Abstract
The authors report a 17-year-female and a 19-year-male with uncontrolled insulin-dependent diabetes mellitus (IDDM) for ≥10 years, treated with insulin-secreting human adipose tissue derived mesenchymal stem cells (IS-h-ADMSC). Both had hypothyroidism and were diagnosed as polyglandular autoimmune syndrome type-3 (PGAS-3). PGAS are rare polyendocrinopathies with ≥2 endocrine disorders mediated by autoimmune mechanisms leading to hypo-function and organ failure. Therapeutic options are hormone replacement, immunosuppression and avoiding infection. The authors administered autologous H-AD-IS-MSC+bone marrow-derived haematopoietic stem cells (HSC) into portal circulation with conditioning of cyclophosphamide, bortezomib, rituximab and rabbit-antithymoglobulin. Over follow-up of 38 and 16 months, respectively, both are doing well with sustained fall of glycosylated haemoglobin (Hb1Ac) from 8.1 to 6.4% and 14.2 to 8.6%, respectively and C-peptide raised from 0.01 to 0.23 ng/ml and 0.1 to 0.34 ng/ml, respectively with sustained 40% decreased insulin requirement. Thus long-term control of IDDM in PGAS-3 with co-transplantation of H-AD-IS-MSC+HSC can be achieved safely and effectively.
Background
This is the first case report to our knowledge where co-transplantation of insulin-secreting human adipose tissue derived mesenchymal stem cells (IS-h-ADMSC) with HSC was successfully carried out to treat insulin-dependent diabetes mellitus (IDDM) in polyglandular autoimmune syndrome (PGAS) type 3. This is a novel therapy which is safe and effective and will open up the avenues for millions of diabetic children all across the world.
Case presentation
A young female of 17 years presented in January,’08 with fatigue, frequent attacks of diabetic ketoacidosis (DKA) and uncontrolled blood sugars. She was known case of IDDM since last 10 years; on Huminsulin 50 IU/day.
A 19-year-male with IDDM of 17 years duration presented in January,’10 with similar complaints. He was on short-acting insulin 50 IU/day and long-acting insulin, 20 IU/day.
No insulin/islet cell/adrenal antibodies were detected in either case. Their clinical examination and growth parameters were unremarkable. They were diagnosed as PGAS type-3.
Investigations
Case 1–serum (s.) T4 was 0.84 μgm/dl (normal range: 4.8–11.6 μgm/dl), serum thyroid stimulating hormone (s.TSH) > 40 μU/ml (normal range: 0.28–6.82 μU/ml), glutamic acid decarboxylase antibodies (GAD Ab) >2000 IU/ml (normal: <10 IU/ml) and antimicrosomal Ab, 1:6400 IU/ml (reference range: >1:100: positive).
Case 2–S.T4 was 0.9 μgm/dl, s.TSH >100 μU/ml, GAD Ab, 740 IU/ ml and antimicrosomal Ab, 816.8 IU/ml.
Fasting blood sugar on admission were 458 mg/dl and 325 mg/dl and postprandial blood sugar, 500 mg/dl and 382 mg/dl, respectively, s.C-peptide, 0.01 and 0.1 ng/ml, respectively and glycosylated haemoglobin (HbA1c), 8.1% and 14.2%, respectively.
Treatment
They were subjected to stem cell transplantation (SCT) and tab thyrox, 50 and 100 mcg/day, respectively. SCT protocol consisted of bortezomib, 1.3 mg/m2 body surface area on days −20, −17, −13 and −10 followed by cyclophosphamide, 20 mg/kg bw on day 10, and granulocyte colony stimulating factor, 300 ug subcutaneously twice daily (to mobilise stem cells) on days −8 and −7 (figure 1). Ten gram fat was resected from anterior abdominal wall on day 14 and subjected to in vitro mesenchymal stem cell (MSC) generation and further differentiation into insulin-secreting stem cells (IS-h-ADMSC). Bone marrow (150 ml) was aspirated from posterior superior iliac crest under local anesthesia on day 6 for in vitro generation of haematopoietic SC (HSC). Rituximab, 8 mg/kg BW and rabbit antithymocyte globulin, 1.5 mg/kg bw were administered on days 3 and 2 to delete autoantibodies. On day 0, IS-h-ADMSC+HSC (100 ml and 76 ml, respectively with CD34+content, 3.8×106/ul each, glucose sensitive insulin levels of 20, 18 ng/ml, respectively) were injected into portal circulation via omental vein under short general anesthesia. SCT was uneventful.
Figure 1.
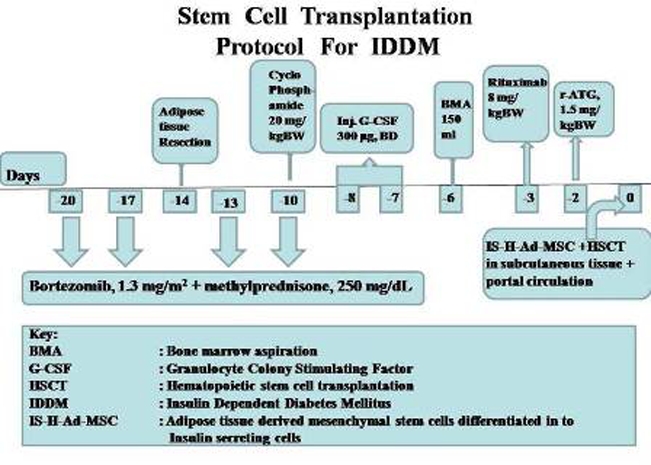
Stem cell transplantation (SCT) protocol for insulin-dependent diabetes mellitus.
Outcome and follow-up
Over follow-up of 38 and 16 months, respectively, both are doing well, maintaining euthyroid state respectively with s.T4, 6.4 μgm/dl and 7 μgm/dl, respectively, s. TSH, 8.43 μU/ml and 0.70 μU/ml, absence of DKA, HbA1c, 6.4% and 8.6% respectively, C-peptide raised to 0.23 and 0.34 ng/ml, respectively and their insulin requirement is sustained to about 40% of original requirement (figure 2).
Figure 2.
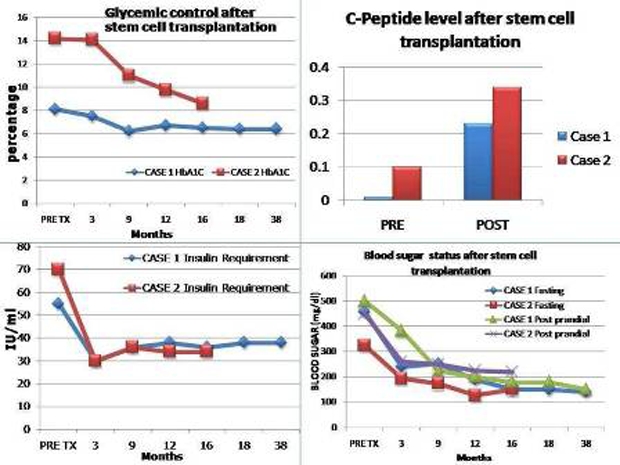
Left upper corner-glycemic control after SCT denoted by glycosylated haemoglobin (Hb1Ac in percentage) in both patients. Left lower corner-insulin requirement after SCT in both patients (in IU/ml). Right upper corner-C-peptide level after SCT in both patients (in ng/ml). Right lower corner-Blood sugar (fasting and post prandial) status (in mg/dl) in both patients.
Discussion
PGAS are rare polyendocrinopathies characterised by association of two or more endocrine disorders mediated by autoimmune mechanisms leading to a hypo-functional state.1–4
Circulating organ/cell-specific autoantibodies and cytotoxic T cells may lead to organ failure.2 Early recognition and replacement therapy is lifesaving. Long-term management includes immunosuppression and avoiding infections.3 4 There is a study of 23 patients treated with haematopoietic stem cell transplantation with 18.8 months follow-up showing sustained insulin-independence.5 We have generated H-AD-MSC in lab and treated 12 IDDM patients previously who have sustained control of IDDM with raised C-peptide levels and controlled Hb1Ac. Hence we decided to explore this protocol which has already given sustained benefits without any adverse effects.6 7 Interestingly, we have also observed good control of thyroid functions also. To our knowledge, this is the first report showing long-term control of IDDM in PGAS-3 using co-transplantation of insulin-secreting stem cells and HSC, which is simple, safe and effective therapy.
Learning points.
-
▶
IDDM is not uncommon in children.
-
▶
PGAS type 3 is a rare polyendocrinopathy and should be looked for in children with IDDM.
-
▶
So far, no therapy other than hormonal replacement was available.
-
▶
Co-transplantation of insulin making stem cells from autologous adipose tissue derived MSC and haematopoietic stem cells is simple, safe and effective therapy for such patients.
Acknowledgments
The authors acknowledge the help of their chief librarian for manuscript writing and providing us literature survey.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Basak RC, Chatterjee M, Rassem MW. Autoimmune polyglandular syndrome (type-lll): case report. Kuwait Med J 2007;39:373–5 [Google Scholar]
- 2.Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metabol 2003;88:2983–92 [DOI] [PubMed] [Google Scholar]
- 3.Kahaly GJ. Polyglandular autoimmune syndromes. Eur J Endocrinol 2009;161:11–20 [DOI] [PubMed] [Google Scholar]
- 4.Husebye ES, Anderson MS. Autoimmune polyendocrine syndromes: clues to type 1 diabetes pathogenesis. Immunity 2010;32:479–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–9 [DOI] [PubMed] [Google Scholar]
- 6.Vanikar AV, Dave SD, Thakkar UG, et al. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int 2010;2010:582382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi HL, Vanikar AV, Thakker U, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc 2008;40:1135–9 [DOI] [PubMed] [Google Scholar]


