Abstract
Significance
The increased activities of free radicals or reactive oxygen species in tissues of exercising humans and animals were first reported ∼30 years ago. A great deal has been learned about the processes that can generate these molecules, but there is little agreement on which are important, how they are controlled, and there are virtually no quantitative data. Superoxide and nitric oxide are generated by skeletal muscle and their reactions lead to formation of secondary species. A considerable amount is known about control of superoxide generation by xanthine oxidase activity, but similar information for other generation systems is lacking.
Recent Advances
Re-evaluation of published data indicates potential approaches to quantification of the hydrogen peroxide concentration in resting and contracting muscle cells. Such calculations reveal that, during contractions, intracellular hydrogen peroxide concentrations in skeletal muscle may only increase by ∼100 nM. The primary effects of this modest increase appear to be in “redox” signaling processes that mediate some of the responses and adaptations of muscle to exercise. These act, in part, to increase the expression of cytoprotective proteins (e.g., heat shock proteins and antioxidant enzymes) that help maintain cell viability. During aging, these redox-mediated adaptations fail and this contributes to age-related loss of skeletal muscle.
Critical Issues and Future Directions
Understanding the control of ROS generation in muscle and the effect of aging and some disease states will aid design of interventions to maintain muscle mass and function, but is dependent upon development of new analytical approaches. The final part of this review indicates areas where such developments are occurring. Antioxid. Redox Signal. 15, 2477–2486.
Introduction
For many years, reactive oxygen species (ROS) and reactive nitrogen species (RNS) were generally thought to exert their major functions in biology though causing (oxidative) damage to DNA, proteins, and lipids (31). Recognition of these deleterious actions of ROS and RNS led to the general idea that intervention strategies to quench their activities would be beneficial to humans and animals (31). The report that exercise (which is acknowledged to have multiple health benefits) induced formation of ROS in tissues such as skeletal muscle and liver (19) was one of the first observations that cast doubt on this universal negative view of ROS, and it is now widely accepted that ROS play important roles in many physiological processes (60). The tissue sources of the ROS generated by exercise are still under investigation, but numerous studies now indicate that skeletal muscle generates multiple ROS and RNS, both at rest and during contractile activity (see ref. 60 for a comprehensive review). Elucidation of the nature of the ROS generated by skeletal muscle, the sites of their generation, and the potential actions of the ROS has involved studies of multiple cell, tissue, and whole animal models, and relied on a number of different analytical, pharmacological, and (more recently) genetic approaches.
The aims of this review are to describe briefly the various ROS and RNS that are generated by skeletal muscle, the potential sites for their generation, and the sparse published data that address the control of ROS generation in skeletal muscle. A detailed understanding of the functions of contraction-induced ROS will require quantitative assessment of their concentrations and activities and a further part of this review describes potential approaches to this. These quantitative considerations illustrate the relatively small increases in intracellular hydrogen peroxide concentration that appear to be induced by contractions in skeletal muscle, and a discussion of potential actions and functions of such low levels of ROS is presented. Aging appears to represent a situation where the control of skeletal muscle ROS activities and actions is modified, and evidence for this will be reviewed. Finally, potential new approaches to monitoring ROS in different subcellular locations in skeletal muscle will be presented as part of a discussion of potential future directions for study.
Nature of the Free Radicals and ROS That Are Generated by Skeletal Muscle
The nature of the molecules generated by skeletal muscle that are known as ROS or RNS, and which include various free radicals, has been extensively reviewed in recent years (38, 60). In brief, skeletal muscle fibers generate superoxide and nitric oxide (NO) as the primary species and these parent molecules lead to formation of several secondary ROS and RNS. Both superoxide and NO are generated from various sources within muscle fibers, and in addition superoxide (49, 63), hydrogen peroxide (77), and NO (6, 45) are released into the interstitial space from muscle fibers (or generated on the extracellular side of the muscle plasma membrane).
The intracellular content or activity of superoxide, hydrogen peroxide, and NO appears to be increased by contractile activity (57, 61, 63, 66) and superoxide, hydrogen peroxide, hydroxyl radical activity, and NO are increased in the muscle interstitial space by contractile activity (49, 58, 74). Since the initial observations in the 1980s (19), most authors have assumed that the ROS generated by contractions are predominantly generated by mitochondria, but recent data argue against this possibility (for discussion, see later and ref. 60). Nonmitochondrial sources for the generation of ROS within skeletal muscle have not been extensively studied, but some potential nonmitochondrial sources, including NAD(P)H oxidase(s), have been described in skeletal muscle (60). NAD(P)H oxidases localized to skeletal muscle plasma membrane (42), sarcoplasmic reticulum (78), and T-tubules (24) have been reported. The activity of the T-tubule localized enzyme has also been claimed to be activated by contractions (24).
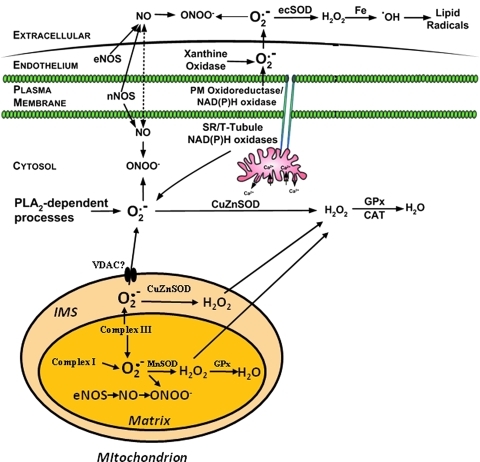
The sources of the extracellular ROS that are released from skeletal muscle cells in culture or isolated muscle preparations are also relatively obscure. Both hydrogen peroxide and NO can theoretically diffuse through the plasma membrane, and hence intracellular sources for these species may play a role (but see later for a further discussion of hydrogen peroxide). Although superoxide has been frequently detected in the extracellular medium surrounding muscle cells and isolated muscles (49, 63), substantial diffusion of this species (or its protonated form) through a plasma membrane seems extremely unlikely (31). In intact muscle preparations, it appears that xanthine oxidase enzymes in the endothelium associated with the muscle play an important role in contraction-induced release of superoxide (28). This source for ROS generation has been claimed to play important roles in adaptations of muscle to contractile activity (27) but has been relatively sparsely studied in recent years. In studies of isolated muscle fibers and myotubes, the role of xanthine oxidase is unclear. Javesghani et al. have reported release of ROS derived from a plasma membrane-localized NAD(P)H oxidase (42). Other NAD(P)H dependent systems have also been implicated (for discussion, see ref. 40). An updated scheme depicting the various sites that have been identified for generation of ROS and NO in skeletal muscle is presented in Figure 1.
FIG. 1.
Schematic representation of the sites and mechanisms proposed for ROS and NO generation in skeletal muscle fibers. Scheme updated from Powers and Jackson (60) to incorporate new data on the potential for hydrogen peroxide release from fibers to the extracellular space (see text for details) and on the role of CuZnSOD in the mitochondrial intermembrane space (IMS). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Control of ROS Production by Skeletal Muscle
Relatively little is known about the factors that control ROS production by skeletal muscle. Initial studies suggested that superoxide generation by skeletal muscle was essentially a by-product of oxygen consumption by mitochondria, and many early authors quoted reports that 2%–5% of the total oxygen consumed by mitochondria undergoes one electron reduction and generates substantial amounts of superoxide (8, 48). This assumption was related to exercise with the belief that the increased ROS generation that occurs during contractile activity was directly related to the elevated oxygen consumption that occurs with increased mitochondrial activity, implying potentially a 50- or 100-fold increase in superoxide generation by skeletal muscle during aerobic contractions (44, 72). As recently reviewed (38, 60), data now argue against such a substantial formation of superoxide within mitochondria. In particular, Brand and colleagues (10) reassessed the rate of production of reactive oxygen species by mitochondria and indicated that the upper estimate of the proportion of the electron flow giving rise to ROS was an order of magnitude lower than the original minimum estimate (71) and this rate of production appears to be further reduced by intrinsic control mechanisms and the metabolic state of the mitochondria (9, 10, 21, 35). Our data indicate that intracellular ROS activity in skeletal muscle cells only increased by a modest 2–4 fold during contractions (50, 74) which appears to support these more recent assessments. In very recent data, investigators have targeted redox-sensitive probes to mitochondria of muscle fibers and have been unable to demonstrate any change in mitochondrial redox potential during contractile activity, which they argue implies a lack of mitochondrial ROS generation during contractions (52), although since the probe was not directly responsive to ROS, the direct relevance of these data remains unclear.
The control of superoxide production by putative NAD(P)H oxidase sources in skeletal muscle is unknown. In neutrophils and other phagocytic cells, the NAD(P)H oxidase complex is assembled on membranes following a stimulus for activation, but different mechanisms apply in many nonphagocytic cells (3). Since the major NAD(P)H oxidase(s) present in skeletal muscle have not been fully characterized, little is known about how superoxide generation by these sources might be controlled. Espinosa et al (24) hypothesized that the T-tubule-localized NAD(P)H oxidase might be activated by depolarization of the T-tubules but this has not been confirmed.
The only source of muscle ROS for which there is information on the control of activity is xanthine oxidase. This enzyme has been recognized to contribute to superoxide generation in ischemia and reperfusion, but recent data also indicate that the xanthine oxidase pathway is important in superoxide formation in the extracellular fluid following a non-damaging protocol of muscle contractions (28). It has been suggested that muscle contraction alters the shear stresses applied to the vascular bed of the muscle, and that this latter stimulus induces superoxide formation and release (67). However, most studies argue that in relatively hypoxic tissues, anaerobic metabolism leads to proteolytic modification of xanthine dehydrogenase to form xanthine oxidase (55) and to the increased availability of the xanthine oxidase substrates, hypoxanthine and xanthine (56). This has led some researchers to argue that superoxide generation by contracting muscle during exercise is greatest at exhaustion (77).
How Much ROS Is Generated by Contracting Skeletal Muscle?
There have been few studies that have attempted to quantify (other than in relative terms) the amounts of different ROS that are generated by skeletal muscle at rest or during contractions. This is because of the labile nature of ROS and difficulties in achieving any true quantification in analyses. Hydrogen peroxide is relatively stable, and recent attempts have been made to quantify the amounts of this ROS in skeletal muscle at rest and during contractions:
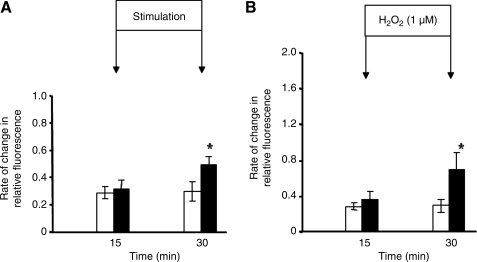
Palomero et al. (57) applied a protocol of electrically stimulated, isometric contractions to single isolated fibers from the mouse flexor digitorum brevis (FDB) muscle using a contraction protocol that had been previously shown (i) to induce release of superoxide and nitric oxide from muscle cells in culture and muscles of mice in vivo (49), (ii) to lead to a fall in muscle glutathione and protein thiol content (75), and (iii) to stimulate redox-regulated adaptive responses (76) when applied to intact muscles in vivo. The increase in intracellular dichlorofluorescein (DCF) fluorescence induced by the contraction protocol was less than that following exposure of the fibers to 1 μM hydrogen peroxide (Fig. 2). Palomero et al. (57) calculated that the likely change in intracellular hydrogen peroxide following addition of 1 μM to the extracellular medium was ∼0.1 μM. Thus it can be inferred that the absolute increase in cytosolic ROS activity in muscle fibers that was achieved following contractile activity was potentially equivalent to ∼100 nM hydrogen peroxide (see ref. 57 for detailed calculations). Previous studies of intracellular hydrogen peroxide concentrations in nonmuscle cells had reported resting concentrations of 10–100 nM (1, 12). Thus, the magnitude of the increase in intracellular hydrogen peroxide concentration calculated to occur during this form of contractile activity is entirely in accord with previous independent calculations in other cell types.
FIG. 2.
Comparison of the rate of increase in CM-DCF fluorescence from single isolated fibers from mouse flexor digitorum brevis muscles subjected to either a 15 min period of electrically stimulated isometric contractions (A) or exposed to 1 μM hydrogen peroxide for 15 min (B). The increase in CM-DCF fluorescence from contracting fibers was less than 50% of that seen following exposure to hydrogen peroxide. Data redrawn from Palomero et al, (57).
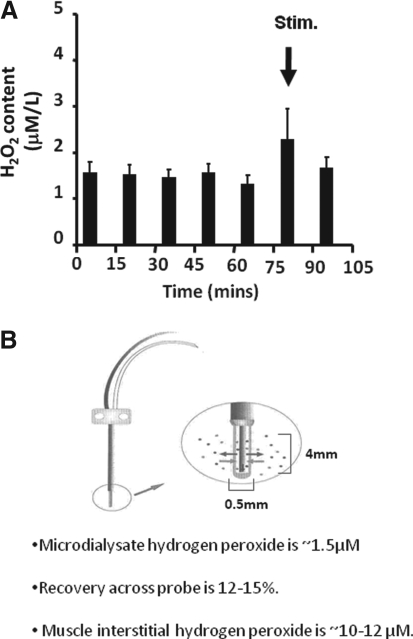
In parallel studies, Vasilaki et al. (75) had previously used microdialysis techniques to examine hydrogen peroxide concentrations in the interstitial fluid of skeletal muscle at rest and following a period of muscle contractions. They measured dialysate hydrogen peroxide concentrations of ∼1.5 μM at rest. Calculations of true interstitial concentrations of analytes from microdialysis experiments depend upon knowledge of the recovery of specific analytes across the microdialysis membrane; Vasilaki and colleagues calculated this to be ∼15% in their experimental model. Thus, they calculated interstitial hydrogen peroxide concentrations to be in the range 10–12 μM at rest and their data indicated that this may increase by ∼100% during contractions (Fig. 3). Using alternative approaches, other studies have reported extracellular hydrogen peroxide concentrations to be 2–4 μM (70) or 5–8 μM (68, 69). Thus, local interstitial concentrations of hydrogen peroxide may be slightly higher than those observed in the peripheral circulation, but again the values calculated are in the same order of those observed by independent analyses in other tissues.
FIG. 3.
The concentration of hydrogen peroxide in microdialysates from the gastrocnemius muscles of mice over five 15 min collections at rest, followed by 15 min of isometric contractions and a further 15 min at rest (A). A schematic diagram of the microdialysis probe is also shown (B), together with the calculation of interstitial hydrogen peroxide from microdialysate values and the values for the recovery of hydrogen peroxide across the probe obtained in preliminary studies. Data derived from Vasilaki et al. (76).
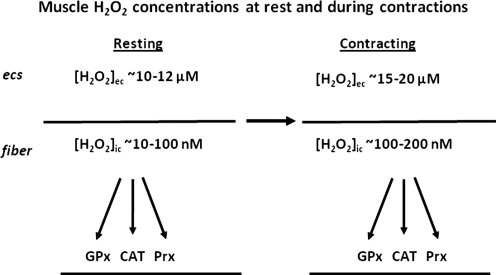
A comparison of these calculated muscle intracellular and interstitial hydrogen peroxide concentrations at rest and following contractile activity is shown in Figure 4 and illustrates the order of magnitude of difference between intracellular and extracellular hydrogen peroxide. The major enzymes for hydrogen peroxide metabolism (glutathione peroxidases, catalase, and peroxiredoxins) are all found at intracellular sites and undoubtedly contribute to this large concentration gradient that is apparently present in muscle cells. It is also clear from these data that simple diffusion of hydrogen peroxide from muscle fibers to the interstitial space cannot occur due to the large adverse concentration gradient. In some previous studies, release of hydrogen peroxide from mitochondria to the cytosol and hence to the muscle interstitial space was postulated as an explanation for data observed (14, 75) but this does not appear feasible. Previous reviews of the sources for ROS generation within muscle have also assumed that this diffusion could readily occur (40, 60).
FIG. 4.
Schematic representation of the concentrations of hydrogen peroxide calculated to occur in the cytosol of muscle fibers ([H2O2]ic) and interstitial space ([H2O2]ec)of fibers at rest and following the period of 15 min contractions. See text for details of calculations. CAT, catalase; ECS, extracellular space; GPx, glutathione peroxidase; Prx: peroxiredoxins.
These data do not preclude skeletal muscle as an important source of the systemic increases in oxidative stress that are seen during some forms of exercise. Muscle is able to release ROS from the plasma membrane or generate ROS on the external face of the plasma membrane (76) and to release superoxide to the extracellular space from endothelium-localized xanthine oxidase (28) in response to contractions. These ROS appear capable of leading to an increased systemic oxidation and oxidation in noncontracting tissues such as the liver (14).
What Physiological Roles May Be Mediated by the Low Concentrations of H2O2 and Other ROS Generated by Contractions in Skeletal Muscle?
While it is clear that oxidative damage to lipids, DNA, and protein may contribute to tissue dysfunction in various situations, it seems likely that oxidative damage is not induced to any substantial extent by the modest changes in ROS concentrations/activities that occur during normal contractions but would be likely to require higher concentrations/activities or more sustained exposure. There has been increasing recognition that ROS mediate physiological processes in tissues, and these molecules have been recognized as important signaling molecules with regulatory functions that modulate changes in cell and tissue homeostasis and gene expression (23, 32, 39). Signaling by these reactive molecules is mainly carried out by targeted modifications of specific residues in proteins (41).
We and others have obtained evidence that ROS modulate a number of physiological responses in skeletal muscle. A single period of contractile activity in mouse muscle was found to increase the activity of muscle antioxidant defense enzymes such as superoxide dismutase (SOD) and catalase, together with HSP60 and HSP70 content (49), changes that were replicated in human muscle studies in our laboratory. We have also characterized the changes in gene expression that occur following an acute period of contractile activity in comparison with those induced by exposure of skeletal muscle cells to hydrogen peroxide. This has identified a number of changes in gene expression that may be regulated directly by the hydrogen peroxide produced during contractile activity in vivo (51). In addition, other studies have implicated redox signaling in diverse processes in muscle such as maintenance of force production during contractions, glucose uptake, and insulin signaling (60).
ROS have become increasingly recognized to mediate some adaptive responses of skeletal muscle to contractile activity through the activation of redox-sensitive transcription factors (29, 39, 43, 64). NFκB is one such factor, along with Activator Protein-1 (AP-1) and Heat Shock Factor 1 (15). NFκB is a redox-regulated factor, and ROS have been proposed to be principal regulators of NFκB activation in many situations (53). NFκB family members expressed in skeletal muscle play critical roles in modulating the specificity of NFκB (7, 34). In skeletal muscle, NFκB modulates expression of a number of genes associated with myogenesis (4, 18), catabolism-related genes (7, 59, 73), and cytoprotective proteins during adaptation to contractile activity (76). Moreover, skeletal muscle has been identified as an endocrine organ producing cytokines via NFκB activation following a number of stresses including systemic inflammation or physical strain (25, 46). The specificity of the responses of skeletal muscle cells to NFκB activation is unclear but it is likely to be largely due to subtle differences in NFκB activation such as κB binding sequences and NFκB dimer formation that regulate expression of specific genes (4). Activation of NFκB by ROS appears to involve oxidation of key cysteine residues in the upstream activators of NFκB and in many situations the process is inhibited by antioxidants or reducing agents (33).
Thus, the increased ROS generated by skeletal muscle during contractions appear to stimulate various adaptive responses in the muscle. Activation of redox-sensitive transcription factors such as NFκB is one pathway by which these changes occur, but many others are feasible (39, 60). Activation of these responses is one of the key functions of the ROS generated during contractions and is essential for maintenance of muscle cell homeostasis during repeated episodes of contractile activity.
Failure of ROS-Mediated Redox Signaling in Skeletal Muscle During Aging
Loss of skeletal muscle mass and function is an important limiting factor in the maintenance of health and well-being in the elderly and a major contributor to frailty (75, 76). A number of publications have reported that the ability of cells and tissues from old mammals to respond to a variety of stresses (including contractile activity) by an increased content of HSPs and an increase in the activity of antioxidant defense enzymes is severely attenuated. The increase in HSP content and antioxidant defense enzyme activity that is evident in muscles of adult rodents following isometric contractions has been shown to be abolished in muscles of old rodents and this inability to adapt was shown to be due to the lack of complete activation of the appropriate transcription factors (76). Further studies from our laboratory have demonstrated that this age-related inability to produce HSPs plays a critical role in the development of functional deficits that occur with aging in skeletal muscle. Studies using transgenic mice, overexpressing HSP70 in skeletal muscle, demonstrated that increased muscle content of this protein provided protection against the fall in specific force associated with aging and facilitated rapid and successful regeneration following contraction-induced damage in muscles of old mice compared with the impaired regeneration and recovery normally observed in old mice. This protection was associated with maintenance of the ability of muscles of old HSP70 overexpressor mice to activate NFκB following contractions (11).
Activation of redox-responsive transcription factors is aberrant in muscles of old humans and mice. These muscles demonstrate both chronic constitutive activation of redox-sensitive transcription factors (17, 76) and an inability to further activate these transcription factors following an acute non-damaging contraction protocol (76). The chronic activation of transcription factors such as NFκB in muscles of old mice is associated with chronic increases in the expression of a number of genes. For example, increased content and activities of antioxidant defense enzymes such as the superoxide dismutases and catalase (11), increased content of HSPs (20, 76), and increased production of pro- and anti-inflammatory cytokines and chemokines by muscle cells (25).
The inability to activate NFκB in response to an acute contraction protocol is associated with severe attenuation of normal changes in expression of cytoprotective genes (36, 47, 62, 76). We have shown that the increases in HSP content and antioxidant enzyme activities stimulated by isometric contractions in muscles of adult rodents were abolished in muscles of old rodents (76).
A diminished ability to respond to the stress of contractions has been reported to play an important role in other age-related defects in muscle function and adaptation. Ljubicic and Hood (47) have reported a severe attenuation of the signaling pathways involved in mitochondrial biogenesis in fast muscle fibers of old rats following contractions compared with that seen in fibers from young rats. ROS are known to play an important role in the activation of signaling cascades (37). These authors suggest that ROS affect mitochondrial biogenesis via the upregulation of transcriptional regulators such as peroxisome proliferator-activated receptor-γ coactivator-1 protein-α (PGC-1α), suggesting that an aberrant activation of ROS generation following contractions may be responsible for the diminished mitochondrial biogenesis in muscles of old rats. This blunted or absent adaptation to stress in muscle of old humans and mice is not limited to the exercise response. Skeletal muscle of healthy elderly humans demonstrates a reduction in anabolic sensitivity and responsiveness of muscle protein synthesis pathways. Cuthbertson et al. (17) demonstrated a reduction in the phosphorylation of mTOR and downstream translational regulators in response to essential amino acid (EAA) ingestion when compared with the young despite greater plasma EAA availability in elderly subjects. The authors concluded that the nutrient signal was not sensed or transduced as well by muscle in the elderly compared with muscle in the young, resulting in a lower protein synthesis response to the same nutrient stimulus.
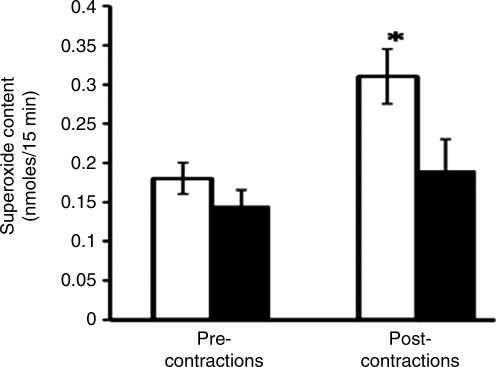
Thus, a modification of redox-regulated adaptive responses to contractile activity is seen in aging and may underlie various critical defects in function that occur in the elderly. Although there are published data that indicate aging is associated with an increased generation of ROS by muscle mitochondria (75), few studies have examined the generation of ROS by muscle of aged animals or man. Vasilaki et al. (75) examined a number of aspects of ROS and RNS generation by muscle from old compared with adult mice (Fig. 5) and found that the contraction-induced release of superoxide was reduced from the muscles of old mice. Thus, taken together, these data on redox-sensitive transcription factor activation and ROS activities suggest that the control of ROS is modified in muscle from old animals, although current data do not allow a precise evaluation of where this defect occurs.
FIG. 5.
Comparison of the effects of contraction on superoxide release from gastrocnemius muscles of old (black bars) compared with muscles of adult mice (open bars). Data are from microdialysis studies and show results from 15 min collections at rest, followed by 15 min of isometric contractions. *P<0.05 compared with the superoxide content in microdialysate from adult mice prior to contractions. Data derived from Vasilaki et al. (76).
Potential Future Directions for the Study of Physiological Generation of ROS and Modification of Subcellular Redox Potentials in Cell Signaling Pathways
The previous discussion of likely intracellular concentrations of hydrogen peroxide relied extensively on considerations of the sensitivity of a nonspecific indicator of ROS (DCFH). In this area, most studies have used indirect approaches to assess ROS activities in contracting skeletal muscle and traditional biochemical analyses to study the redox couples for glutathione etc. have utilized whole tissues. In order to understand the manner in which ROS regulate redox-sensitive processes and cellular adaptations in discrete compartments of the cell, it is necessary to develop approaches that permit analyses of ROS in single cells and cell organelles and of the redox potential in single cells and organelles. This section describes some new analytical approaches that may permit these analyses to be undertaken in skeletal muscle.
Assessment of intracellular ROS activities in single muscle fibers
Several authors have examined the superficial fibers of limb muscles and diaphragm as a means of undertaking single fiber measurements, and others have laboriously dissected single fibers from small rodent muscles (60). We and others have developed approaches to monitor ROS activities in single intact muscle fibers that are readily isolated by collagenase digestion (57, 61) and in single myotubes. To date, these studies have used general indicators of ROS activities (50, 57, 61, 74). The single intact mature fiber preparation has a variety of advantages over other approaches since these fibers are mature in comparison with cultured myotubes and the preparation is relatively uncontaminated with nonmuscle cells. In our studies, fibers have been isolated from the mouse flexor digitorum brevis (FDB) and used to monitor in real-time the changes in ROS activities in skeletal muscle cells, but isolation from other small rodent muscles is also entirely feasible (57, 61). We have demonstrated the use of this preparation with DAF-FM (to monitor NO) and DCFH (or CM-DCFH), an indicator which has been used for a number of years and shown to be sensitive to a number of ROS and reactive nitrogen species including hydrogen peroxide, superoxide, nitric oxide, and peroxynitrite (2, 54). More recent studies have utilized dihydroethidium (DHE) as a probe for superoxide in the single intact mature FDB fibers (see below).
Superoxide activity in cytosol and mitochondria
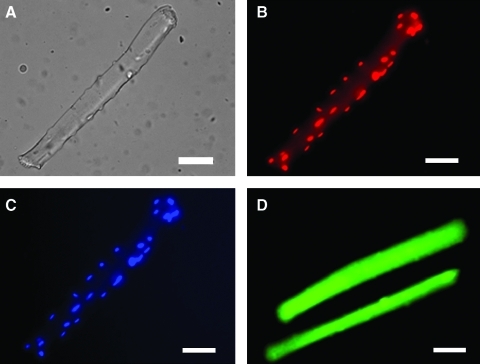
To permit analyses of primary ROS species, a number of new probes have been developed. Measurements of ethidium fluorescence following DHE loading of isolated cells have been used as an assay for intracellular superoxide (80). In the standard technique, oxidation of DHE within all parts of the cell leads to the formation of ethidium that intercalates into nuclear DNA and fluorescence measurements have previously been made from either the cytosol or nuclei of muscle cells (Fig. 6) (80). Our unpublished data illustrate that this technique can be applied to isolated skeletal muscle fibers. A development of this assay to improve specificity involves analysis of the specific product of the reaction of superoxide and 2-hydroxyethidium (DHE) (79) through HPLC approaches or use of an alternative excitation wavelength (maximum 396 nm) for fluorescence microscopy (65).
FIG. 6.
Images of single isolated fibers from mouse FDB muscles loaded with ROS sensitive probes. (A) Brightfield image, bar=50 micron; (B) fiber loaded with DHE; (C) the same fiber as in B stained with DAPI nuclear stain; (D) fiber loaded with DCFH. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
A modification of the above approach potentially permits the analyses of superoxide activity in mitochondria. DHE has been linked through a hexyl carbon chain to a triphenylphosphonium group to produce a compound that accumulates in mitochondria in response to the negative membrane potential. This compound known as MitoSOX or Mito-HE (65) can be used to monitor mitochondrial superoxide generation. In a similar manner to the analysis of 2-hydroxyethidium, a further development of this approach is analysis of the specific product of the reaction of superoxide and MitoSOX (HO-MitoSOX) (65, 79).
Probes to permit the monitoring of other ROS in specific cell compartments have also been developed. In particular, a genetically encoded specific probe for hydrogen peroxide (HyPer) that can be targeted to cytosol, mitochondria, or nuclei is now commercially available (5).
Analysis of subcellular redox couples
In order to understand how ROS interact with signaling processes in specific subcellular sites, it will be necessary to monitor local subcellular redox potentials (26, 33). Analyses of the total cellular reduced and oxidized glutathione content provide an index of the global redox potential of tissues, but do not allow assessment of these in the subcellular organelles that regulate redox signaling pathways. A modification of Western blot analysis (redox Western blotting) allows quantification of reduced and oxidized TRx1 in the cytoplasm and nuclei of cells and TRx2 in mitochondria (26). These very specific techniques provide a measure of the redox potential in cytosol, nuclei, and mitochondria of cells. In addition, new redox-sensitive probes have been developed to monitor the redox potential of specific thiol couples in single cells and at subcellular sites. These include redox-sensitive green fluorescent protein (ro-GFP) (22) and glutaredoxin-tagged ro-GFP (30), both of which can be targeted to different organelles in cells.
Conclusions
Research over the last 30 years has allowed a great deal to be learned about ROS generation by skeletal muscle at rest and during contractions, but there remain big gaps in our understanding of this area that particularly relate to any detailed understanding of the factors controlling muscle ROS generation. New techniques and approaches such as the development of quantitative sensitive ROS or redox-specific probes that can be targeted to specific intra- and extracellular sites are now becoming available (see refs. 5, 30, 65) and should allow these important questions to be answered in the near future with the potential to improve our ability to optimize muscle function during exercise and in a variety of pathological disorders affecting muscle including aging.
Abbreviations Used
- AP-1
activator protein 1
- CAT
catalase
- CM-DCFH
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein
- CuZnSOD
copper, zinc superoxide dismutase (SOD1)
- DAF-FM
4-amino-5-methylamino-2,7-difluorofluorescein
- DCF
2′,7′-dichlorofluorescin
- DCFH
2′,7′-dichlorodihydrofluorescein
- DHE
dihydroethidium
- EAA
essential amino acid
- ecSOD
extracellular superoxide dismutase (SOD3)
- FDB
flexor digitorum brevis
- GPx
glutathione peroxidase
- HSP
heat shock protein
- NAD(P)H
reduced nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor κ-B
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGC-1α
proliferator activated receptor-γ coactivator-1 protein-α
- PLA2
phospholipase A2
- PM
plasma membrane
- ro-GFP
redox-sensitive green fluoresecnt protein
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
References
- 1.Antunes F. Cadenas E. Cellular titration of apoptosis with steady state concentrations of H(2)O(2): Submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med. 2001;30:1008–1018. doi: 10.1016/s0891-5849(01)00493-2. [DOI] [PubMed] [Google Scholar]
- 2.Arbogast S. Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R698–705. doi: 10.1152/ajpregu.00072.2004. [DOI] [PubMed] [Google Scholar]
- 3.Arora S. Vaishya R. Dabla PK. Singh B. NAD(P)H oxidases in coronary artery disease. Adv Clin Chem 2010. 2010;50:65–86. doi: 10.1016/s0065-2423(10)50004-0. [DOI] [PubMed] [Google Scholar]
- 4.Bakkar N. Wang J. Ladner KJ. Wang H. Dahlman JM. Carathers M. Acharyya S. Rudnicki MA. Hollenbach AD. Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belousov VV. Fradkov AF. Lukyanov KA. Staroverov DB. Shakhbazov KS. Terskikh AV. Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 6.Balon TW. Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 7.Bar-Shai M. Carmeli E. Reznick AZ. The role of NF-kappaB in protein breakdown in immobilization, aging, and exercise: From basic processes to promotion of health. Ann NY Acad Sci. 2005;1057:431–447. doi: 10.1196/annals.1356.034. [DOI] [PubMed] [Google Scholar]
- 8.Boveris A. Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brand MD. Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Brand MD. Affourtit C. Esteves TC. Green K. Lambert AJ. Miwa S. Pakay JL. Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Broome CS. Kayani AC. Palomero J. Dillmann WH. Mestril R. Jackson MJ. McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after non-damaging contractile activity. FASEB J. 2006;20:1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- 12.Chance B. Sies H. Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 13.Close GC. Ashton T. McArdle A. Jackson MJ. Microdialysis studies of extracellular reactive oxygen species in skeletal muscle: Factors influencing the reduction of cytochrome c and hydroxylation of salicylate. Free Rad Biol Med. 2005;39:1460–1467. doi: 10.1016/j.freeradbiomed.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Close GL. Kayani AC. Ashton T. McArdle A. Jackson MJ. Release of superoxide from skeletal muscle of adult and old mice: An experimental test of the reductive hotspot hypothesis. Aging Cell. 2007;6:189–95. doi: 10.1111/j.1474-9726.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- 15.Cotto JJ. Morimoto RI. Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors. Biochem Soc Symp. 1999;64:105–118. [PubMed] [Google Scholar]
- 16. This reference has been deleted.
- 17.Cuthbertson D. Smith K. Babraj J. Leese G. Waddell T. Atherton P. Wackerhage H. Taylor PM. Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 18.Dahlman JM. Wang J. Bakkar N. Guttridge DC. The RelA/p65 subunit of NF-kappaB specifically regulates cyclin D1 protein stability: Implications for cell cycle withdrawal and skeletal myogenesis. J Cell Biochem. 2009;106:42–51. doi: 10.1002/jcb.21976. [DOI] [PubMed] [Google Scholar]
- 19.Davies KJ. Quintanilha AT. Brooks GA. Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 20.Demirel HA. Hamilton KL. Shanely RA. Tümer N. Koroly MJ. Powers SK. Age and attenuation of exercise-induced myocardial HSP72 accumulation. Am J Physiol Heart Circ Physiol. 2003;285:H1609–H1615. doi: 10.1152/ajpheart.00982.2002. [DOI] [PubMed] [Google Scholar]
- 21.Di Meo S. Venditti P. Mitochondria in exercise-induced oxidative stress. Biol Signals Recept. 2001;10:125–140. doi: 10.1159/000046880. [DOI] [PubMed] [Google Scholar]
- 22.Dooley CT. Dore TM. Hanson GT. Jackson WC. Remington SJ. Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 23.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 24.Espinosa A. Leiva A. Pena M. Muller M. Debandi A. Hidalgo C. Carrasco MA. Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol. 2006;209:379–388. doi: 10.1002/jcp.20745. [DOI] [PubMed] [Google Scholar]
- 25.Febbraio MA. Pedersen BK. Contraction-induced myokine production and release: Is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Go Y-M. Ziegler TR. Johnson JM. Gu L. Hansen JM. Jones DP. Selective protection of nuclear thioredoxin-1 and glutathione redox systems against oxidation during glucose and glutamine deficiency in human colonic epithelial cells. Free Rad Biol Med. 2007;42:363–370. doi: 10.1016/j.freeradbiomed.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Cabrera MC. Borras C. Pallardo FV. Sastre J. Ji LL. Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Cabrera MC. Close GL. Kayani A. McArdle A. Jackson MJ. Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am J Physiol (Reg Integ Comp Physiol) 2010;298:R2–R8. doi: 10.1152/ajpregu.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Cabrera MC. Domenech E. Romagnoli M. Arduini A. Borras C. Pallardo FV. Sastre J. Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 30.Gutscher M. Pauleau AL. Marty L. Brach T. Wabnitz GH. Samstag Y. Meyer AJ. Dick TP. Real-time imaging of the intracellular glutathione redox potential. Nat Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B. Gutteridge JMC. Free Radical Biology and Medicine. Oxford University Press; 1989. [Google Scholar]
- 32.Haddad JJ. Antioxidant and pro-oxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14:879–897. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 33.Hansen JM. Zhang H. Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci 2006. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 34.Hayden MS. Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Herrero A. Barja G. ADP-regulation of mitochondrial free radical production is different with complex I- or complex II-linked substrates: Implications for the exercise paradox and brain hypermetabolism. J Bioenerg Biomembr. 1997;29:241–249. doi: 10.1023/a:1022458010266. [DOI] [PubMed] [Google Scholar]
- 36.Heydari AR. You S. Takahashi R. Gutsmann-Conrad A. Sarge KD. Richardson A. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res. 2000;256:83–93. doi: 10.1006/excr.2000.4808. [DOI] [PubMed] [Google Scholar]
- 37.Irrcher I. Ljubicic V. Hood Da. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296:C116–123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 38.Jackson MJ. Free radicals generated by contracting muscle: By-products of metabolism or key regulators of muscle function? Free Rad Biol Med. 2008;44:132–141. doi: 10.1016/j.freeradbiomed.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Jackson MJ. Papa S. Bolanos J. Bruckdorfer R. Carlsen H. Elliott RM. Flier J. Griffiths HR. Heales S. Holst B. Lorusso M. Lund E. Oivind Moskaug J. Moser U. Di Paola M. Polidori MC. Signorile A. Stahl W. Vina-Ribes J. Astley SB. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 40.Jackson MJ. Pye D. Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol. 2006;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 41.Janssen-Heininger YM. Mossman BT. Heintz NH. Forman HJ. Kalyanaraman B. Finkel T. Stamler JS. Rhee SG. van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javesghani D. Magder SA. Barreiro E. Quinn MT. Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 43.Ji LL. Gomez-Cabrera MC. Steinhafel N. Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 44.Kanter MM. Free radicals, exercise, and antioxidant supplementation. Int J Sport Nutr. 1994;4:205–220. doi: 10.1123/ijsn.4.3.205. [DOI] [PubMed] [Google Scholar]
- 45.Kobzik L. Reid MB. Bredt DS. Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 46.Lee CE. McArdle A. Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26:524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Ljubicic V. Hood DA. Kinase-specific responsiveness to incremental contractile activity in skeletal muscle with low and high mitochondrial content. Am J Physiol Endocrinol Metab. 2008;295:E195–204. doi: 10.1152/ajpendo.90276.2008. [DOI] [PubMed] [Google Scholar]
- 48.Loschen G. Azzi A. Richter C. Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 49.McArdle A. Pattwell D. Vasilaki A. Griffiths RD. Jackson MJ. Contractile activity-induced oxidative stress: Cellular origin and adaptive responses. Am J Physiol (Cell Physiol) 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- 50.McArdle F. Pattwell DM. Vasilaki A. McArdle A. Jackson MJ. Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med. 2005;39:651–657. doi: 10.1016/j.freeradbiomed.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 51.McArdle F. Spiers S. Aldemir H. Vasilaki A. Beaver A. Iwanejko L. McArdle A. Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004;561:233–244. doi: 10.1113/jphysiol.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michaelson LP. Shi G. Ward CW. Rodney GG. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve. 2010;42:522–529. doi: 10.1002/mus.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran LK. Gutteridge JM. Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem. 2001;8:763–772. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- 54.Murrant CL. Andrade FH. Reid MB. Exogenous reactive oxygen and nitric oxide alter intracellular oxidant status of skeletal muscle fibres. Acta Physiol Scand. 1999;166:111–121. doi: 10.1046/j.1365-201x.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 55.Nishino T. Okamoto K. Eger BT. Pai EF. Nishino T. Mammalian xanthine oxidoreductase: Mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 56.Pacher P. Nivorozhkin A. Szabó C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palomero J. Pye D. Kabayo T. Spiller DG. Jackson MJ. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibres by real-time fluorescence microscopy. Antioxid Redox Signal. 2008;10:1463–1474. doi: 10.1089/ars.2007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pattwell DM. McArdle A. Morgan JE. Patridge TA. Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Peterson JM. Guttridge DC. Skeletal muscle diseases, inflammation, and NF-kappaB signaling: Insights and opportunities for therapeutic intervention. Int Rev Immunol. 2008;27:375–387. doi: 10.1080/08830180802302389. [DOI] [PubMed] [Google Scholar]
- 60.Powers SK. Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pye D. Kabayo T. Palomero J. Jackson MJ. Real-time measurements of nitric oxide in mature skeletal muscle fibres during contractions. J Physiol. 2007;581:309–318. doi: 10.1113/jphysiol.2006.125930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao DV. Watson K. Jones GL. Age-related attenuation in the expression of the major heat shock proteins in human peripheral lymphocytes. Mech Ageing Dev. 1999;107:105–118. doi: 10.1016/s0047-6374(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 63.Reid MB. Shoji T. Moody MR. Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 64.Ristow M. Zarse K. Oberbach A. Klöting N. Birringer M. Kiehntopf M. Stumvoll M. Kahn CR. Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson KM. Janes MS. Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- 66.Silveira LR. Pereira-Da-Silva L. Juel C. Hellsten Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med. 2003;35:455–464. doi: 10.1016/s0891-5849(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 67.Stofan DA. Callahan LA. DiMarco AF. Nethery DE. Supinski GS. Modulation of release of reactive oxygen species by the contracting diaphragm. Am J Respir Crit Care Med. 2000;161:891–898. [PubMed] [Google Scholar]
- 68.Stone JR. Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9:231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 69.Stone JR. Maki JL. Collins T. Basal and hydrogen peroxide stimulated sites of phosphorylation in heterogeneous nuclear ribonucleoprotein C1/C2. Biochemistry. 2003;42:1301–1308. doi: 10.1021/bi0268091. [DOI] [PubMed] [Google Scholar]
- 70.Stone JR. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch Biochem Biophys. 2004;422:119–124. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 71.St-Pierre J. Buckingham JA. Roebuck SJ. Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 72.Urso ML. Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 73.Van Gammeren D. Damrauer JS. Jackman RW. Kandarian SC. The IkappaB kinases IKKalpha and IKKbeta are necessary and sufficient for skeletal muscle atrophy. FASEB J. 2009;23:362–370. doi: 10.1096/fj.08-114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vasilaki A. Csete M. Pye D. Lee S. Palomero J. McArdle F. Van Remmen H. Richardson A. McArdle A. Faulkner JA. Jackson MJ. Genetic modification of the MnSOD/GPx1 pathway influences intracellular ROS generation in quiescent, but not contracting myotubes. Free Radic Biol Med. 2006;41:1719–1725. doi: 10.1016/j.freeradbiomed.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Vasilaki A. Mansouri A. Remmen H. van der Meulen JH. Larkin L. Richardson AG. McArdle A. Faulkner JA. Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: Effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 76.Vasilaki A. McArdle F. Iwanejko LM. McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: The effect of age. Mech Ageing Dev. 2006;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Viña J. Gimeno A. Sastre J. Desco C. Asensi M. Pallardó FV. Cuesta A. Ferrero JA. Terada LS. Repine JE. Mechanism of free radical production in exhaustive exercise in humans and rats; Role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000;49:539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- 78.Xia R. Webb JA. Gnall LL. Cutler K. Abramson JJ. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Physiol. 2003;285:C215–221. doi: 10.1152/ajpcell.00034.2002. [DOI] [PubMed] [Google Scholar]
- 79.Zhao H. Joseph J. Fales HM. Sokoloski EA. Levine RL. Vasquez-Vivar J. Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuo L. Christofi FL. Wright VP. Liu CY. Merola AJ. Berliner LJ. Clanton TL. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:C1058–1066. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]