin (61, 97). NAC could also benefit skeletal muscle dysfunction caused by chemotherapy. In healthy individuals, NAC promotes endurance, improving exercise performance, and slowing the rate of fatigue (136, 137, 148, 199). A few studies published in the early 1980s assessed NAC protection against doxorubicin-induced cardiomyopathy in patients. Dresdale and associates administered NAC to disease-free cancer patients with documented doxorubicin-induced cardiomyopathy (63). NAC did not reverse the abnormal cardiac function in patients, administered over 2 years following chemotherapy. The acute and chronic cardiotoxicity caused by doxorubicin, evident by an increase in tubular area and mitochondrial damage, was not protected with NAC (172, 249). These early studies did not address the timing or dose dependency of NAC, necessitating further studies of the drug, possibly with interventions given pre-chemotherapy exposure.
NAC is not the only antioxidant available to use as a research tool. Further research investigating antioxidants already approved for human use could provide relief for cancer patients from debilitating muscle weakness. An emerging field is developing that requires more systematic testing. As shown in Table 1, patients report fatigue while exposed to multiple different chemotherapeutic agents within the anthracycline class. Fatigue and weakness are common side effects in cancer treatment. These debilitating side effects not only compromise quality of life in patients, but also limit the effectiveness of the treatment. Studies investigating the mechanistic link between chemotherapy-induced oxidative stress and muscle dysfunction lay the groundwork for the development of novel therapies that can lead to improved quality of life and increased physical activity in patients.
Abbreviations Used
- CAT
catalase
- CTCAE
National Cancer Institute Common Toxicity Criteria for Adverse Events
- EORTC QLQ-C30
European Organization for Research and Treatment of Cancer QLQ-C30
- GpX
glutathione peroxidase
- GSH
glutathione
- NAC
N-acetylcysteine
- NCI
National Cancer Institute
- NSCL
non-small cell lung
- QOL
quality of life
- ROS
reactive oxygen species
- SCL
small cell lung
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- TNF
tumor necrosis factor-alpha
- TNFR1
TNF receptor subtype 1
Acknowledgments
The authors thank Erin Wolf for editorial assistance. Our work in this area is supported by the National Institutes of Health Grant R01CA139843 (to D.K. St. Clair) and by the American Heart Association Predoctoral Fellowship 09PRE2020088 (to L.A.A. Gilliam).
References
- 1–2. These references have been deleted.
- 3.Aaronson NK. Ahmedzai S. Bergman B. Bullinger M. Cull A. Duez NJ. Filiberti A. Flechtner H. Fleishman SB. de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 4.Abd El-Gawad HM. El-Sawalhi MM. Nitric oxide and oxidative stress in brain and heart of normal rats treated with doxorubicin: Role of aminoguanidine. J Biochem Mol Toxicol. 2004;18:69–77. doi: 10.1002/jbt.20013. [DOI] [PubMed] [Google Scholar]
- 5.Abramson JJ. Buck E. Salama G. Casida JE. Pessah IN. Mechanism of anthraquinone-induced calcium release from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1988;263:18750–18758. [PubMed] [Google Scholar]
- 6.Adachi T. Nagae T. Ito Y. Hirano K. Sugiura M. Relation between cardiotoxic effect of adriamycin and superoxide anion radical. J Pharmacobiodyn. 1983;6:114–123. doi: 10.1248/bpb1978.6.114. [DOI] [PubMed] [Google Scholar]
- 7.Akbas SH. Timur M. Ozben T. The effect of quercetin on topotecan cytotoxicity in MCF-7 and MDA-MB 231 human breast cancer cells. J Surg Res. 2005;125:49–55. doi: 10.1016/j.jss.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Akerley W. McCoy J. Hesketh PJ. Goodwin JW. Bearden JD. Atkins JN. Chansky K. Crowley JJ. Gandara DR. Gemcitabine and irinotecan for patients with untreated extensive stage small cell lung cancer: SWOG 0119. J Thorac Oncol. 2007;2:526–530. doi: 10.1097/JTO.0b013e318060d2dc. [DOI] [PubMed] [Google Scholar]
- 9.Amato RJ. Sarao H. A phase I study of paclitaxel/doxorubicin/thalidomide in patients with androgen-independent prostate cancer. Clin Genitourin Cancer. 2006;4:281–286. doi: 10.3816/CGC.2006.n.008. [DOI] [PubMed] [Google Scholar]
- 10.Andrade FH. Reid MB. Allen DG. Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol Online. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arcamone FFG. Penco S. Process for the preparation of adriamycin and adriamycinone and adriamycin derivatives. US Patent. 1974;3(803):124. [Google Scholar]
- 12.Argiris A. Agarwala SS. Karamouzis MV. Burmeister LA. Carty SE. A phase II trial of doxorubicin and interferon alpha 2b in advanced, non-medullary thyroid cancer. Invest New Drugs. 2008;26:183–188. doi: 10.1007/s10637-007-9091-2. [DOI] [PubMed] [Google Scholar]
- 13.Argiris A. Buchanan A. Brockstein B. Kolesar J. Ghebremichael M. Pins M. Hahn K. Axelrod R. Forastiere A. Docetaxel and irinotecan in recurrent or metastatic head and neck cancer: A phase 2 trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115:4504–4513. doi: 10.1002/cncr.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arndt V. Stegmaier C. Ziegler H. Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107:2496–2503. doi: 10.1002/cncr.22274. [DOI] [PubMed] [Google Scholar]
- 15.Arthur PG. Grounds MD. Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr Opin Clin Nutr Metab Care. 2008;11:408–416. doi: 10.1097/MCO.0b013e328302f3fe. [DOI] [PubMed] [Google Scholar]
- 16.Avner BS. Hinken AC. Yuan C. Solaro RJ. H2O2 alters rat cardiac sarcomere function and protein phosphorylation through redox signaling. Am J Physiol Heart Circ Physiol. 2010;299:H723–730. doi: 10.1152/ajpheart.00050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barreiro E. Hussain SN. Protein carbonylation in skeletal muscles: Impact on function. Antioxid Redox Signal. 2010;12:417–429. doi: 10.1089/ars.2009.2808. [DOI] [PubMed] [Google Scholar]
- 18.Bender CM. Sereika SM. Berga SL. Vogel VG. Brufsky AM. Paraska KK. Ryan CM. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 19.Berthiaume JM. Oliveira PJ. Fariss MW. Wallace KB. Dietary vitamin E decreases doxorubicin-induced oxidative stress without preventing mitochondrial dysfunction. Cardiovasc Toxicol. 2005;5:257–267. doi: 10.1385/ct:5:3:257. [DOI] [PubMed] [Google Scholar]
- 20.Blazeby JM. Brookes ST. Alderson D. The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut. 2001;49:227–230. doi: 10.1136/gut.49.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonifati DM. Ori C. Rossi CR. Caira S. Fanin M. Angelini C. Neuromuscular damage after hyperthermic isolated limb perfusion in patients with melanoma or sarcoma treated with chemotherapeutic agents. Cancer Chemother Pharmacol. 2000;46:517–522. doi: 10.1007/s002800000175. [DOI] [PubMed] [Google Scholar]
- 22.Borden LS., Jr. Clark PE. Lovato J. Hall MC. Stindt D. Harmon M. Mohler R. Torti FM. Vinorelbine, doxorubicin, and prednisone in androgen-independent prostate cancer. Cancer. 2006;107:1093–1100. doi: 10.1002/cncr.22078. [DOI] [PubMed] [Google Scholar]
- 23.Bos AM. De Vos FY. de Vries EG. Beijnen JH. Rosing H. Mourits MJ. van der Zee AG. Gietema JA. Willemse PH. A phase I study of intraperitoneal topotecan in combination with intravenous carboplatin and paclitaxel in advanced ovarian cancer. Eur J Cancer. 2005;41:539–548. doi: 10.1016/j.ejca.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Bower JE. Ganz PA. Aziz N. Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Brown DJ. McMillan DC. Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103:377–382. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- 26.Bruner DW. Barsevick A. Tian C. Randall M. Mannel R. Cohn DE. Sorosky J. Spirtos NM. Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: A gynecologic oncology group study. Qual Life Res. 2007;16:89–100. doi: 10.1007/s11136-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 27.Byar KL. Berger AM. Bakken SL. Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:E18–26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 28.Cadeddu C. Piras A. Mantovani G. Deidda M. Dessi M. Madeddu C. Massa E. Mercuro G. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. 2010;160:487 e1–7. doi: 10.1016/j.ahj.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Callahan LA. She ZW. Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol. 2001;90:45–54. doi: 10.1152/jappl.2001.90.1.45. [DOI] [PubMed] [Google Scholar]
- 30.Canton M. Neverova I. Menabo R. Van Eyk J. and Di Lisa F. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H870–877. doi: 10.1152/ajpheart.00714.2003. [DOI] [PubMed] [Google Scholar]
- 31.Cella D. Lai JS. Chang CH. Peterman A. Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 32.Cereda S. Passoni P. Reni M. Vigano MG. Aldrighetti L. Nicoletti R. Villa E. The cisplatin, epirubicin, 5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary tract adenocarcinoma. Cancer. 2010;116:2208–2214. doi: 10.1002/cncr.24970. [DOI] [PubMed] [Google Scholar]
- 33.Chabner BA. Ryan DP. Paz-Ares L. Garcia-Carbonero R. Calabresi P. Hardman JG. Limbird LE. Gilman AG. Goodman & Gilman's The pharmacologic basis of therapeutics. New York: McGraw-Hill, Medical Publishing Division; 2001. pp. 1389–1459. [Google Scholar]
- 34.Chaiswing L. Cole MP. St Clair DK. Ittarat W. Szweda LI. Oberley TD. Oxidative damage precedes nitrative damage in adriamycin-induced cardiac mitochondrial injury. Toxicol Pathol. 2004;32:536–547. doi: 10.1080/01926230490502601. [DOI] [PubMed] [Google Scholar]
- 35.Chan JS. Beer TM. Quinn DI. Pinski JK. Garzotto M. Sokoloff M. Dehaze DR. Ryan CW. A phase II study of high-dose calcitriol combined with mitoxantrone and prednisone for androgen-independent prostate cancer. BJU Int. 2008;102:1601–1606. doi: 10.1111/j.1464-410X.2008.08017.x. [DOI] [PubMed] [Google Scholar]
- 36.Chanan-Khan A. Miller KC. Musial L. Padmanabhan S. Yu J. Ailawadhi S. Sher T. Mohr A. Bernstein ZP. Barcos M. Patel M. Iancu D. Lee K. Czuczman MS. Bortezomib in combination with pegylated liposomal doxorubicin and thalidomide is an effective steroid independent salvage regimen for patients with relapsed or refractory multiple myeloma: Results of a phase II clinical trial. Leuk Lymphoma. 2009;50:1096–1101. doi: 10.1080/10428190902912460. [DOI] [PubMed] [Google Scholar]
- 37.Chandran K. Aggarwal D. Migrino RQ. Joseph J. McAllister D. Konorev EA. Antholine WE. Zielonka J. Srinivasan S. Avadhani NG. Kalyanaraman B. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: Cardioprotection by Mito-Q. Biophys J. 2009;96:1388–1398. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang VT. Thaler HT. Polyak TA. Kornblith AB. Lepore JM. Portenoy RK. Quality of life and survival: The role of multidimensional symptom assessment. Cancer. 1998;83:173–179. doi: 10.1002/(sici)1097-0142(19980701)83:1<173::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee K. Zhang J. Honbo N. Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2009;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y. Jungsuwadee P. Vore M. Butterfield DA. St Clair DK. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 41.Chiosi E. Spina A. Sorrentino A. Romano M. Sorvillo L. Senatore G. D'Auria R. Abbruzzese A. Caraglia M. Naviglio S. Illiano G. Change in TNF-alpha receptor expression is a relevant event in doxorubicin-induced H9c2 cardiomyocyte cell death. J Interferon Cytokine Res. 2007;27:589–597. doi: 10.1089/jir.2006.0161. [DOI] [PubMed] [Google Scholar]
- 42.Choong NW. Mauer AM. Hoffman PC. Rudin CM. Winegarden JD., 3rd Villano JL. Kozloff M. Wade JL., 3rd Sciortino DF. Szeto L. Vokes EE. Phase II trial of temozolomide and irinotecan as second-line treatment for advanced non-small cell lung cancer. J Thorac Oncol. 2006;1:245–251. doi: 10.1016/s1556-0864(15)31575-6. [DOI] [PubMed] [Google Scholar]
- 43.Chu E. DeNita VT. Physician's Cancer Chemotherapy Drug Manual. Sudbury, MA: Jones & Bartlett Publishers, Inc.; 2008. [Google Scholar]
- 44.Crohns M. Liippo K. Erhola M. Kankaanranta H. Moilanen E. Alho H. Kellokumpu-Lehtinen P. Concurrent decline of several antioxidants and markers of oxidative stress during combination chemotherapy for small cell lung cancer. Clin Biochem. 2009;42:1236–1245. doi: 10.1016/j.clinbiochem.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Cullu E. Ozkan I. Culhaci N. Alparslan B. A comparison of the effect of doxorubicin and phenol on the skeletal muscle. May doxorubicin be a new alternative treatment agent for spasticity? J Pediatr Orthop B. 2005;14:134–138. doi: 10.1097/01202412-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Cullu E. Ozkan I. Culhaci N. Alparslan B. Dikicioglu E. Savk SO. [Doxorubicin-induced chemomyectomy effects in rat skeletal muscle] Acta Orthop Traumatol Turc. 2003;37:323–329. [PubMed] [Google Scholar]
- 47.Curtis KK. Hartney JT. Jewell RC. Park JW. Lebowitz PF. Griffin PP. Borad MJ. Fitch TR. Northfelt DW. A phase I study to characterize the safety, tolerability, and pharmacokinetics of topotecan at 4 mg/m2 administered weekly as a 30-minute intravenous infusion in patients with cancer. J Clin Pharmacol. 2010;50:268–275. doi: 10.1177/0091270009343699. [DOI] [PubMed] [Google Scholar]
- 48.Danz ED. Skramsted J. Henry N. Bennett JA. Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46:1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Davies KJ. Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261:3060–3067. [PubMed] [Google Scholar]
- 50.de Beer EL. Finkle H. Voest EE. Van Heijst BGV. Schiereck P. Doxorubicin interacts directly with skinned single skeletal muscle fibres. Eur J Pharmacol. 1992;214:97–100. doi: 10.1016/0014-2999(92)90103-b. [DOI] [PubMed] [Google Scholar]
- 51.de Jong N. Kester AD. Schouten HC. Abu-Saad HH. Courtens AM. Course of fatigue between two cycles of adjuvant chemotherapy in breast cancer patients. Cancer Nurs. 2006;29:467–477. doi: 10.1097/00002820-200611000-00007. [DOI] [PubMed] [Google Scholar]
- 52.DeAtley SM. Aksenov MY. Aksenova MV. Harris B. Hadley R. Cole HP. Carney JM. Butterfield DA. Antioxidants protect against reactive oxygen species associated with adriamycin-treated cardiomyocytes. Cancer Lett. 1999;136:41–46. doi: 10.1016/s0304-3835(98)00306-1. [DOI] [PubMed] [Google Scholar]
- 53.Dees EC. O'Neil BH. Lindley CM. Collichio F. Carey LA. Collins J. Riordan WJ. Ivanova A. Esseltine D. Orlowski RZ. A phase I and pharmacologic study of the combination of bortezomib and pegylated liposomal doxorubicin in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2008;63:99–107. doi: 10.1007/s00280-008-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delemasure S. Sicard P. Lauzier B. Moreau D. Vergely C. Rochette L. Acute administration of epirubicin induces myocardial depression in isolated rat heart and production of radical species evaluated by electron spin resonance spectroscopy. J Cardiovasc Pharmacol. 2007;50:647–653. doi: 10.1097/FJC.0b013e31815571f7. [DOI] [PubMed] [Google Scholar]
- 55.Delgado G. Potkul RK. Treat JA. Lewandowski GS. Barter JF. Forst D. Rahman A. A phase I/II study of intraperitoneally administered doxorubicin entrapped in cardiolipin liposomes in patients with ovarian cancer. Am J Obstet Gynecol. 1989;160:812–817. doi: 10.1016/0002-9378(89)90296-2. discussion 817–819, [DOI] [PubMed] [Google Scholar]
- 56.Diaz R. Aparicio J. Molina J. Palomar L. Gimenez A. Ponce J. Segura A. Gomez-Codina J. Clinical predictors of severe toxicity in patients treated with combination chemotherapy with irinotecan and/or oxaliplatin for metastatic colorectal cancer: A single center experience. Med Oncol. 2006;23:347–357. doi: 10.1385/mo:23:3:347. [DOI] [PubMed] [Google Scholar]
- 57.Diotte NM. Xiong Y. Gao J. Chua BH. Ho YS. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta. 2009;1793:427–438. doi: 10.1016/j.bbamcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Donovan KA. Jacobsen PB. Andrykowski MA. Winters EM. Balducci L. Malik U. Kenady D. McGrath P. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doroshow JH. Davies KJ. Comparative cardiac oxygen radical metabolism by anthracycline antibiotics, mitoxantrone, bisantrene, 4′-(9-acridinylamino)-methanesulfon-m-anisidide, and neocarzinostatin. Biochem Pharmacol. 1983;32:2935–2939. doi: 10.1016/0006-2952(83)90399-4. [DOI] [PubMed] [Google Scholar]
- 60.Doroshow JH. Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–3074. [PubMed] [Google Scholar]
- 61.Doroshow JH. Locker GY. Ifrim I. Myers CE. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest. 1981;68:1053–1064. doi: 10.1172/JCI110328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doroshow JH. Tallent C. Schechter JE. Ultrastructural features of adriamycin-induced skeletal and cardiac muscle toxicity. Am J Pathol. 1985;118:288–297. [PMC free article] [PubMed] [Google Scholar]
- 63.Dresdale AR. Barr LH. Bonow RO. Mathisen DJ. Myers CE. Schwartz DE. d'Angelo T. Rosenberg SA. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am J Clin Oncol. 1982;5:657–663. doi: 10.1097/00000421-198212000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Dugan E. Truax R. Meadows KL. Blobe GC. Morse MA. Fernando NH. Gockerman JP. Petros WP. Hurwitz HI. Phase I dose escalation study of gemcitabine plus irinotecan in advanced solid tumors. Anticancer Res. 2009;29:5149–5153. [PMC free article] [PubMed] [Google Scholar]
- 65.Duran I. Siu LL. Chen EX. Oza AM. Sturgeon J. Chin SF. Brown S. Pond GR. Nottage M. Phase I trial of gemcitabine, doxorubicin and cisplatin (GAP) in patients with advanced solid tumors. Anticancer Drugs. 2006;17:81–87. doi: 10.1097/01.cad.0000190282.05748.63. [DOI] [PubMed] [Google Scholar]
- 66.Eckardt JR. von Pawel J. Pujol JL. Papai Z. Quoix E. Ardizzoni A. Poulin R. Preston AJ. Dane G. Ross G. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25:2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 67.el-Missiry MA. Othman AI. Amer MA. Abd el-Aziz MA. Attenuation of the acute adriamycin-induced cardiac and hepatic oxidative toxicity by N-(2-mercaptopropionyl) glycine in rats. Free Radic Res. 2001;35:575–581. doi: 10.1080/10715760100301581. [DOI] [PubMed] [Google Scholar]
- 68.Elbl L. Vasova I. Tomaskova I. Jedlicka F. Kral Z. Navratil M. Smardova L. Wagnerova B. Vorlicek J. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47:843–851. doi: 10.1080/10428190500402559. [DOI] [PubMed] [Google Scholar]
- 69.Enzinger PC. Kulke MH. Clark JW. Ryan DP. Kim H. Earle CC. Vincitore MM. Michelini AL. Mayer RJ. Fuchs CS. A phase II trial of irinotecan in patients with previously untreated advanced esophageal and gastric adenocarcinoma. Dig Dis Sci. 2005;50:2218–2223. doi: 10.1007/s10620-005-3038-2. [DOI] [PubMed] [Google Scholar]
- 70.Enzinger PC. Ryan DP. Clark JW. Muzikansky A. Earle CC. Kulke MH. Meyerhardt JA. Blaszkowsky LS. Zhu AX. Fidias P. Vincitore MM. Mayer RJ. Fuchs CS. Weekly docetaxel, cisplatin, and irinotecan (TPC): Results of a multicenter phase II trial in patients with metastatic esophagogastric cancer. Ann Oncol. 2009;20:475–480. doi: 10.1093/annonc/mdn658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ertunc M. Sara Y. Korkusuz P. Onur R. Differential contractile impairment of fast- and slow-twitch skeletal muscles in a rat model of doxorubicin-induced congestive heart failure. Pharmacology. 2009;84:240–248. doi: 10.1159/000241723. [DOI] [PubMed] [Google Scholar]
- 72.Fabbro M. Gladieff L. Guichard F. El Demery M. Dalenc F. Kerr C. Delannes M. Paraiso D. Pujade-Lauraine E. Kurtz JE. Phase I study of irinotecan and cisplatin in combination with pelvic radiotherapy in the treatment of locally advanced cervical cancer: A GINECO trial. Gynecol Oncol. 2010;117:276–280. doi: 10.1016/j.ygyno.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 73.Fadillioglu E. Oztas E. Erdogan H. Yagmurca M. Sogut S. Ucar M. Irmak MK. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol. 2004;24:47–52. doi: 10.1002/jat.945. [DOI] [PubMed] [Google Scholar]
- 74.Falkenberg JH. Iaizzo PA. McLoon LK. Physiological assessment of muscle strength in vitro after direct injection of doxorubicin into rabbit sternocleidomastoid muscle. Mov Disord. 2001;16:683–692. doi: 10.1002/mds.1125. [DOI] [PubMed] [Google Scholar]
- 75.Falkenberg JH. Iaizzo PA. McLoon LK. Muscle strength following direct injection of doxorubicin into rabbit sternocleidomastoid muscle in situ. Muscle Nerve. 2002;25:735–741. doi: 10.1002/mus.10082. [DOI] [PubMed] [Google Scholar]
- 76.Fang J. Nakamura H. Iyer AK. Tumor-targeted induction of oxystress for cancer therapy. J Drug Target. 2007;15:475–486. doi: 10.1080/10611860701498286. [DOI] [PubMed] [Google Scholar]
- 77.Fekrazad HM. Verschraegen CF. Royce M. Smith HO. Chyi Lee F. Rabinowitz I. A phase I study of flavopiridol in combination with gemcitabine and irinotecan in patients with metastatic cancer. Am J Clin Oncol. 2010;33:393–397. doi: 10.1097/COC.0b013e3181b2043f. [DOI] [PubMed] [Google Scholar]
- 78.Ferreira LF. Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol. 2008;104:853–860. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- 79.Findlay B. Tonkin K. Crump M. Norris B. Trudeau M. Blackstein M. Burnell M. Skillings J. Bowman D. Walde D. Levine M. Pritchard KI. Palmer MJ. Tu D. Shepherd L. A dose escalation trial of adjuvant cyclophosphamide and epirubicin in combination with 5-fluorouracil using G-CSF support for premenopausal women with breast cancer involving four or more positive nodes. Ann Oncol. 2007;18:1646–1651. doi: 10.1093/annonc/mdm277. [DOI] [PubMed] [Google Scholar]
- 80.Frasci G. Comella P. Carreca I. DeCataldis G. Muci D. Brunetti C. Russo A. Palmeri S. D'Aniello R. Giordano R. D'Aiuto M. Comella G. Weekly dose-dense cisplatin-epirubicin-paclitaxel administration with granulocyte colony-stimulating factor support does not substantially improve prognosis in extensive disease small-cell lung cancer. A SICOG phase II study. Oncology. 2005;68:223–229. doi: 10.1159/000086778. [DOI] [PubMed] [Google Scholar]
- 81.Frasci G. D'Aiuto G. Comella P. Thomas R. Botti G. Di Bonito M. De Rosa V. Iodice G. Rubulotta MR. Comella G. Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: Final analysis of a sicog phase III study. Br J Cancer. 2006;95:1005–1012. doi: 10.1038/sj.bjc.6603395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frasci G. D'Aiuto G. Thomas R. Comella P. Di Bonito M. Lapenta L. D'Aiuto M. Botti G. Vallone P. De Rosa V. D'Aniello R. Giordano R. Comella G. Biweekly docetaxel-irinotecan treatment with filgrastim support is highly active in antracycline-paclitaxel-refractory breast cancer patients. Oncology. 2005;68:391–397. doi: 10.1159/000086980. [DOI] [PubMed] [Google Scholar]
- 83.Fukuda M. Soda H. Kinoshita A. Nakamura Y. Nagashima S. Takatani H. Tsukamoto K. Kohno S. Oka M. Irinotecan and cisplatin with concurrent split-course radiotherapy in locally advanced nonsmall-cell lung cancer: A multiinstitutional phase 2 study. Cancer. 2007;110:606–613. doi: 10.1002/cncr.22817. [DOI] [PubMed] [Google Scholar]
- 84.Furuse J. Okusaka T. Funakoshi A. Yamao K. Nagase M. Ishii H. Nakachi K. Ueno H. Ikeda M. Morizane C. Horikawa Y. Mizuno N. Early phase II study of uracil-tegafur plus doxorubicin in patients with unresectable advanced biliary tract cancer. Jpn J Clin Oncol. 2006;36:552–556. doi: 10.1093/jjco/hyl075. [DOI] [PubMed] [Google Scholar]
- 85.Galvao DA. Taaffe DR. Spry N. Joseph D. Turner D. Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: A comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009;12:198–203. doi: 10.1038/pcan.2008.51. [DOI] [PubMed] [Google Scholar]
- 86.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 87.Gilliam LA. Ferreira LF. Bruton JD. Moylan JS. Westerblad H. St Clair DK. Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol. 2009;107:1935–1942. doi: 10.1152/japplphysiol.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilliam LA. Moylan JS. Ann Callahan L. Sumandea MP. Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve. 2011;43:94–102. doi: 10.1002/mus.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gilliam LA. Moylan JS. Ferreira LF. Reid MB. TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm weakness. Am J Physiol Lung Cell Mol Physiol. 2011;300:L225–231. doi: 10.1152/ajplung.00264.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goel A. Grossbard ML. Malamud S. Homel P. Dietrich M. Rodriguez T. Mirzoyev T. Kozuch P. Pooled efficacy analysis from a phase I-II study of biweekly irinotecan in combination with gemcitabine, 5-fluorouracil, leucovorin and cisplatin in patients with metastatic pancreatic cancer. Anticancer Drugs. 2007;18:263–271. doi: 10.1097/CAD.0b013e3280121334. [DOI] [PubMed] [Google Scholar]
- 91.Gupta A. Srivastava S. Prasad R. Natu SM. Mittal B. Negi MP. Srivastava AN. Oxidative stress in non-small cell lung cancer patients after chemotherapy: Association with treatment response. Respirology. 2010;15:349–356. doi: 10.1111/j.1440-1843.2009.01703.x. [DOI] [PubMed] [Google Scholar]
- 92.Hahn CA. Jones EL. Blivin JL. Sanders LL. Yu D. Dewhirst MW. Secord AA. Prosnitz LR. Prospective assessment of quality of life in ovarian cancer patients receiving whole abdomen hyperthermia and liposomal doxorubicin. Int J Hyperthermia. 2005;21:349–357. doi: 10.1080/02656730400022260. [DOI] [PubMed] [Google Scholar]
- 93.Hamza A. Amin A. Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24:63–73. doi: 10.1007/s10565-007-9016-z. [DOI] [PubMed] [Google Scholar]
- 94.Harada Y. Kato S. Komiya H. Shirota T. Mukai K. Hayashi T. Primary omental gamma/delta T-cell lymphoma involving the central nervous system. Leuk Lymphoma. 2004;45:1947–1950. doi: 10.1080/10428190410001697368. [DOI] [PubMed] [Google Scholar]
- 95.Hardin BJ. Campbell KS. Smith JD. Arbogast S. Smith J. Moylan JS. Reid MB. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol. 2008;104:694–699. doi: 10.1152/japplphysiol.00898.2007. [DOI] [PubMed] [Google Scholar]
- 96.Hayes S. Battistutta D. Newman B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast Cancer Res Treat. 2005;94:1–10. doi: 10.1007/s10549-005-5991-z. [DOI] [PubMed] [Google Scholar]
- 97.Herman EH. Ferrans VJ. Myers CE. Van Vleet JF. Comparison of the effectiveness of (+/-)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) and N-acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res. 1985;45:276–281. [PubMed] [Google Scholar]
- 98.Hilder TL. Carlson GM. Haystead TA. Krebs EG. Graves LM. Caspase-3 dependent cleavage and activation of skeletal muscle phosphorylase b kinase. Mol Cell Biochem. 2005;275:233–242. doi: 10.1007/s11010-005-2411-y. [DOI] [PubMed] [Google Scholar]
- 99.Hockenberry MJ. Hooke MC. Gregurich M. McCarthy K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J Pediatr Hematol Oncol. 2009;31:664–669. doi: 10.1097/MPH.0b013e3181b259a7. [DOI] [PubMed] [Google Scholar]
- 100.Hofheinz RD. Gnad-Vogt U. Wein A. Saussele S. Kreil S. Pilz L. Hehlmann R. Hochhaus A. Irinotecan and capecitabine as second-line treatment after failure for first-line infusional 24-h 5-fluorouracil/folinic acid in advanced colorectal cancer: A phase II study. Anticancer Drugs. 2005;16:39–45. doi: 10.1097/00001813-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 101.Honda M. Miura A. Izumi Y. Kato T. Ryotokuji T. Monma K. Fujiwara J. Egashira H. Nemoto T. Doxorubicin, cisplatin, and fluorouracil combination therapy for metastatic esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:641–645. doi: 10.1111/j.1442-2050.2010.01070.x. [DOI] [PubMed] [Google Scholar]
- 102.Hong YS. Lee HR. Park S. Lee SC. Hwang IG. Park BB. Lee J. Ahn JS. Ahn MJ. Lim HY. Park K. Three-week schedule of irinotecan plus cisplatin in patients with previously untreated extensive-stage small-cell lung cancer. Br J Cancer. 2006;95:1648–1652. doi: 10.1038/sj.bjc.6603500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Horenstein MS. Vander Heide RS. L'Ecuyer TJ. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab. 2000;71:436–444. doi: 10.1006/mgme.2000.3043. [DOI] [PubMed] [Google Scholar]
- 104.Hurley J. Reis I. Silva O. Gomez C. DeZarraga F. Velez P. Welsh C. Powell J. Doliny P. Weekly docetaxel/carboplatin as primary systemic therapy for HER2-negative locally advanced breast cancer. Clin Breast Cancer. 2005;5:447–454. doi: 10.3816/cbc.2005.n.003. [DOI] [PubMed] [Google Scholar]
- 105.Il'yasova D. Mixon G. Wang F. Marcom PK. Marks J. Spasojevich I. Craft N. Arredondo F. DiGiulio R. Markers of oxidative status in a clinical model of oxidative assault: A pilot study in human blood following doxorubicin administration. Biomarkers. 2009;14:321–325. doi: 10.1080/13547500902946757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iqbal M. Dubey K. Anwer T. Ashish A. Pillai KK. Protective effects of telmisartan against acute doxorubicin-induced cardiotoxicity in rats. Pharmacol Rep. 2008;60:382–390. [PubMed] [Google Scholar]
- 107.Jackson MJ. Pye D. Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol. 2007;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 108.Jacobsen PB. Hann DM. Azzarello LM. Horton J. Balducci L. Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 109.Jansen CE. Cooper BA. Dodd MJ. Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2010 doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 110.Jhamb R. Gupta N. Garg S. Kumar S. Gulati S. Mishra D. Beniwal P. Diffuse lymphomatous infiltration of kidney presenting as renal tubular acidosis and hypokalemic paralysis: case report. Croat Med J. 2007;48:860–863. doi: 10.3325/cmj.2007.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jimeno A. Rudek MA. Purcell T. Laheru DA. Messersmith WA. Dancey J. Carducci MA. Baker SD. Hidalgo M. Donehower RC. Phase I and pharmacokinetic study of UCN-01 in combination with irinotecan in patients with solid tumors. Cancer Chemother Pharmacol. 2008;61:423–433. doi: 10.1007/s00280-007-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 113.Joshi G. Hardas S. Sultana R. St Clair DK. Vore M. Butterfield DA. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. J Neurosci Res. 2007;85:497–503. doi: 10.1002/jnr.21158. [DOI] [PubMed] [Google Scholar]
- 114.Kadia TM. Yang H. Ferrajoli A. Maddipotti S. Schroeder C. Madden TL. Holleran JL. Egorin MJ. Ravandi F. Thomas DA. Newsome W. Sanchez-Gonzalez B. Zwiebel JA. Espinoza-Delgado I. Kantarjian HM. Garcia-Manero G. A phase I study of vorinostat in combination with idarubicin in relapsed or refractory leukaemia. Br J Haematol. 2010;150:72–82. doi: 10.1111/j.1365-2141.2010.08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanski J. Behring A. Pelling J. Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: Effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288:H371–381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 116.Kato T. Mishima H. Ikenaga M. Murata K. Ishida H. Fukunaga M. Ota H. Tominaga S. Ohnishi T. Amano M. Ikeda K. Ikeda M. Sekimoto M. Sakamoto J. Monden M. A phase II study of irinotecan in combination with doxifluridine, an intermediate form of capecitabine, in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2008;61:275–281. doi: 10.1007/s00280-007-0471-2. [DOI] [PubMed] [Google Scholar]
- 117.Kebieche M. Lakroun Z. Lahouel M. Bouayed J. Meraihi Z. Soulimani R. Evaluation of epirubicin-induced acute oxidative stress toxicity in rat liver cells and mitochondria, and the prevention of toxicity through quercetin administration. Exp Toxicol Pathol. 2009;61:161–167. doi: 10.1016/j.etp.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Kelly MK. Wicker RJ. Barstow TJ. Harms CA. Effects of N-acetylcysteine on respiratory muscle fatigue during heavy exercise. Respir Physiol Neurobiol. 2009;165:67–72. doi: 10.1016/j.resp.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Khawli FA. Reid MB. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol. 1994;77:317–324. doi: 10.1152/jappl.1994.77.1.317. [DOI] [PubMed] [Google Scholar]
- 120.Knobel H. Havard Loge J. Brit Lund M. Forfang K. Nome O. Kaasa S. Late medical complications and fatigue in Hodgkin's disease survivors. J Clin Oncol. 2001;19:3226–3233. doi: 10.1200/JCO.2001.19.13.3226. [DOI] [PubMed] [Google Scholar]
- 121.Kuo HH. Chiu MJ. Liao WC. Hwang SL. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc. 2006;105:64–69. doi: 10.1016/S0929-6646(09)60110-8. [DOI] [PubMed] [Google Scholar]
- 122.Lamb GD. Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 124.Lefrak EA. Pitha J. Rosenheim S. Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 125.Li T. Danelisen I. Singal PK. Early changes in myocardial antioxidant enzymes in rats treated with adriamycin. Mol Cell Biochem. 2002;232:19–26. doi: 10.1023/a:1014862912783. [DOI] [PubMed] [Google Scholar]
- 126.Lin CM. Chen CH. Chang JW. Tsao TC. Phase II study of epirubicin in combination with weekly docetaxel for patients with advanced NSCLC who have failed or relapsed after the frontline platinum-based chemotherapy. Am J Clin Oncol. 2009;32:169–173. doi: 10.1097/COC.0b013e31817eebdc. [DOI] [PubMed] [Google Scholar]
- 127.Lipton A. Campbell-Baird C. Witters L. Harvey H. Ali S. Phase II trial of gemcitabine, irinotecan, and celecoxib in patients with advanced pancreatic cancer. J Clin Gastroenterol. 2010;44:286–288. doi: 10.1097/MCG.0b013e3181cda097. [DOI] [PubMed] [Google Scholar]
- 128.Liu J. Tu D. Dancey J. Reyno L. Pritchard KI. Pater J. Seymour LK. Quality of life analyses in a clinical trial of DPPE (tesmilifene) plus doxorubicin versus doxorubicin in patients with advanced or metastatic breast cancer: NCIC CTG Trial MA.19. Breast Cancer Res Treat. 2006;100:263–271. doi: 10.1007/s10549-006-9257-1. [DOI] [PubMed] [Google Scholar]
- 129.Liu L. Zhang XJ. Qian B. Min XY. Cheng YL. [Heat shock protein 27 attenuated doxorubicin-induced myocardial damage by reducing cardiomyocyte apoptosis, mitochondria damage and protein carbonylation] Zhonghua Xin Xue Guan Bing Za Zhi. 2008;36:1021–1026. [PubMed] [Google Scholar]
- 130.LoConte NK. Thomas JP. Alberti D. Heideman J. Binger K. Marnocha R. Utecht K. Geiger P. Eickhoff J. Wilding G. Kolesar J. A phase I pharmacodynamic trial of bortezomib in combination with doxorubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2008;63:109–115. doi: 10.1007/s00280-008-0719-5. [DOI] [PubMed] [Google Scholar]
- 131.Loesch D. Greco FA. Senzer NN. Burris HA. Hainsworth JD. Jones S. Vukelja SJ. Sandbach J. Holmes F. Sedlacek S. Pippen J. Lindquist D. McIntyre K. Blum JL. Modiano MR. Boehm KA. Zhan F. Asmar L. Robert N. Phase III multicenter trial of doxorubicin plus cyclophosphamide followed by paclitaxel compared with doxorubicin plus paclitaxel followed by weekly paclitaxel as adjuvant therapy for women with high-risk breast cancer. J Clin Oncol. 2010;28:2958–2965. doi: 10.1200/JCO.2009.24.1000. [DOI] [PubMed] [Google Scholar]
- 132.Luctkar-Flude M. Groll D. Woodend K. Tranmer J. Fatigue and physical activity in older patients with cancer: A six-month follow-up study. Oncol Nurs Forum. 2009;36:194–202. doi: 10.1188/09.ONF.194-202. [DOI] [PubMed] [Google Scholar]
- 133.Mahadevan D. Dreisbach L. Kristedja T. Williams D. Obregon Y. Kurtin S. Von Hoff DD. Phase I study of fixed dose gemcitabine plus epirubicin in patients with advanced solid malignancies. Am J Clin Oncol. 2009;32:607–611. doi: 10.1097/COC.0b013e31819cc9ed. [DOI] [PubMed] [Google Scholar]
- 134.Mantovani G. Madeddu C. Cadeddu C. Dessi M. Piras A. Massa E. Serpe R. Antoni G. Mercuro G. Persistence, up to 18 months of follow-up, of epirubicin-induced myocardial dysfunction detected early by serial tissue Doppler echocardiography: Correlation with inflammatory and oxidative stress markers. Oncologist. 2008;13:1296–1305. doi: 10.1634/theoncologist.2008-0151. [DOI] [PubMed] [Google Scholar]
- 135.Mariappan N. Soorappan RN. Haque M. Sriramula S. Francis J. TNF-{alpha}-induced mitochondrial oxidative stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol. 2007;293:H2726–H2737. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- 136.Matuszczak Y. Farid M. Jones J. Lansdowne S. Smith MA. Taylor AA. Reid MB. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve. 2005;32:633–638. doi: 10.1002/mus.20385. [DOI] [PubMed] [Google Scholar]
- 137.McKenna MJ. Medved I. Goodman CA. Brown MJ. Bjorksten AR. Murphy KT. Petersen AC. Sostaric S. Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McLoon LK. Bauer G. Wirtschafter J. Quantification of muscle loss in the doxorubicin-treated orbicularis oculi of the monkey. Effect of local injection of doxorubicin into the eyelid. Invest Ophthalmol Vis Sci. 1991;32:1667–1673. [PubMed] [Google Scholar]
- 139.McLoon LK. Ekern M. Wirtschafter J. Verapamil substantially increases the chemomyectomy effect of doxorubicin injected into rabbit or monkey eyelid. Invest Ophthalmol Vis Sci. 1992;33:3228–3234. [PubMed] [Google Scholar]
- 140.McLoon LK. Falkenberg JH. Dykstra D. Iaizzo PA. Doxorubicin chemomyectomy as a treatment for cervical dystonia: Histological assessment after direct injection into the sternocleidomastoid muscle. Muscle Nerve. 1998;21:1457–1464. doi: 10.1002/(sici)1097-4598(199811)21:11<1457::aid-mus14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 141.McLoon LK. Kirsch JD. Cameron S. Wirtschafter JD. Injection of doxorubicin into rabbit eyelid does not result in loss of facial motor neurons. Brain Res. 1994;641:105–110. doi: 10.1016/0006-8993(94)91821-x. [DOI] [PubMed] [Google Scholar]
- 142.McLoon LK. Luo XX. Wirtschafter J. Acute morphologic changes in orbicularis oculi muscle after doxorubicin injection into the eyelid. Muscle Nerve. 1993;16:737–743. doi: 10.1002/mus.880160708. [DOI] [PubMed] [Google Scholar]
- 143.McLoon LK. Ozel B. Wirtschafter J. Cyclosporin protects the eyelid skin from injury after injection of doxorubicin. Invest Ophthalmol Vis Sci. 1995;36:1433–1440. [PubMed] [Google Scholar]
- 144.McLoon LK. Wirtschafter J. Doxorubicin chemomyectomy: Injection of monkey orbicularis oculi results in selective muscle injury. Invest Ophthalmol Vis Sci. 1988;29:1854–1859. [PubMed] [Google Scholar]
- 145.McLoon LK. Wirtschafter JD. Doxorubicin chemomyectomy in orbicularis oculi: Increasing drug infiltration at the injection site. Curr Eye Res. 1996;15:883–889. doi: 10.3109/02713689609017630. [DOI] [PubMed] [Google Scholar]
- 146.McLoon LK. Wirtschafter JD. Doxil-induced chemomyectomy: Effectiveness for permanent removal of orbicularis oculi muscle in monkey eyelid. Invest Ophthalmol Vis Sci. 2001;42:1254–1257. [PubMed] [Google Scholar]
- 147.McLoon LK. Wirtschafter JD. Cameron JD. Muscle loss from doxorubicin injections into the eyelids of a patient with blepharospasm. Am J Ophthalmol. 1993;116:646–648. doi: 10.1016/s0002-9394(14)73213-1. [DOI] [PubMed] [Google Scholar]
- 148.Medved I. Brown MJ. Bjorksten AR. Murphy KT. Petersen AC. Sostaric S. Gong X. McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 2004;97:1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- 149.Meeske K. Smith AW. Alfano CM. McGregor BA. McTiernan A. Baumgartner KB. Malone KE. Reeve BB. Ballard-Barbash R. Bernstein L. Fatigue in breast cancer survivors two to five years post diagnosis: A HEAL Study report. Qual Life Res. 2007;16:947–960. doi: 10.1007/s11136-007-9215-3. [DOI] [PubMed] [Google Scholar]
- 150.Mercuro G. Cadeddu C. Piras A. Dessi M. Madeddu C. Deidda M. Serpe R. Massa E. Mantovani G. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: Correlation with inflammatory and oxidative stress markers. Oncologist. 2007;12:1124–1133. doi: 10.1634/theoncologist.12-9-1124. [DOI] [PubMed] [Google Scholar]
- 151.Merski J. Daskal Y. Busch H. Comparison of adriamycin-induced nucleolar segregation in skeletal muscle, cardiac muscle, and liver cells. Cancer Treat Rep. 1978;62:771–778. [PubMed] [Google Scholar]
- 152.Miller AA. Case D. Atkins JN. Giguere JK. Bearden JD. Phase II study of carboplatin, irinotecan, and thalidomide in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2006;1:832–836. [PubMed] [Google Scholar]
- 153.Miller AA. Wang XF. Bogart JA. Hodgson LD. Rocha Lima CM. Radford JE. Vokes EE. Green MR. Phase II trial of paclitaxel-topotecan-etoposide followed by consolidation chemoradiotherapy for limited-stage small cell lung cancer: CALGB 30002. J Thorac Oncol. 2007;2:645–651. doi: 10.1097/JTO.0b013e318074bbf5. [DOI] [PubMed] [Google Scholar]
- 154.Mingyan E. Hongli L. Shufeng L. Bo Y. Effects of pyrrolidine dithiocarbamate on antioxidant enzymes in cardiomyopathy induced by adriamycin in rats. Cardiology. 2008;111:119–125. doi: 10.1159/000119699. [DOI] [PubMed] [Google Scholar]
- 155.Minotti G. Menna P. Salvatorelli E. Cairo G. Gianni L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 156.Mita MM. Ochoa L. Rowinsky EK. Kuhn J. Schwartz G. Hammond LA. Patnaik A. Yeh IT. Izbicka E. Berg K. Tolcher AW. A phase I, pharmacokinetic and biologic correlative study of oblimersen sodium (Genasense, G3139) and irinotecan in patients with metastatic colorectal cancer. Ann Oncol. 2006;17:313–321. doi: 10.1093/annonc/mdj067. [DOI] [PubMed] [Google Scholar]
- 157.Mock V. Atkinson A. Barsevick A. Cella D. Cimprich B. Cleeland C. Donnelly J. Eisenberger MA. Escalante C. Hinds P. Jacobsen PB. Kaldor P. Knight SJ. Peterman A. Piper BF. Rugo H. Sabbatini P. Stahl C. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology (Williston Park) 2000;14:151–161. [PubMed] [Google Scholar]
- 158.Mohamed HE. Asker ME. Ali SI. el-Fattah TM. Protection against doxorubicin cardiomyopathy in rats: Role of phosphodiesterase inhibitors type 4. J Pharm Pharmacol. 2004;56:757–768. doi: 10.1211/0022357023565. [DOI] [PubMed] [Google Scholar]
- 159.Molina JR. Jett JR. Foster N. Lair BS. Carroll TJ. Tazelaar HD. Hillman S. Mailliard JA. Bernath AM., Jr. Nikcevich D. Phase II NCCTG trial of oral topotecan and paclitaxel with G-CSF (filgrastim) support in patients with previously untreated extensive-stage small cell lung cancer. Am J Clin Oncol. 2006;29:246–251. doi: 10.1097/01.coc.0000217566.11742.b5. [DOI] [PubMed] [Google Scholar]
- 160.Molina JR. Kaufmann SH. Reid JM. Rubin SD. Galvez-Peralta M. Friedman R. Flatten KS. Koch KM. Gilmer TM. Mullin RJ. Jewell RC. Felten SJ. Mandrekar S. Adjei AA. Erlichman C. Evaluation of lapatinib and topotecan combination therapy: Tissue culture, murine xenograft, and phase I clinical trial data. Clin Cancer Res. 2008;14:7900–7908. doi: 10.1158/1078-0432.CCR-08-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Molina JR. Nikcevich D. Hillman S. Geyer S. Drevyanko T. Jett J. Verdirame J. Tazelaar H. Rowland K. Wos E. Kutteh L. Nair S. Fitch T. Flynn P. Stella P. Adjei AA. A Phase II NCCTG study of irinotecan and docetaxel in previously treated patients with non-small cell lung cancer. Cancer Invest. 2006;24:382–389. doi: 10.1080/07357900600705318. [DOI] [PubMed] [Google Scholar]
- 162.Monsuez JJ. Charniot JC. Vignat N. Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 163.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moopanar TR. Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 degrees C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moopanar TR. Allen DG. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol. 2006;571:191–200. doi: 10.1113/jphysiol.2005.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morris R. Alvarez RD. Andrews S. Malone J. Bryant C. Heilbrun LK. Smith D. Schimp V. Munkarah A. Topotecan weekly bolus chemotherapy for relapsed platinum-sensitive ovarian and peritoneal cancers. Gynecol Oncol. 2008;109:346–352. doi: 10.1016/j.ygyno.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morsi MI. Hussein AE. Mostafa M. El-Abd E. El-Moneim NA. Evaluation of tumour necrosis factor-alpha, soluble P-selectin, gamma-glutamyl transferase, glutathione S-transferase-pi and alpha-fetoprotein in patients with hepatocellular carcinoma before and during chemotherapy. Br J Biomed Sci. 2006;63:74–78. doi: 10.1080/09674845.2006.11732724. [DOI] [PubMed] [Google Scholar]
- 168.Movsas B. Langer CJ. Ross HJ. Wang L. Jotte RM. Feigenberg S. Xu F. Huang CH. Monberg MJ. Obasaju CK. Randomized phase II trial of cisplatin, etoposide, and radiation followed by gemcitabine alone or by combined gemcitabine and docetaxel in stage III A/B unresectable non-small cell lung cancer. J Thorac Oncol. 2010;5:673–679. doi: 10.1097/JTO.0b013e3181d60e8f. [DOI] [PubMed] [Google Scholar]
- 169.Mukherjee S. Banerjee SK. Maulik M. Dinda AK. Talwar KK. Maulik SK. Protection against acute adriamycin-induced cardiotoxicity by garlic: Role of endogenous antioxidants and inhibition of TNF-alpha expression. BMC Pharmacol. 2003;3:16. doi: 10.1186/1471-2210-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Munster PN. Marchion D. Thomas S. Egorin M. Minton S. Springett G. Lee JH. Simon G. Chiappori A. Sullivan D. Daud A. Phase I trial of vorinostat and doxorubicin in solid tumours: Histone deacetylase 2 expression as a predictive marker. Br J Cancer. 2009;101:1044–1050. doi: 10.1038/sj.bjc.6605293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Murad AM. Skare NG. Vinholes J. Lago S. Pecego R. Phase II multicenter trial of docetaxel, epirubicin, and 5-fluorouracil (DEF) in the treatment of advanced gastric cancer: A novel, safe, and active regimen. Gastric Cancer. 2006;9:99–105. doi: 10.1007/s10120-006-0361-z. [DOI] [PubMed] [Google Scholar]
- 172.Myers C. Bonow R. Palmeri S. Jenkins J. Corden B. Locker G. Doroshow J. Epstein S. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. Semin Oncol. 1983;10:53–55. [PubMed] [Google Scholar]
- 173.Nashawati E. DiMarco A. Supinski G. Effects produced by infusion of a free radical-generating solution into the diaphragm. Am Rev Respir Dis. 1993;147:60–65. doi: 10.1164/ajrccm/147.1.60. [DOI] [PubMed] [Google Scholar]
- 173a.National Cancer Institute Common Toxicity Criteria for Adverse Events. 2010.
- 174.Neidhart JA. Gochnour D. Roach R. Hoth D. Young D. A comparison of mitoxantrone and doxorubicin in breast cancer. J Clin Oncol. 1986;4:672–677. doi: 10.1200/JCO.1986.4.5.672. [DOI] [PubMed] [Google Scholar]
- 175.Neidhart JA. Gochnour D. Roach RW. Young D. Steinberg JA. A comparative trial of mitoxantrone and doxorubicin in patients with minimally pretreated breast cancer. Semin Oncol. 1984;11:11–14. [PubMed] [Google Scholar]
- 176.Neri B. Cipriani G. Fulignati C. Turrini M. Ponchietti R. Bartoletti R. Della Melina A. Di Cello V. Dominici A. Maleci D. Raugei A. Villari D. Nicita G. Weekly paclitaxel and epirubicin in the treatment of symptomatic hormone-refractory advanced prostate carcinoma: Report of a phase II trial. Anticancer Drugs. 2005;16:63–66. doi: 10.1097/00001813-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 177.Neri B. Cipriani G. Grifoni R. Molinara E. Pantaleo P. Rangan S. Vannini A. Tonelli P. Valeri A. Pantalone D. Taddei A. Bechi P. Gemcitabine plus irinotecan as first-line weekly therapy in locally advanced and/or metastatic pancreatic cancer. Oncol Res. 2009;17:559–564. doi: 10.3727/096504009789745610. [DOI] [PubMed] [Google Scholar]
- 177a.NHLBI Workshop summary. Respiratory muscle fatigue. Report of the Respiratory Muscle Fatigue Workshop Group. Am Rev Respir Dis. 1990;142:474–480. doi: 10.1164/ajrccm/142.2.474. [DOI] [PubMed] [Google Scholar]
- 178.Orlowski RZ. Nagler A. Sonneveld P. Blade J. Hajek R. Spencer A. San Miguel J. Robak T. Dmoszynska A. Horvath N. Spicka I. Sutherland HJ. Suvorov AN. Zhuang SH. Parekh T. Xiu L. Yuan Z. Rackoff W. Harousseau JL. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 179.Oshita F. Saito H. Murakami S. Kondo T. Yamada K. Phase II study of paclitaxel and irinotecan with intercalated gefitinib in patients with advanced non-small-cell lung cancer. Am J Clin Oncol. 2010;33:66–69. doi: 10.1097/COC.0b013e31819ccc6d. [DOI] [PubMed] [Google Scholar]
- 180.Park SH. Cho EK. Kim Y. Kyung SY. An CH. Lee SP. Park JW. Jeong SH. Lee JI. Choi SJ. Park J. Shin DB. Lee JH. Salvage treatment with topotecan in patients with irinotecan-refractory small cell lung cancer. Cancer Chemother Pharmacol. 2008;62:1009–1014. doi: 10.1007/s00280-008-0690-1. [DOI] [PubMed] [Google Scholar]
- 181.Pater JL. Loeb M. Nonanatomic prognostic factors in carcinoma of the lung: A multivariate analysis. Cancer. 1982;50:326–331. doi: 10.1002/1097-0142(19820715)50:2<326::aid-cncr2820500227>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 182.Patrick DL. Ferketich SL. Frame PS. Harris JJ. Hendricks CB. Levin B. Link MP. Lustig C. McLaughlin J. Reid LD. Turrisi AT., III Unutzer J. Vernon SW. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: Pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst Monogr. 2004:9–16. doi: 10.1093/jncimonographs/djg014. [DOI] [PubMed] [Google Scholar]
- 183.Pectasides D. Pectasides E. Papaxoinis G. Psyrri A. Pliarchopoulou K. Koumarianou A. Macheras A. Athanasas G. Xiros N. Economopoulos T. Carboplatin/gemcitabine alternating with carboplatin/pegylated liposomal doxorubicin and carboplatin/cyclophosphamide in platinum-refractory/resistant paclitaxel - pretreated ovarian carcinoma. Gynecol Oncol. 2010;118:52–57. doi: 10.1016/j.ygyno.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 184.Pectasides D. Samantas E. Fountzilas G. Briasoulis E. Kosmidis P. Skarlos D. Dimopoulos MA. Kalofonos HP. Economopoulos T. Syrigos K. Combination chemotherapy with cisplatin, etoposide and irinotecan in patients with extensive small-cell lung cancer: A phase II study of the Hellenic Co-operative Oncology Group. Lung Cancer. 2007;58:355–361. doi: 10.1016/j.lungcan.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 185.Pectasides D. Xiros N. Papaxoinis G. Aravantinos G. Sykiotis C. Pectasides E. Psyrri A. Koumarianou A. Gaglia A. Gouveris P. Economopoulos T. Gemcitabine and pegylated liposomal doxorubicin alternating with cisplatin plus cyclophosphamide in platinum refractory/resistant, paclitaxel-pretreated, ovarian carcinoma. Gynecol Oncol. 2008;108:47–52. doi: 10.1016/j.ygyno.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 186.Penson RT. Seiden MV. Matulonis UA. Appleman LJ. Fuller AF. Goodman A. Campos SM. Clark JW. Roche M. Eder JP. A phase I clinical trial of continual alternating etoposide and topotecan in refractory solid tumours. Br J Cancer. 2005;93:54–59. doi: 10.1038/sj.bjc.6602671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pfeiffer T. Krause U. Thome U. Rajewski A. Skorzek M. Scheulen ME. Tissue toxicity of doxorubicin in first and second hyperthermic isolated limb perfusion. An experimental study in dogs. Eur J Surg Oncol. 1997;23:439–444. doi: 10.1016/s0748-7983(97)93727-6. [DOI] [PubMed] [Google Scholar]
- 188.Piscitelli SC. Rodvold KA. Rushing DA. Tewksbury DA. Pharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancer. Clin Pharmacol Ther. 1993;53:555–561. doi: 10.1038/clpt.1993.69. [DOI] [PubMed] [Google Scholar]
- 189.Plant DR. Gregorevic P. Williams DA. Lynch GS. Redox modulation of maximum force production of fast-and slow-twitch skeletal muscles of rats and mice. J Appl Physiol. 2001;90:832–838. doi: 10.1152/jappl.2001.90.3.832. [DOI] [PubMed] [Google Scholar]
- 190.Posterino GS. Lamb GD. Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1996;496:809–325. doi: 10.1113/jphysiol.1996.sp021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Powers SK. Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Prochniewicz E. Lowe DA. Spakowicz DJ. Higgins L. O'Conor K. Thompson LV. Ferrington DA. Thomas DD. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–626. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Prue G. Allen J. Gracey J. Rankin J. Cramp F. Fatigue in gynecological cancer patients during and after anticancer treatment. J Pain Symptom Manage. 2010;39:197–210. doi: 10.1016/j.jpainsymman.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 194.Puduvalli VK. Giglio P. Groves MD. Hess KR. Gilbert MR. Mahankali S. Jackson EF. Levin VA. Conrad CA. Hsu SH. Colman H. de Groot JF. Ritterhouse MG. Ictech SE. Yung WK. Phase II trial of irinotecan and thalidomide in adults with recurrent glioblastoma multiforme. Neuro Oncol. 2008;10:216–222. doi: 10.1215/15228517-2007-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Puri A. Maulik SK. Ray R. Bhatnagar V. Electrocardiographic and biochemical evidence for the cardioprotective effect of vitamin E in doxorubicin-induced acute cardiotoxicity in rats. Eur J Pediatr Surg. 2005;15:387–391. doi: 10.1055/s-2005-872923. [DOI] [PubMed] [Google Scholar]
- 196.Ramalingam SS. Foster J. Gooding W. Evans T. Sulecki M. Belani CP. Phase 2 study of irinotecan and paclitaxel in patients with recurrent or refractory small cell lung cancer. Cancer. 2010;116:1344–1349. doi: 10.1002/cncr.24753. [DOI] [PubMed] [Google Scholar]
- 197.Reid MB. Haack KE. Franchek KM. Valberg PA. Kobzik L. West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 198.Reid MB. Lannergren J. Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: Involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–484. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 199.Reid MB. Stokic DS. Koch SM. Khawli FA. Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest. 1994;94:2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Reni M. Cereda S. Bonetto E. Vigano MG. Passoni P. Zerbi A. Balzano G. Nicoletti R. Staudacher C. Di Carlo V. Dose-intense PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2007;59:361–367. doi: 10.1007/s00280-006-0277-7. [DOI] [PubMed] [Google Scholar]
- 201.Rietjens SJ. Beelen M. Koopman R. LJ VANL. Bast A. Haenen GR. A single session of resistance exercise induces oxidative damage in untrained men. Med Sci Sports Exerc. 2007;39:2145–2151. doi: 10.1249/mss.0b013e318157936d. [DOI] [PubMed] [Google Scholar]
- 202.Ringdal GI. Gotestam KG. Kaasa S. Kvinnsland S. Ringdal K. Prognostic factors and survival in a heterogeneous sample of cancer patients. Br J Cancer. 1996;73:1594–1599. doi: 10.1038/bjc.1996.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rom J. von Minckwitz G. Eiermann W. Sievert M. Schlehe B. Marme F. Schuetz F. Scharf A. Eichbaum M. Sinn HP. Kaufmann M. Sohn C. Schneeweiss A. Oblimersen combined with docetaxel, adriamycin and cyclophosphamide as neo-adjuvant systemic treatment in primary breast cancer: Final results of a multicentric phase I study. Ann Oncol. 2008;19:1698–1705. doi: 10.1093/annonc/mdn280. [DOI] [PubMed] [Google Scholar]
- 204.Ruan J. Martin P. Coleman M. Furman RR. Cheung K. Faye A. Elstrom R. Lachs M. Hajjar KA. Leonard JP. Durable responses with the metronomic rituximab and thalidomide plus prednisone, etoposide, procarbazine, and cyclophosphamide regimen in elderly patients with recurrent mantle cell lymphoma. Cancer. 2010;116:2655–2664. doi: 10.1002/cncr.25055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ryan DP. O'Neil BH. Supko JG. Rocha Lima CM. Dees EC. Appleman LJ. Clark J. Fidias P. Orlowski RZ. Kashala O. Eder JP. Cusack JC., Jr A Phase I study of bortezomib plus irinotecan in patients with advanced solid tumors. Cancer. 2006;107:2688–2697. doi: 10.1002/cncr.22280. [DOI] [PubMed] [Google Scholar]
- 206.Safra T. Menczer J. Bernstein R. Shpigel S. Inbar MJ. Grisaru D. Golan A. Levy T. Efficacy and toxicity of weekly topotecan in recurrent epithelial ovarian and primary peritoneal cancer. Gynecol Oncol. 2007;105:205–210. doi: 10.1016/j.ygyno.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 207.Sarid D. Ron IG. Sperber F. Stadler Y. Kahan P. Kovner F. Ben-Yosef R. Marmor S. Grinberg Y. Maimon N. Weinstein J. Yaal-Hahoshen N. Neoadjuvant treatment with paclitaxel and epirubicin in invasive breast cancer: A phase II study. Clin Drug Investig. 2006;26:691–701. doi: 10.2165/00044011-200626120-00003. [DOI] [PubMed] [Google Scholar]
- 208.Sarvazyan N. Visualization of doxorubicin-induced oxidative stress in isolated cardiac myocytes. Am J Physiol. 1996;271:H2079–2085. doi: 10.1152/ajpheart.1996.271.5.H2079. [DOI] [PubMed] [Google Scholar]
- 209.Sawyer DB. Peng X. Chen B. Pentassuglia L. Lim CC. Mechanisms of anthracycline cardiac injury: Can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–113. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sayar H. Shen Z. Lee SJ. Royce M. Rabinowitz I. Lee F. Smith H. Eberhardt S. Maestas A. Lu H. Verschraegen C. Phase I study of capecitabine in combination with cisplatin and irinotecan in patients with advanced solid malignancies. Invest New Drugs. 2009;27:153–158. doi: 10.1007/s10637-008-9172-x. [DOI] [PubMed] [Google Scholar]
- 211.Schelman WR. Morgan-Meadows S. Marnocha R. Lee F. Eickhoff J. Huang W. Pomplun M. Jiang Z. Alberti D. Kolesar JM. Ivy P. Wilding G. Traynor AM. A phase I study of Triapine in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;63:1147–1156. doi: 10.1007/s00280-008-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schneeweiss A. Schuetz F. Rudlowski C. Hahn M. Lauschner I. Sinn HP. von Fournier D. Sohn C. Dose-dense primary systemic chemotherapy with gemcitabine plus epirubicin sequentially followed by docetaxel for early breast cancer: Final results of a phase I/II trial. Anticancer Drugs. 2005;16:1023–1028. doi: 10.1097/01.cad.0000176508.73090.fb. [DOI] [PubMed] [Google Scholar]
- 213.Schwartz AL. Winters-Stone K. Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34:627–633. doi: 10.1188/07.ONF.627-633. [DOI] [PubMed] [Google Scholar]
- 214.Sessa C. Perotti A. Noberasco C. De Braud F. Gallerani E. Cresta S. Zucchetti M. Vigano L. Locatelli A. Jimeno J. Feilchenfeldt JW. D'Incalci M. Capri G. Ielmini N. Gianni L. Phase I clinical and pharmacokinetic study of trabectedin and doxorubicin in advanced soft tissue sarcoma and breast cancer. Eur J Cancer. 2009;45:1153–1161. doi: 10.1016/j.ejca.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 215.Shah C. Ready N. Perry M. Kirshner J. Gajra A. Neuman N. Garziano S. A multi-center phase II study of weekly topotecan as second-line therapy for small cell lung cancer. Lung Cancer. 2007;57:84–88. doi: 10.1016/j.lungcan.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 216.Shah MA. Kortmansky J. Motwani M. Drobnjak M. Gonen M. Yi S. Weyerbacher A. Cordon-Cardo C. Lefkowitz R. Brenner B. O'Reilly E. Saltz L. Tong W. Kelsen DP. Schwartz GK. A phase I clinical trial of the sequential combination of irinotecan followed by flavopiridol. Clin Cancer Res. 2005;11:3836–3845. doi: 10.1158/1078-0432.CCR-04-2651. [DOI] [PubMed] [Google Scholar]
- 217.Shuai Y. Guo JB. Peng SQ. Zhang LS. Guo J. Han G. Dong YS. Metallothionein protects against doxorubicin-induced cardiomyopathy through inhibition of superoxide generation and related nitrosative impairment. Toxicol Lett. 2007;170:66–74. doi: 10.1016/j.toxlet.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 218.Smith MA. Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 219.Sobrero A. Ackland S. Clarke S. Perez-Carrion R. Chiara S. Gapski J. Mainwaring P. Langer B. Young S. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 220.Society AC. Atlanta: American Cancer Society; 2010. Cancer Facts & Figures 2010. [Google Scholar]
- 221.Socinski MA. Smit EF. Lorigan P. Konduri K. Reck M. Szczesna A. Blakely J. Serwatowski P. Karaseva NA. Ciuleanu T. Jassem J. Dediu M. Hong S. Visseren-Grul C. Hanauske AR. Obasaju CK. Guba SC. Thatcher N. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27:4787–4792. doi: 10.1200/JCO.2009.23.1548. [DOI] [PubMed] [Google Scholar]
- 222.Sohn JH. Moon YW. Lee CG. Kim GE. Chung KY. Chang J. Kim SK. Kim YS. Choi BW. Choi HJ. Kim JH. Phase II trial of irinotecan and cisplatin with early concurrent radiotherapy in limited-disease small-cell lung cancer. Cancer. 2007;109:1845–1950. doi: 10.1002/cncr.22621. [DOI] [PubMed] [Google Scholar]
- 223.Spallarossa P. Altieri P. Garibaldi S. Ghigliotti G. Barisione C. Manca V. Fabbi P. Ballestrero A. Brunelli C. Barsotti A. Matrix metalloproteinase-2 and −9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res. 2006;69:736–745. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 224.Spallarossa P. Garibaldi S. Altieri P. Fabbi P. Manca V. Nasti S. Rossettin P. Ghigliotti G. Ballestrero A. Patrone F. Barsotti A. Brunelli C. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37:837–846. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 225.Spigel DR. Greco FA. Zubkus JD. Murphy PB. Saez RA. Farley C. Yardley DA. Burris HA., 3rd Hainsworth JD. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol. 2009;4:1555–1560. doi: 10.1097/JTO.0b013e3181bbc540. [DOI] [PubMed] [Google Scholar]
- 226.Spigel DR. Hainsworth JD. Gandhi JG. Gian VG. Peyton JD. West-Osterfield K. Clark BL. Vazquez ER. Jones SF. Burris HA. Greco FA. A phase II trial of carboplatin and weekly topotecan in the first-line treatment of patients with extensive stage small cell lung cancer. J Thorac Oncol. 2010;5:862–866. doi: 10.1097/jto.0b013e3181d86a4f. [DOI] [PubMed] [Google Scholar]
- 227.Spigel DR. Hainsworth JD. Simons L. Meng C. Burris HA., 3rd Yardley DA. Grapski R. Schreeder M. Mallidi PV. Greco FA. Irinotecan, carboplatin, and imatinib in untreated extensive-stage small-cell lung cancer: A phase II trial of the Minnie Pearl Cancer Research Network. J Thorac Oncol. 2007;2:854–861. doi: 10.1097/JTO.0b013e31814617b7. [DOI] [PubMed] [Google Scholar]
- 228.Sprangers MA. Cull A. Bjordal K. Groenvold M. Aaronson NK. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: Guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res. 1993;2:287–295. doi: 10.1007/BF00434800. [DOI] [PubMed] [Google Scholar]
- 229.Stathopoulos GP. Tsavdaridis D. Malamos NA. Rigatos SK. Kosmas C. Pergantas N. Stathopoulos JG. Xynotroulas J. Irinotecan combined with docetaxel in pre-treated metastatic breast cancer patients: A phase II study. Cancer Chemother Pharmacol. 2005;56:487–491. doi: 10.1007/s00280-005-1006-3. [DOI] [PubMed] [Google Scholar]
- 230.Stone P. Hardy J. Broadley K. Tookman AJ. Kurowska A. A'Hern R. Fatigue in advanced cancer: A prospective controlled cross-sectional study. Br J Cancer. 1999;79:1479–1486. doi: 10.1038/sj.bjc.6690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Stone P. Richards M. A'Hern R. Hardy J. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol. 2000;11:561–567. doi: 10.1023/a:1008331230608. [DOI] [PubMed] [Google Scholar]
- 232.Stone RM. DeAngelo DJ. Janosova A. Galinsky I. Canning C. Ritz J. Soiffer RJ. Low dose interleukin-2 following intensification therapy with high dose cytarabine for acute myelogenous leukemia in first complete remission. Am J Hematol. 2008;83:771–777. doi: 10.1002/ajh.21253. [DOI] [PubMed] [Google Scholar]
- 233.Stromgren AS. Goldschmidt D. Groenvold M. Petersen MA. Jensen PT. Pedersen L. Hoermann L. Helleberg C. Sjogren P. Self-assessment in cancer patients referred to palliative care: A study of feasibility and symptom epidemiology. Cancer. 2002;94:512–520. doi: 10.1002/cncr.10222. [DOI] [PubMed] [Google Scholar]
- 234.Sugarbaker PH. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for the treatment of advanced primary and recurrent ovarian cancer. Curr Opin Obstet Gynecol. 2009;21:15–24. doi: 10.1097/GCO.0b013e32831f8f32. [DOI] [PubMed] [Google Scholar]
- 235.Supinski GS. Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102:2056–2063. doi: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- 236.Swain SM. Whaley FS. Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 237.Takemura G. Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 238.Tangpong J. Cole MP. Sultana R. Joshi G. Estus S. Vore M. St CW. Ratanachaiyavong S. St Clair DK. Butterfield DA. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis. 2006;23:127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 239.Tew WP. Radovich D. O'Reilly E. Schwartz G. Schrag D. Saltz LB. Kelsen DP. Kepler S. Ilson DH. Phase I trial of weekly cisplatin, irinotecan and paclitaxel in patients with advanced gastrointestinal cancer. Invest New Drugs. 2009;27:366–373. doi: 10.1007/s10637-008-9194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tewey KM. Rowe TC. Yang L. Halligan BD. Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466–8. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- 241.Tian Q. Katz AM. Kim DH. Effects of azumolene on doxorubicin-induced Ca2+ release from skeletal and cardiac muscle sarcoplasmic reticulum. Biochim Biophys Acta. 1991;1094:27–34. doi: 10.1016/0167-4889(91)90022-p. [DOI] [PubMed] [Google Scholar]
- 242.Timolati F. Ott D. Pentassuglia L. Giraud MN. Perriard JC. Suter TM. Zuppinger C. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:845–854. doi: 10.1016/j.yjmcc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 243.Timur M. Akbas SH. Ozben T. The effect of topotecan on oxidative stress in MCF-7 human breast cancer cell line. Acta Biochim Pol. 2005;52:897–902. [PubMed] [Google Scholar]
- 244.Tokarska-Schlattner M. Zaugg M. Zuppinger C. Wallimann T. Schlattner U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J Mol Cell Cardiol. 2006;41:389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 245.Tozer RG. Tai P. Falconer W. Ducruet T. Karabadjian A. Bounous G. Molson JH. Droge W. Cysteine-rich protein reverses weight loss in lung cancer patients receiving chemotherapy or radiotherapy. Antioxid Redox Signal. 2008;10:395–402. doi: 10.1089/ars.2007.1919. [DOI] [PubMed] [Google Scholar]
- 246.Tsavaris N. Kosmas C. Skopelitis H. Dimitrakopoulos A. Kopterides P. Bougas D. Stravodimos K. Mitropoulos D. Alamanis C. Giannopoulos A. Methotrexate-paclitaxel-epirubicin-carboplatin (M-TEC) combination chemotherapy in patients with advanced bladder cancer: An open label phase II study. J Chemother. 2005;17:441–448. [PubMed] [Google Scholar]
- 247.Tsimberidou AM. Moulder S. Fu S. Wen S. Naing A. Bedikian AY. Daring S. Uehara C. Ng C. Wallace M. Camacho L. Kurzrock R. Phase I clinical trial of hepatic arterial infusion of cisplatin in combination with intravenous liposomal doxorubicin in patients with advanced cancer and dominant liver involvement. Cancer Chemother Pharmacol. 2010;66:1087–1093. doi: 10.1007/s00280-010-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Turner-Gomes SO. Lands LC. Halton J. Hanning RM. Heigenhauser GJ. Pai M. Barr R. Cardiorespiratory status after treatment for acute lymphoblastic leukemia. Med Pediatr Oncol. 1996;26:160–165. doi: 10.1002/(SICI)1096-911X(199603)26:3<160::AID-MPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 249.Unverferth DV. Jagadeesh JM. Unverferth BJ. Magorien RD. Leier CV. Balcerzak SP. Attempt to prevent doxorubicin-induced acute human myocardial morphologic damage with acetylcysteine. J Natl Cancer Inst. 1983;71:917–920. [PubMed] [Google Scholar]
- 250.Usta Y. Ismailoglu UB. Bakkaloglu A. Orhan D. Besbas N. Sahin-Erdemli I. Ozen S. Effects of pentoxifylline in adriamycin-induced renal disease in rats. Pediatr Nephrol. 2004;19:840–843. doi: 10.1007/s00467-004-1538-5. [DOI] [PubMed] [Google Scholar]
- 251.Valerio MR. Tagliaferri P. Raspagliesi F. Fulfaro F. Badalamenti G. Arcara C. Cicero G. Russo A. Venuta S. Guarneri G. Gebbia N. A phase II study of pegylated liposomal doxorubicin oxaliplatin and cyclophosphamide as second-line treatment in relapsed ovarian carcinoma. Int J Gynecol Cancer. 2006;16(Suppl 1):79–85. doi: 10.1111/j.1525-1438.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 252.Van der Speeten K. Stuart OA. Mahteme H. Sugarbaker PH. A pharmacologic analysis of intraoperative intracavitary cancer chemotherapy with doxorubicin. Cancer Chemother Pharmacol. 2009;63:799–805. doi: 10.1007/s00280-008-0800-0. [DOI] [PubMed] [Google Scholar]
- 253.van Norren K. van Helvoort A. Argiles JM. van Tuijl S. Arts K. Gorselink M. Laviano A. Kegler D. Haagsman HP. van der Beek EM. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer. 2009;100:311–314. doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Van Vleet JF. Ferrans VJ. Clinical and pathologic features of chronic adriamycin toxicosis in rabbits. Am J Vet Res. 1980;41:1462–1469. [PubMed] [Google Scholar]
- 255.Villani F. Busia A. Villani M. Laffranchi A. Viviani S. Bonfante V. Cardiopulmonary response to exercise in patients with different degrees of lung toxicity after radio-chemotherapy for Hodgkin's disease. Anticancer Res. 2009;29:777–783. [PubMed] [Google Scholar]
- 256.Villani F. Galimberti M. Monti E. Piccinini F. Lanza E. Rozza A. Favalli L. Poggi P. Zunino F. Effect of glutathione and N-acetylcysteine on in vitro and in vivo cardiac toxicity of doxorubicin. Free Radic Res Commun. 1990;11:145–151. doi: 10.3109/10715769009109677. [DOI] [PubMed] [Google Scholar]
- 257.Vincenzi B. Santini D. Rabitti C. Coppola R. Beomonte Zobel B. Trodella L. Tonini G. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: A single centre phase II trial. Br J Cancer. 2006;94:792–797. doi: 10.1038/sj.bjc.6603018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Vodermaier A. Breast cancer treatment and cognitive function: The current state of evidence, underlying mechanisms and potential treatments. Womens Health (Lond Engl) 2009;5:503–516. doi: 10.2217/whe.09.36. [DOI] [PubMed] [Google Scholar]
- 259.Vrdoljak AL. Berend S. Zeljezic D. Piljac-Zegarac J. Plestina S. Kuca K. Radic B. Mladinic M. Kopjar N. Irinotecan side effects relieved by the use of HI-6 oxime: In vivo experimental approach. Basic Clin Pharmacol Toxicol. 2009;105:401–409. doi: 10.1111/j.1742-7843.2009.00460.x. [DOI] [PubMed] [Google Scholar]
- 260.Wajant H. Pfizenmaier K. Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 261.Wang GW. Klein JB. Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J Pharmacol Exp Ther. 2001;298:461–468. [PubMed] [Google Scholar]
- 262.Waters SH. Gillibrand A. Berry H. Kumar S. Velikova G. Dodwell DJ. Perren TJ. Dose-finding study of weekly docetaxel, epirubicin and capecitabine, as first-line treatment in advanced breast cancer. Cancer Chemother Pharmacol. 2009;64:407–412. doi: 10.1007/s00280-009-1021-x. [DOI] [PubMed] [Google Scholar]
- 263.Weiss RB. The anthracyclines: Will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–686. [PubMed] [Google Scholar]
- 264.Wessner B. Strasser EM. Koitz N. Schmuckenschlager C. Unger-Manhart N. Roth E. Green tea polyphenol administration partly ameliorates chemotherapy-induced side effects in the small intestine of mice. J Nutr. 2007;137:634–640. doi: 10.1093/jn/137.3.634. [DOI] [PubMed] [Google Scholar]
- 265.William WN., Jr. Lee JL. Shin DM. Hong WK. Liu S. Lee JJ. Lippman SM. Khuri FR. Kim ES. Phase I trial of weekly topotecan and gemcitabine in patients with solid tumors. Am J Clin Oncol. 2009;32:15–19. doi: 10.1097/COC.0b013e318178e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.William WN., Jr. Uyeki J. Johnson FM. Feng L. Peeples BO. Fossella FV. Karp DD. Blumenschein GR. Stewart DJ. Glisson BS. Weekly alternating therapy with irinotecan plus cisplatin and etoposide plus cisplatin in the treatment of patients with extensive small cell lung carcinoma. Cancer. 2010;116:2409–2415. doi: 10.1002/cncr.25076. [DOI] [PubMed] [Google Scholar]
- 267.Wirtschafter JD. McLoon LK. Long-term efficacy of local doxorubicin chemomyectomy in patients with blepharospasm and hemifacial spasm. Ophthalmology. 1998;105:342–346. doi: 10.1016/s0161-6420(98)93484-4. [DOI] [PubMed] [Google Scholar]
- 268.Wojnowski L. Kulle B. Schirmer M. Schluter G. Schmidt A. Rosenberger A. Vonhof S. Bickeboller H. Toliat MR. Suk EK. Tzvetkov M. Kruger A. Seifert S. Kloess M. Hahn H. Loeffler M. Nurnberg P. Pfreundschuh M. Trumper L. Brockmoller J. Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 269.Wright MJ. Halton JM. Martin RF. Barr RD. Long-term gross motor performance following treatment for acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;31:86–90. doi: 10.1002/(sici)1096-911x(199808)31:2<86::aid-mpo7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 270.Wu HS. McSweeney M. Assessing fatigue in persons with cancer: An instrument development and testing study. Cancer. 2004;101:1685–1695. doi: 10.1002/cncr.20540. [DOI] [PubMed] [Google Scholar]
- 271.Xiong Y. Liu X. Lee CP. Chua BH. Ho YS. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic Biol Med. 2006;41:46–55. doi: 10.1016/j.freeradbiomed.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 272.Yamada K. Sugiyama S. Kosaka K. Hayakawa M. Ozawa T. Early appearance of age-associated deterioration in mitochondrial function of diaphragm and heart in rats treated with doxorubicin. Exp Gerontol. 1995;30:581–593. doi: 10.1016/0531-5565(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 273.Yamamoto Y. Hoshino Y. Ito T. Nariai T. Mohri T. Obana M. Hayata N. Uozumi Y. Maeda M. Fujio Y. Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 2008;79:89–96. doi: 10.1093/cvr/cvn076. [DOI] [PubMed] [Google Scholar]
- 274.Yamanaka S. Tatsumi T. Shiraishi J. Mano A. Keira N. Matoba S. Asayama J. Fushiki S. Fliss H. Nakagawa M. Amlodipine inhibits doxorubicin-induced apoptosis in neonatal rat cardiac myocytes. J Am Coll Cardiol. 2003;41:870–878. doi: 10.1016/s0735-1097(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 275.Yavuzsen T. Davis MP. Ranganathan VK. Walsh D. Siemionow V. Kirkova J. Khoshknabi D. Lagman R. LeGrand S. Yue GH. Cancer-related fatigue: Central or peripheral? J Pain Symptom Manage. 2009;38:587–596. doi: 10.1016/j.jpainsymman.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 276.Yen HC. Oberley TD. Vichitbandha S. Ho YS. St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98:1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yong WP. Desai AA. Innocenti F. Ramirez J. Shepard D. Kobayashi K. House L. Fleming GF. Vogelzang NJ. Schilsky RL. Ratain MJ. Pharmacokinetic modulation of oral etoposide by ketoconazole in patients with advanced cancer. Cancer Chemother Pharmacol. 2007;60:811–819. doi: 10.1007/s00280-007-0428-5. [DOI] [PubMed] [Google Scholar]
- 278.Zauderer M. Patil S. Hurria A. Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat. 2009;117:205–210. doi: 10.1007/s10549-008-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zellars RC. Stearns V. Frassica D. Asrari F. Tsangaris T. Myers L. DiPasquale S. Lange JR. Jacobs LK. Emens LA. Armstrong DK. Fetting JH. Garrett-Mayer E. Davidson NE. Wolff AC. Feasibility trial of partial breast irradiation with concurrent dose-dense doxorubicin and cyclophosphamide in early-stage breast cancer. J Clin Oncol. 2009;27:2816–2822. doi: 10.1200/JCO.2008.20.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhu AX. Fuchs CS. Clark JW. Muzikansky A. Taylor K. Sheehan S. Tam K. Yung E. Kulke MH. Ryan DP. A phase II study of epirubicin and thalidomide in unresectable or metastatic hepatocellular carcinoma. Oncologist. 2005;10:392–398. doi: 10.1634/theoncologist.10-6-392. [DOI] [PubMed] [Google Scholar]
- 281.Ziotopoulos P. Androulakis N. Mylonaki E. Chandrinos V. Zachariadis E. Boukovinas I. Agelidou A. Kentepozidis N. Ignatiadis M. Vossos A. Georgoulias V. Front-line treatment of advanced non-small cell lung cancer with irinotecan and docetaxel: A multicentre phase II study. Lung Cancer. 2005;50:115–122. doi: 10.1016/j.lungcan.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 282.Zorzato F. Salviati G. Facchinetti T. Volpe P. Doxorubicin induces calcium release from terminal cisternae of skeletal muscle. A study on isolated sarcoplasmic reticulum and chemically skinned fibers. J Biol Chem. 1985;260:7349–7355. [PubMed] [Google Scholar]