Abstract
Lava caves contain a wealth of yellow, white, pink, tan, and gold-colored microbial mats; but in addition to these clearly biological mats, there are many secondary mineral deposits that are nonbiological in appearance. Secondary mineral deposits examined include an amorphous copper-silicate deposit (Hawai‘i) that is blue-green in color and contains reticulated and fuzzy filament morphologies. In the Azores, lava tubes contain iron-oxide formations, a soft ooze-like coating, and pink hexagons on basaltic glass, while gold-colored deposits are found in lava caves in New Mexico and Hawai‘i. A combination of scanning electron microscopy (SEM) and molecular techniques was used to analyze these communities. Molecular analyses of the microbial mats and secondary mineral deposits revealed a community that contains 14 phyla of bacteria across three locations: the Azores, New Mexico, and Hawai‘i. Similarities exist between bacterial phyla found in microbial mats and secondary minerals, but marked differences also occur, such as the lack of Actinobacteria in two-thirds of the secondary mineral deposits. The discovery that such deposits contain abundant life can help guide our detection of life on extraterrestrial bodies. Key Words: Biosignatures—Astrobiology—Bacteria—Caves—Life detection—Microbial mats. Astrobiology 11, 601–618.
1. Introduction
The recognition that early Mars was quite similar to early Earth, warmer and with liquid water (Baker et al., 1991), led to the suggestion that life may have evolved on Mars at about the same time as it did on Earth (Westall et al., 2000; Beaty et al., 2005). Testing this hypothesis has focused on two rather separate paths. First, researchers have looked at early Precambrian deposits on Earth and searched for traces of life (Buick, 1990; Gibson et al., 1999; Westall, 1999; Westall et al., 2001). The examination of Precambrian deposits has expanded into mining those traces of life for evidence of environmental conditions that might illuminate the conditions needed for life to evolve and survive (Omelon, 2008). Second, efforts have included searching Earth for Mars analogues: places that mimic in some way an environment known or suspected to have been present on ancient Mars. Potential analogues have expanded as conditions on Mars are better understood and include the Antarctic Dry Valleys (Friedmann, 1982; Ascaso and Wierzchos, 2003; Wierzchos et al., 2005; Omelon, 2008), hypersaline environments (Douglas, 2004; Mancinelli et al., 2004; Blackhurst et al., 2005; Barbieri et al., 2006; Benison et al., 2008; Sadooni et al., 2010), hot springs (Glamoclija et al., 2004; Preston et al., 2008; Parenteau and Cady, 2010), Fe-rich environments (Gillan and De Ridder, 2001; Schelble et al., 2004; Villar et al., 2006; Fernández-Remolar et al., 2008; Izawa et al., 2010), sulfur-rich surface habitats (Boston et al., 2006; Engel, 2007), impact deposits (e.g., impact-induced hydrothermal systems, Hode et al., 2008), and subsurface environments (McKay and Stoker, 1989; Boston et al., 2001; Fernández-Remolar et al., 2008; Izawa et al., 2010).
Subsurface environments that are also Fe-rich, such as basaltic lava caves, are considered particularly appealing for several reasons. First, large areas of Mars are underlain by basaltic rocks, which thus provides a large target area. Second, life in the subsurface has a high preservation potential in that it is protected from damaging solar radiation (Westall et al., 2000; Villar et al., 2006; Izawa et al., 2010; Léveillé and Datta, 2010). The subsurface of Mars likely retained liquid water for longer than the surface, which would have provided a potential refuge for life (Westall et al., 2000). Researchers also have suggested that alteration of mafic rocks could provide chemicals, such as hydrogen, to fuel chemosynthetic life (Boston et al., 1992; Stevens and McKinley, 1995; Kelley et al., 2005; Fernández-Remolar et al., 2008; Blank et al., 2009).
Accessing subsurface environments on Earth to develop an array of potential biosignatures and on other planets to test for extinct or extant life has been a key target. Blank et al. (2009) examined an active microbial community in an alkaline spring system within an ophiolite as a possible analogue for Mars. Villar et al. (2006) sampled basalt on the surface and found extremophile communities in small cavities and under protective minerals. Fernández-Remolar et al. (2008) went further in their study of the Río Tinto area in Spain by drilling a series of cores to access the subsurface. Many of the examples of microbes in basaltic glass also come from cores (Izawa et al., 2010, and references therein). An alternative way to access the subsurface is via caves (Boston, 2000), specifically lava caves (Boston et al., 2003; Léveillé and Datta, 2010).
Lava caves have been recognized on Mars and elsewhere by using a variety of orbiter data (see summary in Léveillé and Datta, 2010). Lava caves are common on Earth wherever basaltic lava occurs. The most common are lava tubes, which form when a fluid lava flow cools on the top from contact with the cool atmosphere but keeps flowing underneath (Fig. 1). A lava tube forms when the molten lava drains out, leaving a cave. Entrances to lava caves occur where the roof has collapsed; multiple entrances are common (Palmer, 2007). Despite their frequency, relatively little work has been done on either the microbiology or mineralogy of lava caves (Forti, 2005; Northup et al., 2008; White, 2010). To target lava caves in the search for life on Mars, a better understanding of life in Earth's lava caves is needed.
FIG. 1.
Photos illustrate an opening to a lava cave in a volcanic trench (A), the entrance to Four Windows Cave from the inside of the cave (B), a skylight in Four Windows Cave (C), a typical lava cave shape in El Malpais (D). All photos are from El Malpais National Monument and are copyright Kenneth Ingham; used with permission. Color images available online at www.liebertonline.com/ast
Our study revealed a large array of microbial mats in volcanic lava caves on Earth (Northup et al., 2008; Garcia et al., 2009; Moya et al., 2009; Snider et al., 2009). In addition to these clearly biological deposits, there are many mineral deposits that appear to be nonbiological in origin. However, a combination of scanning electron microscopy (SEM) and molecular techniques revealed diverse microbial communities that inhabit both the microbial mats and the mineral-like deposits. The discovery that such deposits contain abundant life can help guide our detection of life on other extraterrestrial bodies.
2. Materials and Methods
2.1. Site description and sample collection
Basaltic lava caves (mostly lava tubes) from different climate conditions were sampled, including one tropical example (Hawai‘i), one temperate (the Azores), and one from a semi-arid environment (New Mexico) (Table 1).
Table 1.
Overview of Samples and Analyses Performed
| Location | Cave name | Sample nature | Sample color | Analyses performed | Relevant figures |
|---|---|---|---|---|---|
| Microbial mats | |||||
| Hawai‘i | Bird Park | Microbial mat | White and yellow | Molecular and SEM | |
| Hawai‘i | Epperson's | Microbial mat | White and yellow | Molecular and SEM | |
| Hawai‘i | Kaumana | Microbial mat | White and yellow | Molecular | |
| Hawai‘i | Kula Kai | Microbial mat | White and yellow | Molecular and SEM | |
| Azores | Branca Opala | Microbial mat | White and yellow | Molecular | Fig. 2 |
| Azores | Balcões | Microbial mat | White and yellow | Molecular | Fig. 2 |
| Azores | Achada | Microbial mat | White and yellow | Molecular | Fig. 2 |
| Azores | Principiantes | Microbial mat | White and yellow | Molecular | Fig. 2 |
| New Mexico | ELMA2 | Microbial mat | White and yellow | Molecular and SEM | |
| New Mexico | ELMA3 | Microbial mat | White and yellow | Molecular and SEM | |
| New Mexico | ELMA4 | Microbial mat | White and yellow | Molecular | |
| New Mexico | Four Windows | Microbial mat | White and yellow | Molecular and SEM | |
| Secondary mineral deposits | |||||
| Hawai‘i | Maelstrom | Copper silicate | Blue-green | Molecular and SEM | Figs. 3, 9, 11 |
| Hawai‘i | Thurston | Gold-colored veins | Gold | Molecular and SEM | Figs. 8, 9, 12 |
| Hawai‘i | Epperson's | Pointillistic colonies | White | SEM | Fig. 4 |
| Azores | Algar do Carvão | Hexagons | Pink | Molecular and SEM | Figs. 7, 9, 13 |
| Azores | Buracos | Fe oxide | Red | Molecular | Figs. 5, 9 |
| Azores | Principiantes | Organic ooze | Butterscotch | Molecular | Figs. 6, 9 |
| New Mexico | Four Windows | Gold-colored veins | Gold | Molecular and SEM | Figs. 8, 9, 12 |
In each cave, 3–10 small rock chips, covered with either microbial mats or secondary minerals, were aseptically chipped from the surface of each sample site for DNA analysis and SEM. Samples were collected from the New Mexican and Hawaiian Island lava caves, under National Park Service collecting permits or permission of landowners. Azorean samples were collected on the island of Terceira, under the auspices of Os Montanheiros, an Azorean organization that supervises access to many of the caves. Samples were selected for collection based on uniformity of color or the presence of colored secondary mineral deposition. Samples for DNA analysis were stored in sucrose lysis buffer on site to preserve the DNA (Giovannoni et al., 1990) and transported to the laboratory for storage in a −80°C freezer until DNA extraction.
2.1.1. Hawai‘i
The Big Island of Hawai‘i, USA, in the Pacific Ocean located at 19°43′N 155°5′W, is composed of five shield volcanoes, mainly composed of tholeiitic basalt. The central portion of the island is formed from the two largest shield volcanoes, Mauna Kea (dormant) and Mauna Loa (active) with Kohala (dormant) to the northwest, Hualalai (active) to the west, and Kilauea (very active) to the southeast. Lava caves were sampled on Mauna Loa and Kilauea flows as noted below.
We sampled four lava cave walls for yellow and white microbial mats, including three on Mauna Loa (Bird Park Cave, also known as Kipuka Puaulu, Hawai‘i Volcanoes National Park; Kula Kai Caverns in the Kipuka Kanohina Cave Preserve; and Kaumana Cave, near Hilo), and one on Kilauea (Epperson's Cave, on the eastern side of the Big Island). Substrates underlying microbial mats were gray basaltic rock that varied from smooth to rough textures and often included lavacicles (i.e., small lava stalactites). Blue-green stalactites and deposits were sampled from the Maelstrom section of the Kipuka Kanohina Cave Preserve, on the south flank of Mauna Loa. The blue-green stalactites are forming in ceiling fractures in very vesicular, dark gray basalt. Gold-colored secondary minerals were collected from the dark side of Thurston Lava Tube on Kilauea, Hawai‘i Volcanoes National Park, and occurred on dark gray basaltic rock walls with surface roughness, or in cracks in the wall. Hawaiian lava caves sampled occur between 304 and 1219 m in elevation, experience a surface precipitation of 1016–4013 mm/year on average, and have an internal cave temperature that varies from 14°C to 19°C.
2.1.2. The Azores
Terceira is located in the Atlantic Ocean, approximately 1500 km west of the coast of Portugal, in the center of the Azores island chain at 38°44′N, 27°17′W. Terceira is built by four main volcanic complexes (Serra de Santa Bárbara, Serra do Morião, Pico Alto, and Serra do Cume) (Nunes, 2000, 2004). Recent work has documented 69 lava caves on Terceira, mostly lava tubes (Nunes et al., 2008). White and yellow microbial mat samples from Terceira were collected from Gruta dos Principiantes, Gruta da Achada, Gruta Branca Opala, and Gruta da Balcões. Samples of black volcanic glass with pink hexagons were collected from Algar do Carvão, a volcanic chimney, from a rock in the middle of the tourist trail. The patches of basaltic glass occur scattered across the face of a gray, vesicular basaltic boulder. Iron-oxide muds/rock samples were collected from Gruta dos Buracos, where the iron-oxide columns, stalactites, and stalagmites form at, or below, an actively dripping area at the back of this short lava cave. Butterscotch-colored organic ooze filigree was collected from the ceiling of a low passage in Gruta dos Principiantes, where it covered the entire ceiling area. The Azorean caves sampled occur at an elevation that varies between 255 and 585 m, have a surface precipitation of 1400–2303 mm/year on average, and have a relatively constant cave temperature of 15–16°C, except for Algar do Carvão, which is cooler at 11°C.
2.1.3. New Mexico
El Malpais National Monument, approximately 55 km south-southwest of Grants, New Mexico, USA, is part of the Zuni-Bandera volcanic field. The Zuni-Bandera volcanic field is a basaltic lava field on the edge of the Rio Grande Rift (Sims et al., 2007). There are three eruptive episodes: the oldest dating to ca. 700 ka, a middle one at ca. 150 ka, and the youngest at ca. 80 ka to 3 ka or younger (Laughlin et al., 1994; Sims et al., 2007). Numerous lava caves occur in the national monument; those sampled are from the Bandera flow, which has been dated at approximately 10–12 ka (Sims et al., 2007) or the Hoya de Cibola flow, which occurred at approximately 18 ka (Polyak, personal communication). Samples were collected from white microbial mats, gold-colored secondary mineral deposits, and yellow ooze-mats, all of which occur on walls of Four Windows, ELMA2, and ELMA3 Caves located in the Bandera flow. Samples were also collected from ELMA4, located in the Hoya de Cibola flow. Substrates underlying these deposits are dark gray basalt and roughly textured. The caves occur at approximately 2200 m in elevation in a region that is semi-arid (precipitation averaging ∼381 mm/year) with hot summers and cold winters. Cave temperatures vary from −2°C to +9°C.
2.2. Molecular phylogeny
2.2.1. Extraction of nucleic acids
Genomic DNA was extracted from rock chips with microbial mats or secondary mineral deposits stored in sucrose lysis buffer with the Power Soil DNA Extraction kit (MoBio Laboratories, Inc.).
2.2.2. 16S rDNA PCR amplification and clone library construction
The 16S rRNA gene was amplified from environmental DNA by PCR with universal primers, p46 forward (5′-GCYTAAYACATGCAAGTCG-3′) and p1409 reverse (5′-GTGACGGGCRGTGTGTRCAA-3′; Northup et al., 2010) and AmpliTaq LD (Applied Biosystems) with an MJ thermal cycler as follows: 4 min denaturation at 94°C followed by 35 cycles of 45 s annealing at 55°C, 2 min at 72°C (extension), and 30 s at 94°C (denaturation), with a final 45 s 55°C annealing and 20 min 72°C extension step after cycling was complete. Amplification products were cloned with the TOPO TA Cloning kit (Invitrogen), and plated on LB/ampicillin agar plates (Sambrook et al., 1989).
2.2.3. 16S rDNA sequencing
In initial sequencing, 125–300 ng of purified DNA was used as a template in cycle sequencing reactions of 96 clones/sample with ABI PRISM dye terminator cycle sequencing kit (Perkin-Elmer-Applied Biosystems) at the University of New Mexico Molecular Biology Facility. Primers used for sequencing of the 16S rRNA gene were T3 and T7. Additional sequencing was done through the Washington University Sequencing Facility in St. Louis, Missouri, with the T3 and T7 primers.
2.2.4. Phylogenetic analysis
Sequences were edited and assembled with Sequencher 4.8. (Gene Codes, Ann Arbor, MI). Orientation of each sequence was checked with OrientationChecker (www.bioinformatics-toolkit.org/Squirrel/index.html). To detect the presence of possible chimeras, sequences were analyzed with the Mallard/Pintail software (www.bioinformatics-toolkit.org; Ashelford et al., 2006). Alignment was accomplished with Greengenes (greengenes.lbl.gov; DeSantis et al., 2006) and manually corrected with the BioEdit editor (www.mbio.ncsu.edu/BioEdit/bioedit.html), guided by 16S rRNA secondary structure considerations. Sequences were then classified at the phylum level by using the Ribosomal Database Project Classifier (http://rdp.cme.msu.edu/classifier/classifier.jsp; Maidak et al., 2001). All sequences were analyzed by BLAST (National Center for Biotechnology Information; Altschul et al., 1997) and the Ribosomal Database Project Classifier (Maidak et al., 2001) to determine the taxonomic groupings of clone sequences.
2.3. Microscopy
Samples were examined on a JEOL 5800 scanning electron microscope equipped with an energy-dispersive X-ray (EDX) analyzer. Rock chips covered with microbial mats, secondary mineral deposits, or pieces of the blue-green stalactites were mounted directly on scanning electron microscope sample stubs in the field then air-dried and coated with Au-Pd metal in the laboratory for imaging.
3. Results
3.1. Field observations
Lava caves on Earth contain a colorful and diverse array of microbial mats (Fig. 2) that vary in color from white to yellow to pink, with various shades in between (Northup et al., 2008; Garcia et al., 2009; Moya et al., 2009; Snider et al., 2009). The microbial mats range in size from small scattered colonies to extensive areas that cover walls or ceilings of caves. The coverage of the visible microbial mats tends to be much more extensive in moist Hawaiian and Azorean caves than in the more arid caves, both on Hawai‘i and in New Mexico.
FIG. 2.
Microbial mats from Azorean lava caves. (A) Yellow microbial mats coat the lava cave wall. (B) White and pinkish-tan microbial colonies from a lava cave wall. (C) White and tan colonies on a wall that is dripping water. (D) White and tan microbial colonies adorn a lavacicle on the cave ceiling. (E) Yellow and white microbial mats from a lava cave wall. Field of view is approximately 2.5 m (A), 5 cm (B), 6 cm (C), 2 cm (D), and 4 cm (E) across. Images courtesy of Kenneth Ingham; used with permission. Color images available online at www.liebertonline.com/ast
In addition to the clearly biological deposits of microbial mats, there are many apparent mineral deposits, including blue-green copper silicate, white dots, iron oxides, gold-colored veins, and pink hexagons. The blue-green copper-silicate deposit (Fig. 3, identified as chrysocolla; see below) contains many biological morphologies discussed below. These copper silicate deposits occur in two portions (Maelstrom and Lower Tapa) of the Kipuka Kanohina Cave Preserve on Hawai‘i, as either dripping stalactites associated with cracks in the ceiling (Fig. 3) or as dry deposits on the floor beneath the stalactites (White, 2010).
FIG. 3.
Blue-green, copper-containing deposits from the Maelstrom entrance of the Kipuka Kanohina Cave Preserve, Hawai‘i. Width of drip≈0.5 cm. Image courtesy of Kenneth Ingham; used with permission. Color images available online at www.liebertonline.com/ast
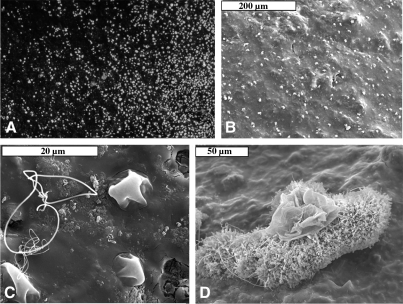
A recent discovery in Epperson's Cave is the presence of small, >1 mm sized white dots on the wall (Fig. 4A). Because of their ambiguous appearance, a small sample was examined with SEM. Scanning electron microscopy examination revealed that the smooth areas between white dots are an iron-oxide biofilm (Fig. 4B) with emerging colonies that when erupted show a variety of filaments, rods, and Actinobacteria-like morphologies (Fig. 4C, 4D). No molecular work has been done on these features to date due to the lack of available samples.
FIG. 4.
Pointillistic features from the wall of Epperson's Cave. (A) Two-centimeter overview of macroscopic deposits. Image courtesy of Kenneth Ingham; used with permission. (B) Overview of iron-oxide biofilm with unerupted colonies. (C) Scanning electron micrograph close-up view of the iron-oxide (FeOX) biofilm with colonies that are getting ready to erupt. (D) Newly erupted colony.
In the Azores, Gruta dos Buracos contains an impressive iron-oxide formation that includes iron-oxide stalactites above the formation (Fig. 5). On one side of the red iron-oxide formation, rimstone dams and gours have formed and hardened into rock. Much of the rest of the formation is softer and much less lithified. All caves sampled in the Azores contain extensive soft, ooze coatings that are clearly different from microbial mats, which show defined colony edges. In Gruta dos Principiantes, the ooze has hardened into a filigree (Fig. 6). These oozes, which cover up to 75% of the surfaces in some areas, are generally butterscotch in color but are occasionally more red-brown or cream. These oozes may represent surface materials that have seeped down through overlying cracks and fissures. In Algar do Carvão, pink hexagons (Fig. 7), approximately 0.2–2 mm in diameter, were observed on several patchy areas of black basaltic glass.
FIG. 5.
Iron-oxide formations in Gruta dos Buracos in the Azores. Image courtesy of Kenneth Ingham; used with permission. Color images available online at www.liebertonline.com/ast
FIG. 6.
Butterscotch-colored ooze forming a filigree in Gruta dos Principiantes in the Azores. Color images available online at www.liebertonline.com/ast
FIG. 7.
Pink hexagons on basaltic glass in Algar do Carvão, the Azores. (A) Overview of the pink hexagons; (B) close-up view from (A). Color images available online at www.liebertonline.com/ast
One mineral-like deposit that occurs in two locations is the gold-colored veins and scattered gold-colored deposits found in Four Windows Cave in New Mexico and Thurston Lava Tube in Hawai‘i (Fig. 8A, 8B, 8C, respectively). These deposits are often veinlike, approximately 1–3 mm in diameter, cover larger stretches of the wall, but are relatively invisible because of their size. In one instance in each of these caves, the deposits cover several meters of the wall in an alcove or reside in cracks in the lava walls.
FIG. 8.
Gold-colored secondary minerals on the walls and in cracks of Four Windows Cave in El Malpais National Monument, New Mexico (A, B) and in Thurston Lava Tube, in Hawai‘i Volcanoes National Park (C). Images courtesy of Kenneth Ingham; used with permission. Color images available online at www.liebertonline.com/ast
3.2. Bacterial phyla present
To compare obvious microbial features with apparent mineralogical features, we analyzed two sets of samples. First, we analyzed the microbial mats with clearly defined colonies visible to the unaided eye, and in particular the dominant yellow and white mats, which occur across all the caves we sampled. Four caves each from Hawai‘i, the Azores, and New Mexico were chosen for characterization (Table 1). Second, we analyzed six examples of mineral-like deposits, including the blue-green copper-silicate deposit from Hawai‘i; the gold-colored veins from Hawai‘i and New Mexico; and the pink hexagons, red iron oxides, and butterscotch-colored oozes from the Azores (Table 1).
Molecular analysis of white and yellow microbial mats revealed the presence of 13 phyla across these deposits (Table 2), with sequences that fall within four of the Proteobacteria subdivisions (Alpha-, Beta-, Gamma-, and Deltaproteobacteria). At the phylum level, a great deal of overlap was seen across caves and the three locations. All caves sampled in all three locations were found to contain Actinobacteria, Proteobacteria, and Acidobacteria. All but one of the caves (ELMA3) contained Nitrospirae. Nine of the 12 caves contained Gemmatimonadetes and Chloroflexi, while eight of the caves contained Verrucomicrobia and Bacteroidetes. Fewer cave microbial mats contained Firmicutes (seven caves), Planctomycetes (six caves), Chlamydiae (four caves), OP10 (four caves), and TM7 (three caves). The number of phyla per cave ranged from 5 to 11. Overall, there tended to be slightly more diversity at the phyla level in the yellow mats than in the white mats, but not in all phyla. At the operational taxonomic unit level (roughly species), major differences existed between the Azores and Hawai‘i locations, with New Mexico sample analysis still in progress (Hathaway et al., unpublished data; Moya et al., unpublished data). Pico Island studies have added a 14th phylum, Ktedonobacteria.
Table 2.
Comparison of Bacterial Phyla Found in Yellow (Y) and White (W) Microbial Mats in Hawai‘i, the Azores, and New Mexico
| Cave | Act | αP | βP | γP | δP | Acid | Clfx | TM7 | Nit | Ver | Gem | Planc | Bact | Chlam | OP10 | Firm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4W | W | W | W | W | W | W | W | W | W | |||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||
| ELMA2 | ||||||||||||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||
| ELMA3 | W | W | W | W | W | W | ||||||||||
| Y | Y | Y | Y | Y | Y | Y | Y | |||||||||
| ELMA4 | W | W | W | W | W | W | W | W | ||||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||
| Bird Park | W | W | W | W | W | W | W | W | W | |||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||
| Epperson's | W | W | W | W | W | W | W | W | W | |||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||
| Kaumana | W | W | W | W | W | W | W | W | W | W | ||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||
| Kula Kai | W | W | W | W | W | W | W | W | ||||||||
| Y | Y | Y | Y | Y | Y | |||||||||||
| Branca Opala | W | W | W | W | W | W | W | |||||||||
| Y | Y | Y | Y | Y | Y | Y | Y | |||||||||
| Balcões | W | W | W | W | W | W | W | W | W | |||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||
| Achada | W | W | W | W | W | W | W | W | W | W | ||||||
| Y | Y | Y | Y | Y | Y | Y | Y | |||||||||
| Principiantes | W | W | W | W | W | W | W | W | W | W | W | W | W | W | ||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||
4W=Four Windows Cave, New Mexico; ELMA=El Malpais National Monument; Act=Actinobacteria; αP=Alphaproteobacteria; βP=Betaproteobacteria; γP=Gammaproteobacteria; δP=Deltaproteobacteria; Acid=Acidobacteria; Clfx=Chloroflexi; TM7=TM7; Nit=Nitrospirae; Ver=Verrucomicrobia; Gem=Gemmatimonadetes; Planc=Planctomycetes; Bact=Bacteroidetes; Chlam=Chlamydiae; OP10=OP10; Firm=Firmicutes.
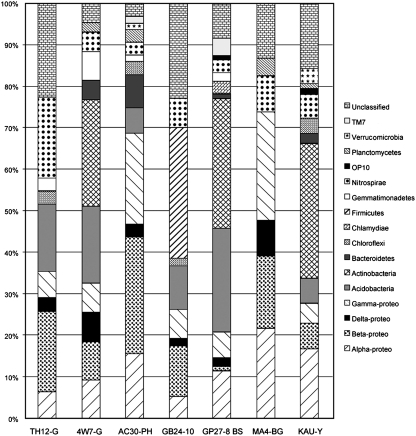
In comparing microbial mat samples with secondary mineral deposit samples, there were some marked differences (Fig. 9). The mineral-like deposits also contained 13 phyla across all the samples, including the same phyla found in the microbial mats. One representative microbial mat sample is provided on the right of Fig. 9 to allow for comparison. As in the microbial mat samples, Proteobacteria were ubiquitous. Nitrospirae and Acidobacteria, found across almost all microbial mat samples, were found in all but one (Maelstrom blue-green deposits) of the mineral-like deposits. Unlike the microbial mats, where they are almost ubiquitous, Actinobacteria were found in only two of the six mineral deposit samples. The iron-oxide formation contained several Bacillus spp., in the phylum Firmicutes, which were not present in the other secondary mineral deposits, including in the pink hexagons that also contain iron oxides.
FIG. 9.
Comparison of the phyla present in the secondary mineral deposits. TH12-G=gold-colored deposits in Thurston Lava Tube, Hawai‘i; 4W7-G=Gold deposits in Four Windows Cave, New Mexico; AC30-PH=Pink hexagons in Algar do Carvão, Azores; GB24-10=Iron-oxide formation in Gruta dos Buracos; GP27-8 BS=Butterscotch filigree in Gruta dos Principiantes, the Azores; MA4-BG=Blue-green deposits in the Maelstrom section of Kipuka Kanohina Cave Preserve, Hawai‘i; KAU-Y=Kaumana Cave, Hawai‘i.
3.3. Biological morphologies revealed by scanning electron microscopy
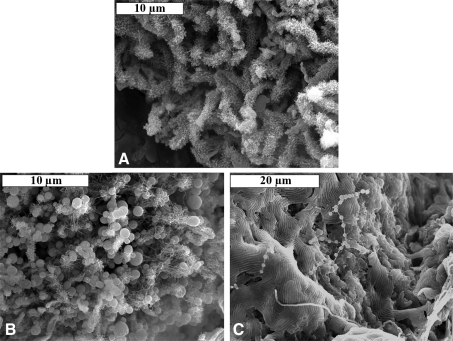
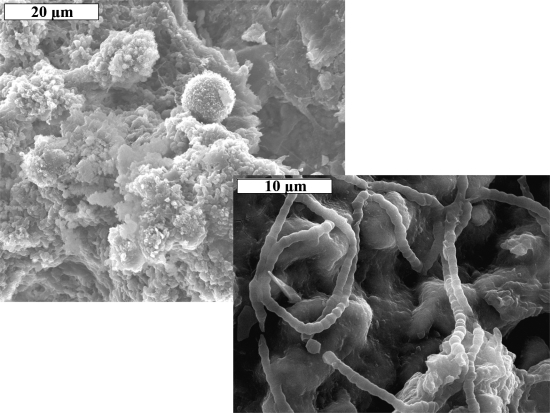
Microbial mats from the lava caves sampled were found to contain a variety of biological morphologies, including filaments with extensive putative pili covering their surfaces (Fig. 10A), as well as coccoid forms (Fig. 10B), beads-on-a-string (Fig. 10C), and rods arranged in rows (Fig. 10C). SEM also revealed a variety of biological morphologies in the secondary mineral deposits, including reticulated filaments in the blue-green deposits (Fig. 11A, 11B; Melim et al., 2008), filaments resembling a concertina or accordion in the gold-colored veins (Fig. 12), and iron-oxide coated and uncoated filaments in the pink hexagons (Fig. 13).
FIG. 10.
Scanning electron microscope images of microbial mats showing a variety of biological morphologies. (A) Filaments with extensive putative pili covering their surfaces, (B) coccoid forms with pili or filamentous extracellular polymeric substances, and (C) beads-on-a-string and rods arranged in rows.
FIG. 11.
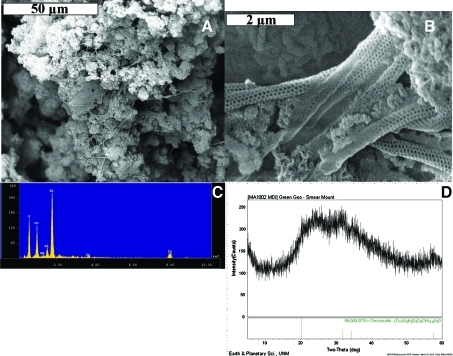
Details of the blue-green deposits. (A) Overview of reticulated filaments overlying Actinobacteria-like morphologies. (B) Closer view showing reticulated morphology from one of the clusters of filamentous forms in (A) (Melim et al., 2008). (C) EDX of blue-green deposit showing Si, O, Cu, Al. Traces of Ca and Mg are also present. (D) X-ray diffraction pattern of blue-green deposit compared to pattern for chrysocolla; pattern indicates the blue-green deposit is amorphous chrysocolla. Color images available online at www.liebertonline.com/ast
FIG. 12.
Scanning electron microscope images of gold-colored vein deposits. (Left) Filaments and fuzzy coccoid/filament morphologies from Thurston Lava Tube, Hawai‘i. (Right) Filamentous morphologies from Four Windows Cave, New Mexico.
FIG. 13.
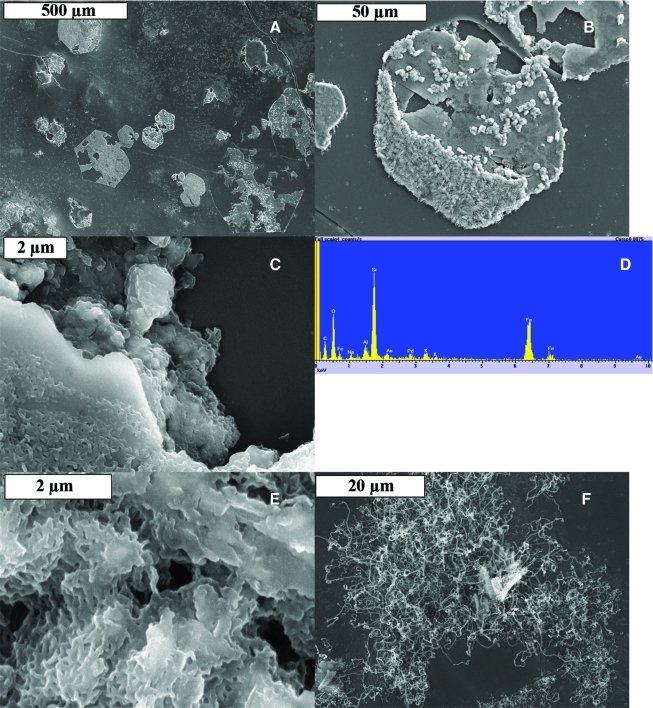
Scanning electron microscope images of pink hexagons. (A) Overview of a field of pink hexagons. (B) Closer view of one of the hexagons that shows some degradation of the hexagon. (C) Close-up view of detail from (B), showing the coated filaments inhabiting the upper surface of the hexagon. (D) EDX analysis of the hexagons showing the presence of iron oxides and a possible opal coating (Si, O). (E) Close-up view of the coated filaments that inhabit the surfaces of hexagons. (F) Small- and large-diameter filaments have colonized the basaltic glass between hexagons. Color images available online at www.liebertonline.com/ast
X-ray diffraction and EDX analyses (Fig. 11C, 1D) of the blue-green stalactites and floor deposits from the Maelstrom portion of the Kipuka Kanohina Cave Preserve on the Big Island suggested that these are poorly crystalline crysocolla, a hydrated copper silicate. EDX analysis provided a Al:Cu:Si ratio of 0.15:1.8:1 compared with a typical analysis of chrysocolla with a ratio of 0.12:1.98:1 (Anthony et al., 1990). Examination with SEM revealed that morphologies consistent with Actinobacteria-like organisms are present in abundance in these blue-green deposits (Fig. 11A). The filaments shown among the Actinobacteria-like morphologies in Fig. 11A are reticulated filaments (Fig. 11B) that resemble those described by Melim et al. (2008).
Although superficially similar in appearance at the macroscopic scale, the gold-colored secondary mineral deposits from Four Windows Cave (New Mexico) and Thurston Lava Tube (Hawai‘i) differed in appearance when viewed with SEM. The gold-colored, vein-like deposits from Four Windows Cave (Fig. 8A, 8B) exhibited a distinctive filamentous nature with a segmented appearance (Fig. 12 Right). The gold-colored deposits from Thurston Lava Tube were seen to contain biofilm-like layers and filamentous and coccoid shapes (Fig. 12 Left) when viewed with SEM.
The pink hexagons found on the basaltic glass from Algar do Carvão (Fig. 7) showed a number of different types of filaments (Fig. 13B, 13C). The hexagons themselves have very straight edges (Fig. 13A) and, where broken, appear to be incised down into the basaltic glass (Fig. 13B). The surface of the hexagons is covered with what appear to be coated and uncoated filaments (Fig. 13C, 13E). EDX analysis of the hexagons revealed the presence of iron oxide and carbon from the filaments on the surface (Fig. 13D). Tangled masses of filaments of differing sizes were observed between hexagons in many instances (Fig. 13F) and differed in morphology from the filaments found on the hexagons. Structures observed on the hexagons included coated filaments and coccoid shapes, but evidence supporting their biological nature was lacking. Hematite has a hexagonal structure, and it is likely that the hexagons originally formed abiotically and have subsequently been colonized. Future studies utilizing the electron microprobe and transmission electron microscope with electron energy loss spectroscopy capability to examine thin sections will help to ascertain the oxidation state of the iron present in the basaltic glass and the distribution of iron and other elements.
4. Discussion
4.1. Microbial diversity
This study expands our knowledge of the diversity of microorganisms in lava caves and the diversity of microorganisms associated with secondary mineral deposits in lava caves. Microbial mats in lava caves have received very little attention from researchers (Northup and Welbourn, 1997). The earliest descriptions of these microbial mats (Staley and Crawford, 1975; Stoner and Howarth, 1981), based on cultivation studies, suggested that the organisms in the mats included bacteria, especially Actinobacteria, and fungi. One of the first molecular studies of these microbial mats (Northup et al., 2008) documented four phyla of bacteria present in the microbial mats of Four Windows Cave (New Mexico) but was very limited in the samples examined. Additional studies of New Mexican and Hawaiian lava cave microbial mats (Garcia et al., 2009; Moya et al., 2009; Snider et al., 2009) have since expanded these molecular analyses extensively. This study incorporates results from the Azorean Islands (Hathaway, unpublished data) and further expands the number of phyla found in the microbial mats.
Lava cave microbial mats from substantially different climatic regimes (semi-arid to tropical) had very similar composition at the phylum level. Each of the 13 phyla found across the three locations and 12 caves occurred in at least two of the three locations. Lava caves have dramatically different temperature and humidity conditions inside than the surface conditions (Cropley, 1965; Thakur and Momoh, 1983; Smithson, 1991). Some lava caves are cold traps and have permanent or seasonal ice (e.g., Four Windows Cave, in New Mexico), in spite of the fact that the mean annual surface temperature is well above freezing (http://weather.nmsu.edu/News/climate-in-NM.htm). Once in the deep zone of the cave, relative humidity approaches 100% and temperatures are generally cool, ranging from 14°C to 19°C (Hawai‘i), 11°C to 16°C (Azores), to −2°C to 9°C (New Mexico) in our sampled caves. The overlying basaltic rock acts as an effective insulator, buffering the cave from surface influences. Such conditions may contribute to the similarities observed at the phylum level in bacteria composition across the three locations. Studies are underway to examine what phylogenetic differences exist at finer phylogenetic levels and in temperature ranges seasonally.
Energy sources available in lava caves in the three locations also share commonalities. The basaltic rock in which the caves occur contains reduced iron and manganese, potential energy sources for chemolithoautotrophs, such as some of the species found in the Firmicutes, and sulfur, which has been shown to be important in other cave systems (Boston et al., 2006; reviewed in Engel, 2007). Lava caves contain numerous cracks and fissures, through which organic matter seeps; they also often possess skylights, through which organic matter falls. Air that flows into lava tubes, particularly those with multiple entrances, transports fine soil particles and organic matter, and some lava caves in New Mexico have seasonal populations of bats and pack rats that contribute guano and other organic detritus. Some of the phyla, such as the Actinobacteria, were ubiquitous across samples and demonstrate the utilization of a heterotrophic lifestyle by lava cave residents that utilize the organic matter that seeps or falls into lava caves.
Studies of volcanic habitats are important in discovering new microbial diversity (Donachie et al., 2004; Gomez-Alvarez et al., 2007; King, 2007; Stott et al., 2008, Cockell et al., 2009). The study by Gomez-Alvarez et al. (2007) of surface volcanic terrain in Hawai‘i revealed that Acidobacteria, Alpha- and Gammaproteobacteria, Actinobacteria, and Cyanobacteria dominate bacterial communities. The first four of these groups also appear to dominate clone libraries from the subsurface microbial mats. Because of the lack of sunlight beyond the Twilight Zone (Howarth, 1982), Cyanobacteria are rarely found in caves except for this zone (Martinez and Asencio, 2010). Studies of soils, in general, have found that soils are dominated by Proteobacteria, Acidobacteria, Actinobacteria, Verrucomicrobia, Bacteroidetes, Chloroflexi, Planctomycetes, Gemmatimonadetes, and Firmicutes (Janssen, 2006). The Proteobacteria, in particular, make up the greatest percentage (averaging 39%) of soil bacterial communities in studies reviewed by Janssen (2006). Our results from the lava caves across our three locations mirror the results of Janssen (2006), with all of these common soil groups occurring in our study sites (Fig. 8). As we add more sites to our study, we will investigate the degree to which this pattern holds. These results also suggest that a careful comparison with soils that overlie the lava caves may provide insight into the colonization of these lava caves. Gomez-Alvarez et al. (2007) suggested that differences in the local environment and elemental composition of the volcanic deposits themselves may control bacterial community composition. In future studies, we will analyze the basaltic rock elemental composition to look for factors that may contribute to community compositions within lava cave microbial mats.
When we compared lava cave microbial diversity to other cave types, we observed a great deal of overlap (Table 3). In particular, the Actinobacteria, Proteobacteria (Alpha-, Beta-, Gamma-, and Deltaproteobacteria), Acidobacteria, Verrucomicrobia, Planctomycetes, Nitrospirae, and Bacteroidetes have been found in 6–11 of the other cave studies examined (Table 3). The only phylum found in lava caves that were not documented in the studies we examined were the Chlamydiae and the Ktedonobacteria. The other cave studies examined in the construction of Table 3 also revealed the presence of additional phyla that have not yet been observed in our lava cave studies: for example, Spirochaetes, SPAM, SR1, WS3, BRC1. Several of these phyla are candidate phyla that have been relatively recently discovered in natural environments through genetic sequencing. It is worth noting that the more recent the study, the more bacterial phyla were found. This is a reflection of the increased ease and lowered cost of sequencing, which allows for the generation of more genetic sequences from environmental samples. The comparison of these studies with our lava caves suggests that we have additional biodiversity to discover and that caves, in general, contain a core set of bacterial phyla.
Table 3.
Comparison of Bacterial Phyla Found in Lava Caves versus Those Found in Carbonate Cave Studies by Other Authors
|
Reference |
This paper |
Barton et al., 2007 |
Portillo et al., 2008, 2009 |
Chelius and Moore, 2004 |
Holmes et al., 2001 |
Macalady et al., 2008 |
Pašić et al., 2010 |
Schabereiter-Gurtner et al., 2002 |
Shabarova and Pernthaler, 2010 |
Chen et al., 2009 |
Porter et al., 2009 |
Jones et al., 2008 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location/Bacterial phylum | Lava Caves | Carlsbad Cavern, rock | Altamira Cave | Wind Cave | Nullarbor Cave | Frasassi Cave | Slovenia Karst Cave | Tito Bustillo Cave | Swiss karst cave pools | Movile Cave | Multiple caves | Frasassi Cave |
| Actinobacteria | X | X | X | X | X | X | X | X | X | |||
| Alphaproteobacteria | X | X | X | X | X | X | X | X | X | X | X | |
| Betaproteobacteria | X | X | X | X | X | X | X | X | X | X | X | |
| Gammaproteobacteria | X | X | X | X | X | X | X | X | X | X | X | X |
| Deltaproteobacteria | X | X | X | X | X | X | X | X | X | |||
| Epsilonproteobacteria | X | X | X | |||||||||
| Acidobacteria | X | X | X | X | X | X | X | X | ||||
| Chloroflexi | X | X | X | X | X | X | ||||||
| Nitrospirae | X | X | X | X | X | X | X | |||||
| Verrucomicrobia | X | X | X | X | X | X | X | X | ||||
| Gemmatimonadetes | X | X | X | |||||||||
| Planctomycetes | X | X | X | X | X | X | X | X | X | |||
| Bacteroidetes | X | CFB | X | CFB | CFB | CFB | X | X | CFB | CFB | ||
| Chlamydiae | X | |||||||||||
| OD1/OP10 | X | X | ||||||||||
| Firmicutes | X | X | “Low GC” | X | X | X | ||||||
| TM7 | X | X | X | |||||||||
| Ktedonobacteria | X | |||||||||||
| Candidate division BD | X | |||||||||||
| Fibrobacteres | X | |||||||||||
| BRC1 | X | |||||||||||
| OP11 | X | X | ||||||||||
| OP5 | X | |||||||||||
| OP3 | X | |||||||||||
| Spirochaetes | X | |||||||||||
| Cyanobacteria | X | |||||||||||
| SPAM | X | X | ||||||||||
| SR1 | X | |||||||||||
| RCP2-18 | X | |||||||||||
| WS3 | X |
CFB, Cytophaga-Flexibacter-Bacteroides.
4.2. Lava cave secondary mineral composition
A wide variety of secondary minerals occur within lava caves (Palmer, 2007; White, 2010), and indeed most microbial mats appear, to the casual visitor, to be mineral in nature in lava caves. The molecular biology investigation of secondary mineral deposits revealed a rich diversity that overlaps that of the microbial mats to a great extent. As with the microbial mats, the Proteobacteria and Nitrospirae appear frequently in clone libraries, and the Acidobacteria also occur frequently among clones (Fig. 9). The blue-green copper deposits are a notable exception to the presence of the Acidobacteria. Although higher levels of copper are often toxic to life, several extremophilic bacteria and archaea are resistant to high levels of copper through a variety or mechanisms (Orell et al., 2010). This resistance to copper may help explain the diversity of morphologies seen with SEM and the diversity of genetic sequences recovered in our molecular studies.
Other notable differences include the lack of Actinobacteria in four of the six secondary mineral deposits (Fig. 9). Actinobacteria are considered to be the most ubiquitous of the microbial cave inhabitants in general and are important members of the ecosystem (Groth et al., 1999; Lazzarini et al., 2000). The lack of Actinobacteria in these deposits sets them apart from the microbial mats.
The iron deposits that occasionally form stalactites, stalagmites, or columns in the lava caves (Fig. 5) are a rarer phenomenon, and their microbial makeup is somewhat different. Bacillus spp. in the phylum Firmicutes are found in the clone library from this sample (Fig. 9). Several Bacillus spp. are known to oxidize iron (Ferris et al., 1988), and this may indicate an active geomicrobiological role for these organisms within the cave, which is worth further exploration. These same deposits were recently examined by de los Ríos et al. (2011), who documented the presence of morphologies that resembled the iron-oxidizing bacteria Leptothrix and Gallionella. However, they were unable to recover these or Bacillus spp. in their sequencing of denaturing gradient gel electrophoresis bands from two samples. Their results suggest a biogenic origin for these iron speleothems.
While the diversity associated with these secondary minerals is of note, our future studies will also focus on the role that these microbial inhabitants play (if any) in the formation of these secondary mineral deposits.
4.3. Implications for life detection on extraterrestrial bodies
Considering the difficulty of simply getting there, the search for life on extraterrestrial bodies is expensive and difficult (Westall et al., 2000; Boston et al., 2003). Therefore, target sites need to be chosen such that they have the highest potential possible for life (past or present) and for conditions conducive to preservation of biosignatures from that life. The extraterrestrial subsurface has been suggested as potentially harboring life due to the protection from harsh surface conditions (Boston et al., 1992, 2001; Westall et al., 2000). Recent evidence from Mars and other bodies in the Solar System suggests the presence of volcanic lava caves (Léveillé and Datta, 2010). Such caves have the added benefit of being an accessible way into the subsurface that does not require extensive drilling (Boston, 2000; Boston et al., 2003).
Once inside a lava cave, researchers need criteria with which to select specific locations for analysis. Microbial mats are relatively easy to recognize for the trained observer (Fig. 2). Mineral coatings, however, are usually assumed to form from abiological processes (Forti, 2005). Our work has shown that many features that look purely mineralogical actually contain extensive microbial communities, based on the molecular characterization. Our SEM results suggest that the microorganisms are alive, but further studies are needed to verify that these communities are metabolically active. These results lead us to wonder how many other “mineral” coatings found in volcanic lava caves and elsewhere also have a biological component (Benzerara and Menguy, 2009). A better understanding of these features will enhance our ability to detect biosignatures in the rock record of Earth and on other planets. As we work to detect life on other bodies, we need a better catalogue of such biological deposits on Earth that do not immediately appear to be biological in nature.
Acknowledgments
The authors thank Hawai‘i Volcanoes National Park and El Malpais National Park for collecting permits and support of our research, and landowners in Hawai‘i and the Azores for permission to collect samples. The authors thank Don Coons, Emily Davis, Mike Warner, Larry Flemming, Jim Werker, Val Hildreth Werker, Ara Kooser, Jessica Snider, Fernando Pereira, Airidas Dapkevicius, Rita Varela, and many more who assisted with fieldwork. Funding was provided by the Cave Conservancy of the Virginias Undergraduate Research Grant, Alliance for Minority Participation, T&E Inc., Western National Park Association, Fundação para a Ciência e a Tecnologia, grant number PTDC/AMB/7081/2006, Kenneth Ingham Consulting, New Mexico Space Grant Consortium, the National Speleological Society, and the University of New Mexico Biology Department. This project is partially supported by the National Science Foundation under Grant NSF-DEB 0731350 starting 08/01/07 and continuing through 08/01/12. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author or authors and do not necessarily reflect the views of the National Science Foundation. We also acknowledge technical support from the Molecular Biology Facility, which is supported by NIH grant number P20RR018754. Ali Ghadimi provided scanning electron micrographs of the pink hexagons. The authors gratefully acknowledge the photographic contributions of Kenneth Ingham.
Author Disclosure Statement
No competing financial interests exist.
Abbreviations
EDX, energy-dispersive X-ray; SEM, scanning electron microscopy.
References
- Altschul S.F. Madden T.L. Schäffer A.A. Zhang J. Zhang Z. Miller W. Lipman D.J. Gapped BLAST and PSI-B LAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony J.W. Bideaux R.A. Bladh K.W. Nichols M. Handbook of Mineralogy. Vol. 2. Mineral Data Publishing; Tucson, AZ: 1990. Part 1, [Google Scholar]
- Ascaso C. Wierzchos J. The search for biomarkers and microbial fossils in Antarctic rock microhabitats. Geomicrobiol J. 2003;20:439–450. [Google Scholar]
- Ashelford K.E. Chuzhanova N.A. Fry J.C. Jones A.J. Weightman A.J. New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl Environ Microbiol. 2006;72:5734–5741. doi: 10.1128/AEM.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker V. Strom R.G. Gulick V.R. Kargel J.S. Komatsu G. Kale V.S. Ancient oceans, ice sheets and hydrological cycle on Mars. Nature. 1991;352:589–594. [Google Scholar]
- Barbieri R. Stivaletta N. Marinangeli L. Ori G.G. Microbial signatures in sabhka evaporite deposits of Chott el Gharsa (Tunisia) and their astrobiological implications. Planet Space Sci. 2006;54:726–736. [Google Scholar]
- Barton H.A. Taylor N.M. Kreate M.P. Springer A.C. Oehrle S.A. Bertog J.L. The impact of host rock geochemistry on bacterial community structure in oligotrophic cave environments. Int J Speleol. 2007;36:93–104. [Google Scholar]
- Beaty D.W. Clifford S.M. Borg L.E. Catling D.C. Craddock R.A. Des Marais D.J. Farmer J.D. Frey H.M. Haberle R.M. McKay C.P. Newsom H.E. Parker T.J. Segura T. Tanaka K.L. Key science questions from the second conference on early Mars: geologic, hydrologic, and climatic evolution and the implications for life. Astrobiology. 2005;5:663–689. doi: 10.1089/ast.2005.5.663. [DOI] [PubMed] [Google Scholar]
- Benison K.C. Jagniecki E.A. Edwards T.B. Mormile M.R. Storrie-Lombardi M.C. “Hairy blobs:” microbial suspects preserved in modern and ancient extremely acid lake evaporites. Astrobiology. 2008;8:807–821. doi: 10.1089/ast.2006.0034. [DOI] [PubMed] [Google Scholar]
- Benzerara K. Menguy N. Looking for traces of life in minerals. Comptes Rendus Palevol. 2009;8:617–628. [Google Scholar]
- Blackhurst R.L. Genge M.J. Kearsley A.T. Grady M.M. Cryptoendolithic alteration of Antarctic sandstones: pioneers or opportunists? J Geophys Res. 2005;110 doi: 10.1029/2005JE002463. [DOI] [Google Scholar]
- Blank J.G. Green S.J. Blake D. Valley J.W. Kita N.T. Treiman A. Dobson P.F. An alkaline spring system within the Del Puerto Ophiolite (California, USA): a Mars analog site. Planet Space Sci. 2009;57:533–540. [Google Scholar]
- Boston P.J. Life below and life “out there.”. Geotimes. 2000;45:14–17. [Google Scholar]
- Boston P.J. Ivanov M.V. McKay C.P. On the possibility of chemosynthetic ecosystems in subsurface habitats on Mars. Icarus. 1992;95:300–308. doi: 10.1016/0019-1035(92)90045-9. [DOI] [PubMed] [Google Scholar]
- Boston P.J. Spilde M.N. Northup D.E. Melim L.A. Soroka D.A. Kleina L.G. Lavoie K.H. Hose L.D. Mallory L.M. Dahm C.N. Crossey L.J. Scheble R.T. Cave biosignature suites: microbes, minerals and Mars. Astrobiology. 2001;1:25–55. doi: 10.1089/153110701750137413. [DOI] [PubMed] [Google Scholar]
- Boston P.J. Frederick R.D. Welch S.W. Werker J. Meyer T.R. Sprungman B. Hildreth-Werker V. Thompson S.L. Murphy D.L. Human utilization of subsurface extraterrestrial environments. Gravit Space Biol Bull. 2003;16:121–131. [PubMed] [Google Scholar]
- Boston P.J. Hose L.D. Northup D.E. Spilde M.N. The microbial communities of sulfur caves: a newly appreciated geologically driven system on Earth and potential model for Mars. In: Harmon R.S., editor; Wicks C.M., editor. Perspectives on Karst Geomorphology, Hydrology, and Geochemistry: A Tribute Volume to Derek C. Ford and William B. White, GSA Special Paper 404. Geological Society of America; Boulder, CO: 2006. pp. 331–344. [Google Scholar]
- Buick R. Microfossil recognition in Archean rocks: an appraisal of spheroids and filaments from a 3500 m.y. old chert-barite unit at North Pole, Western Australia. Palaios. 1990;5:441–459. [Google Scholar]
- Chelius M.K. Moore J.C. Molecular phylogenetic analysis of archaea and bacteria in Wind Cave, South Dakota. Geomicrobiol J. 2004;21:123–134. [Google Scholar]
- Chen Y. Wu L. Boden R. Hillebrand A. Kumaresan D. Moussard H. Baciu M. Lu Y. Murrell J.C. Life without light: microbial diversity and evidence of sulfur- and ammonium-based chemolithotrophy in Movile Cave. ISME J. 2009;3:1093–1104. doi: 10.1038/ismej.2009.57. [DOI] [PubMed] [Google Scholar]
- Cockell C.S. Olsson-Francis K. Herrera A. Meunier A. Alteration textures in terrestrial volcanic glass and the associated bacterial community. Geobiology. 2009;7:50–65. doi: 10.1111/j.1472-4669.2008.00184.x. [DOI] [PubMed] [Google Scholar]
- Cropley J.B. Influence of surface conditions on temperatures in large cave systems. Bulletin of the National Speleological Society. 1965;27:1–10. [Google Scholar]
- de los Ríos A. Bustillo M.A. Ascaso C. Carvalho M.R. Bioconstructions in ochreous speleothems from lava tubes on Terceira Island (Azores) Sediment Geol. 2011;236:117–128. [Google Scholar]
- DeSantis T. Hugenholtz P. Keller K. Brodie E.L. Larsen N. Piceno Y.M. Phan R. Andersen G.L. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 2006;34:W394–W399. doi: 10.1093/nar/gkl244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie S.P. Hou S. Lee K.S. Riley C.W. Pikina A. Belisle C. Kempe S. Gregory T.S. Bossuyt A. Boerema J. Liu J. Freitas T.A. Malahoff A. Alam M. The Hawaiian Archipelago: a microbial diversity hotspot. Microb Ecol. 2004;48:509–520. doi: 10.1007/s00248-004-0217-1. [DOI] [PubMed] [Google Scholar]
- Douglas S. Microbial biosignatures in evaporite deposits: evidence from Death Valley, California. Planet Space Sci. 2004;52:223–227. [Google Scholar]
- Engel A.S. Observations on the biodiversity of sulfidic karst habitats. Journal of Cave and Karst Studies. 2007;69:187–206. [Google Scholar]
- Fernández-Remolar D.C. Prieto-Ballesteros O. Rodríguez N. Gómez F. Amils R. Gómez-Elvira J. Stoker C.R. Underground habitats in the Río Tinto basin: a model for subsurface life habitats on Mars. Astrobiology. 2008;8:1023–1047. doi: 10.1089/ast.2006.0104. [DOI] [PubMed] [Google Scholar]
- Ferris F.G. Fyfe W.S. Beveridge T.J. Metallic ion binding by Bacillus subtilis: implications for the fossilization of microorganisms. Geology. 1988;16:149–152. [Google Scholar]
- Forti P. Genetic processes of cave minerals in volcanic environments: an overview. Journal of Cave and Karst Studies. 2005;76:3–13. [Google Scholar]
- Friedmann E.I. Endolithic microorganisms in the Antarctic cold desert. Science. 1982;215:1045–1053. doi: 10.1126/science.215.4536.1045. [DOI] [PubMed] [Google Scholar]
- Garcia M.G. Moya M. Spilde M.N. Stone F.D. Northup D.E. Discovering new diversity in Hawaiian lava tube microbial mats. Proceedings of the 15th International Congress of Speleology. 2009;1:364–369. [Google Scholar]
- Gibson E.K. McKay D.S. Thomas-Keprta K. Westall F. Romanek C.A. It's dead Jim. But was it ever alive? Ad Astra. 1999;11:31–33. [Google Scholar]
- Gillan D.C. De Ridder C. Accumulation of a ferric mineral in the biofilm of Montacuta ferruginosa (Mollusca, Bivalvia). Biomineralization, bioaccumulation, and inference of paleoenvironments. Chem Geol. 2001;177:371–379. [Google Scholar]
- Giovannoni S.J. DeLong E.F. Schmidt T.M. Pace N.R. Tangential flow filtration and preliminary phylogenetic analysis of marine picoplankton. Appl Environ Microbiol. 1990;56:2572–2575. doi: 10.1128/aem.56.8.2572-2575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glamoclija M. Garrel L. Berthon J. López-García P. Biosignatures and bacterial diversity in hydrothermal deposits of Sofatara Crater, Italy. Geomicrobiol J. 2004;21:529–541. [Google Scholar]
- Gomez-Alvarez V. King G.M. Nusslein K. Comparative bacterial diversity in recent Hawaiian volcanic deposits of different ages. FEMS Microbiol Ecol. 2007;60:60–73. doi: 10.1111/j.1574-6941.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- Groth I. Vetterman R. Schuetze B. Schumann P. Saiz-Jimenez C. Actinomycetes in Karstic caves of northern Spain (Altamira and Tito Bustillo) J Microbiol Methods. 1999;36:115–122. doi: 10.1016/s0167-7012(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Hode T. Cady S.L. von Dalwigk I. Kristiansson P. Evidence of ancient microbial life in an impact structure and its implications for astrobiology. In: Seckbach J., editor; Walsh M., editor. From Fossils to Astrobiology: Records of Life on Earth and the Search for Extraterrestrial Biosignatures. Springer; London: 2008. pp. 249–274. [Google Scholar]
- Holmes A.J. Tujula N.A. Holley M. Contos A. James J.M. Rogers P. Gillings M.R. Phylogenetic structure of unusalaquatic microbial formations in Nullarbor caves, Australia. Environ Microbiol. 2001;3:256–264. doi: 10.1046/j.1462-2920.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- Howarth F.G. Bioclimatic and geologic factors governing the evolution and distribution of Hawaiian cave insects. Entomologia generalis. 1982;8:17–26. [Google Scholar]
- Izawa M.R.M. Banerjee N.R. Flemming R.L. Bridge N.J. Schultz C. Basaltic glass as a habitat for microbial life: implications for astrobiology and planetary exploration. Planet Space Sci. 2010;58:583–591. [Google Scholar]
- Janssen P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol. 2006;72:1719–1728. doi: 10.1128/AEM.72.3.1719-1728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.S. Lyon E.H. Macalady J.L. Geomicrobiology of biovermiculations from the Frasassi cave system, Italy. Journal of Cave and Karst Studies. 2008;70:78–93. [Google Scholar]
- Kelley D.S. Karson J.A. Frueh-Green G.L. Yoerger D.R. Shank T.M. Butterfield D.A. Hayes J.M. Schrenk M.O. Olson E.J. Proskurowski G. A serpentinite-hosted ecosystem; the Lost City hydrothermal field. Science. 2005;307:1428–1434. doi: 10.1126/science.1102556. [DOI] [PubMed] [Google Scholar]
- King G.M. Chemolithotrohic bacteria: distributions, functions and significance in volcanic environments. Microbes and Environment. 2007;22:309–319. [Google Scholar]
- Laughlin A.W. Poths J. Healey H.A. Reneau S. WoldeGabriel G. Dating of Quaternary basalts using the cosmogenic 3He and 14C methods with implications for excess 40Ar. Geology. 1994;22:135–138. [Google Scholar]
- Lazzarini A. Cavaletti L. Toppo G. Marinelli F. Rare genera of actinomycetes as potential producers of new antibiotics. Antonie Van Leeuwenhoek. 2000;78:399–405. [PubMed] [Google Scholar]
- Léveillé R.J. Datta S. Lava tubes and basaltic caves as astrobiological targets on Earth and Mars: a review. Planet Space Sci. 2010;58:592–598. [Google Scholar]
- Macalady J.L. Dattagupta S. Schaperdoth I. Jones D.S. Druschel G.K. Eastman D. Niche differentiation among sulfur-oxidizing bacterial populations in cave waters. ISME J. 2008;2:590–601. doi: 10.1038/ismej.2008.25. [DOI] [PubMed] [Google Scholar]
- Maidak B.L. Cole J.R. Lilburn T.G. Parker C.T., Jr. Saxman P.R. Farris R.J. Garrity G.M. Olsen G.J. Schmidt T.M. Tiedje J.M. The RDP-II (Ribosomal Database Project) Nucleic Acids Res. 2001;29:173–174. doi: 10.1093/nar/29.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli R.L. Fahlen T.F. Landheim R. Klovstad M.R. Brines and evaporites: analogs for martian life. Adv Space Res. 2004;33:1244–1246. [Google Scholar]
- Martinez A. Asencio A.D. Distribution of cyanobacteria at the Gelada Cave (Spain) by physical parameters. Journal of Cave and Karst Studies. 2010;72:11–20. [Google Scholar]
- McKay C.P. Stoker C.R. The early environment and its evolution on Mars: implications for life. Rev Geophys. l989;27:189–214. [Google Scholar]
- Melim L.A. Northup D.E. Spilde M.N. Jones B. Boston P.J. Bixby R.J. Reticulated filaments in cave pool speleothems: microbe or mineral? Journal of Cave and Karst Studies. 2008;70:135–141. [Google Scholar]
- Moya M. Garcia M.G. Spilde M.N. Northup D.E. Composition of bacterial mats in El Malpais, National Monument, New Mexico, USA: comparison and contrasts with bacterial communities in Hawai‘i lava tubes. Proceedings of the 15th International Congress of Speleology. 2009;2:709–713. [Google Scholar]
- Northup D.E. Welbourn W.C. Life in the Twilight Zone: lava tube ecology. New Mexico Bureau of Mines & Mineral Resources Bulletin. 1997;156:69–82. [Google Scholar]
- Northup D.E. Connolly C.A. Trent A. Peck V.M. Spilde M.N. Welbourn W.C. Natvig D.O. The nature of bacterial communities in Four Windows Cave, El Malpais National Monument, New Mexico, USA. AMCS Bulletin. 2008;19:119–125. [Google Scholar]
- Northup D.E. Snider J.R. Spilde M.N. Porter M.L. Van de Kamp J.L. Boston P.J. Nyberg A.M. Barger J.R. Diversity of rock varnish bacterial communities from Black Canyon, New Mexico. J Geophys Res. 2010;115 doi: 10.1029/2009JG001107. [DOI] [Google Scholar]
- Nunes J.C. Notas sobre a geologia da Terceira. Açoreana. 2000;9:205–215. [Google Scholar]
- Nunes J.C. Geologia. In: Forjaz V.H., editor. Atlas Básico dos Açores: ObservatórioVulcanológico e Geotérmico dos Açores. Ponta Delgada; Azores: 2004. pp. 60–62. [Google Scholar]
- Nunes J.C. Garcia P. Lima E.A. Costa M.P. Pereira F. New geological insights for the Azores Islands (Portugal) lava caves. 13th International Symposium on Vulcanospeleology, International Union of Speleology.2008. [Google Scholar]
- Omelon C.R. Endolithic microbial communities in polar desert habitats. Geomicrobiol J. 2008;25:404–414. [Google Scholar]
- Orell A. Navarro C.A. Arancibia R. Mobarec J.C. Jerez C.A. Life in blue: copper resistance mechanisms of bacteria and archaea used in industrial biomining of minerals. Biotechnol Adv. 2010;28:839–848. doi: 10.1016/j.biotechadv.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Palmer A.N. Cave Geology. Cave Books; Dayton, OH: 2007. [Google Scholar]
- Parenteau M.N. Cady S.L. Palaios. Vol. 25. Yellowstone National Park; United States: 2010. Microbial biosignatures in iron-mineralized phototrophic mats at Chocolate Pots Hot Springs; pp. 97–11. [Google Scholar]
- Pašić L. Kovče B. Sket B. Herzog-Velikonja B. Diversity of microbial communities colonizing the walls of a karstic cave in Slovenia. FEMS Microbiol Ecol. 2010;71:50–60. doi: 10.1111/j.1574-6941.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Porter M.L. Engel A.S. Kane T.C. Kinkle B.K. Productivity-diversity relationships from chemolithoautotrophically based sulfidic karst systems. Int J Speleol. 2009;38:27–40. [Google Scholar]
- Portillo M.C. Gonzalez J.M. Saiz-Jimenez C. Metabolically active microbial communities of yellow and grey colonizations on the walls of Altamira Cave, Spain. J Appl Microbiol. 2008;104:681–691. doi: 10.1111/j.1365-2672.2007.03594.x. [DOI] [PubMed] [Google Scholar]
- Portillo M.C. Saiz-Jimenez C. Gonzalez J.M. Molecular characterization of total and metabolically active bacterial communities of “white colonizations” in the Altamira Cave, Spain. Res Microbiol. 2009;160:41–47. doi: 10.1016/j.resmic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Preston L.J. Benedix G.K. Genge M.J. Sephton M.A. A mulitdiscipinary study of silica sinter deposits with applications to silica identification and detection of fossil life on Mars. Icarus. 2008;198:331–350. [Google Scholar]
- Sadooni F.N. Howari F. Edwards H.G.M. El-Saiy A. Lithology, mineral assemblages and microbial fingerprints of the evaporite-carbonate sediments of the coastal sabkha of Abu Dhabi and their extraterrestrial implications. International Journal of Astrobiology. 2010;9:147–156. [Google Scholar]
- Sambrook J. Fritsch E.F. Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; NY: 1989. [Google Scholar]
- Schabereiter-Gurtner C. Saiz-Jimenez C. Piñar G. Lubitz W. Rölleke S. Phylogenetic 16S rRNA analysis reveals the presence of complex and partly unknown bacterial communities in Tito Bustillo cave, Spain, and on its Palaeolithic paintings. Environ Microbiol. 2002;4:392–400. doi: 10.1046/j.1462-2920.2002.00303.x. [DOI] [PubMed] [Google Scholar]
- Schelble R.T. Westall F. Allen C.C. ∼1.8 Ga iron-mineralized microbiota from the Gunflint Iron Formation, Ontario, Canada: implications for Mars. Adv Space Res. 2004;33:1268–1273. [Google Scholar]
- Shabarova T. Pernthaler J. Karst pools in subsurface environments: collectors of microbial diversity or temporary residence between habitat types. Environ Microbiol. 2010;12:1061–1074. doi: 10.1111/j.1462-2920.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- Sims K.W.W. Ackert R.P., Jr. Ramos F.C. Sohn R.A. Murrell M.T. DePaolo D.J. Determining eruption ages and erosion rates of Quaternary basaltic volcanism from combined U-series disequilibria and cosmogenic exposure ages. Geology. 2007;35:471–474. [Google Scholar]
- Smithson P.A. Inter-relationships between cave and outside air temperature. Theoretical and Applied Climatology. 1991;44:65–73. [Google Scholar]
- Snider J.R. Moya M. Garcia M.G. Spilde M.N. Northup D.E. Identification of the microbial communities associated with roots in lava tubes in New Mexico and Hawai‘i. Proceedings of the 15th International Congress of Speleology. 2009;2:718–723. [Google Scholar]
- Staley J.T. Crawford R. The biologist's chamber: lava tube slime. Cascade Caver. 1975;14:20–21. [Google Scholar]
- Stevens T.O. McKinley J.P. Geochemically produced hydrogen supports microbial ecosystems in deep basalt aquifers. Science. 1995;270:450–454. [Google Scholar]
- Stoner M.F. Howarth F.G. Community structure and niche differentiation in Hawaiian lava tubes. In: Mueller-Dombois D., editor; Bridges K.W., editor; Carson H.L., editor. Island Ecosystems: Biological Organization in Selected Hawaiian Communities. Hutchinson Ross Publishing Company; Stroudsburg, PA: 1981. pp. 318–336. [Google Scholar]
- Stott M.B. Crowe M.A. Mountain B.W. Smirnova A.V. Hou S.B. Alam M. Dunfield P.F. Isolation of novel bacteria, including a candidate division, from geothermal soils in New Zealand. Environ Microbiol. 2008;10:2030–2041. doi: 10.1111/j.1462-2920.2008.01621.x. [DOI] [PubMed] [Google Scholar]
- Thakur A.K.S. Momoh M.M. Temperature variation in upper Earth crust due to periodic nature of solar insolation. Energy Conversion and Management. 1983;23:131–134. [Google Scholar]
- Villar S.E.J. Edwards H.G.M. Benning L.G. Raman spectroscopic and scanning electron microscopic analysis of a novel biological colonisation of volcanic rocks. Icarus. 2006;184:158–169. [Google Scholar]
- Westall F. The nature of fossil bacteria: a guide to the search for extraterrestrial life. J Geophys Res. 1999;107:16437–16451. [Google Scholar]
- Westall F. Brack A. Hofmann B.A. Horneck G. Kurat G. Maxwell J. Ori G.G. Pillinger C. Raulin F. Thomas N. Fitton B. Clancy P. Prieur D. Vassaux D. An ESA study for the search for life on Mars. Planet Space Sci. 2000;48:181–202. doi: 10.2187/bss.12.119. [DOI] [PubMed] [Google Scholar]
- Westall F. de Wit M.J. Dann J. van der Gaast S. de Ronde C.E.J. Gerneke D. Early Archean fossil bacteria and biofilms in hydrothermally influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Res. 2001;106:93–116. [Google Scholar]
- White W.B. Secondary minerals in volcanic caves: data from Hawai‘i. Journal of Cave and Karst Studies. 2010;72:75–85. [Google Scholar]
- Wierzchos J. Sancho L.G. Ascaso C. Biomineralization of endolithic microbes in rocks from the McMurdo Dry Valleys of Antarctica: implications for microbial fossil formations and their detection. Environ Microbiol. 2005;7:566–575. doi: 10.1111/j.1462-2920.2005.00725.x. [DOI] [PubMed] [Google Scholar]