Abstract
Buerger´s disease or Thromboangiitis obliterans is a segmental inflammatory disease that affects the vessels and nerves of the extremities. It usually affects men below 45 years old and correlates with tobacco, as a predisposing factor. The authors present the case of a 34-year-old male, with ulcers in the fingertips with progressive worsening: acrocyanosis, slow healing, necrosis and finally loss of substance. Dorsalis pedis and posterior tibial pulses were not palpable. Personal history of heavy smoking was (20 pack-years). The angiography revealed proximal occlusion of the left posterior tibial and interosseal arteries, with distal circulation by the anterior tibial artery. He was submitted to disarticulation of the second left toe and therapy with pentoxifyline and iloprost infusion, calcium antagonist, antiplatelet drugs, statin and low molecular weight heparin (later replaced by oral anticoagulation). Improvement was seen of active vascular lesions and pain symptoms.
Background
Buerger’s disease or Thromboangiitis obliterans is a type of vasculitis in young, mostly male subjects- remains strangely linked to smoking, which determines its ocurrence, progression and prognosis by yet unknown mechanisms.1 Sometimes it presents with multisystemic complaints.
The diagnosis and treatment of this entity is challenging, since it requires the exclusion of many other causes and a multidisciplinary approach. An illustrative case-report and literature review is presented.
Case presentation
A 34-year-old black male presented in the medicine clinics with a 6 year history of ulcers in the fingertips of both hands and feet with progressive worsening: acrocyanosis, slow healing, necrosis and finally loss of substance. Raynaud’s phenomenon was absent. Sympaticectomy was unsuccessfully tried. He also referred recurrent episodes of ‘migratory thrombophlebitis’, preferentially involving the legs bilaterally and left forearm. A biopsy made in the past was consistent with local thrombophlebitis.
The patient had a known history of smoking (20 pack-year), controlled asthma, sinusitis and primary syphilis (1995). No other vascular risk factor was detected, namely diabetes, dyslipidemia or drug abuse.
On clinical examination, the patient presented a cyanotic ulcerated lesion involving the two distal thirds of the second left toe (figure 1). The patient had an ulcerated lesion on the first right toe with purulent discharge. Healed lesions with loss of substance in the first left toe and second and fourth fingers of right hand were present (figures 2 and 3). Dorsalis pedis and posterior tibial pulses were absent.
Figure 1.
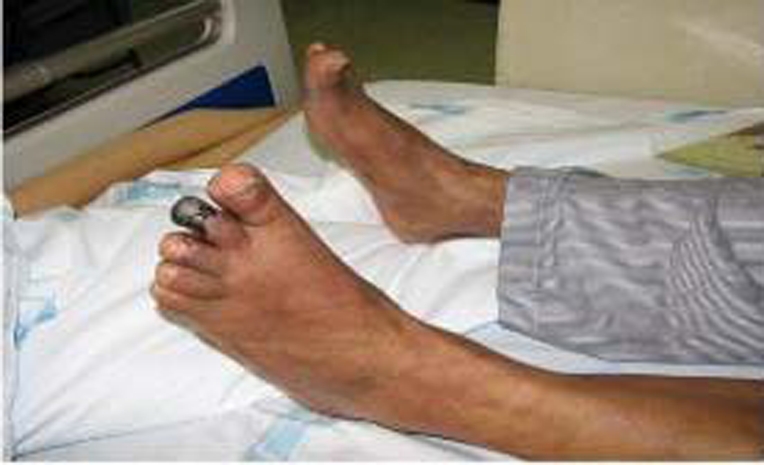
Ulcerated and cyanotic lesion of the second left toe.
Figure 2.
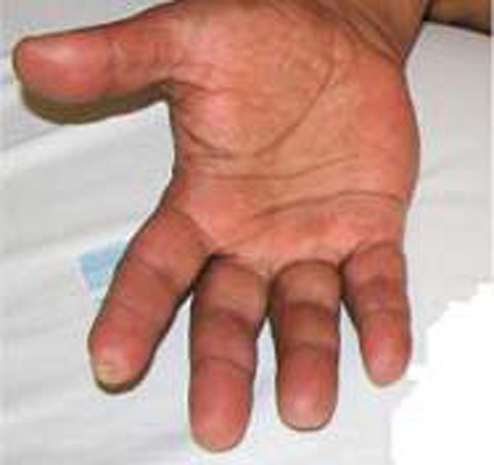
Scar lesions with substance loss of the second and fourth right fingers.
Figure 3.
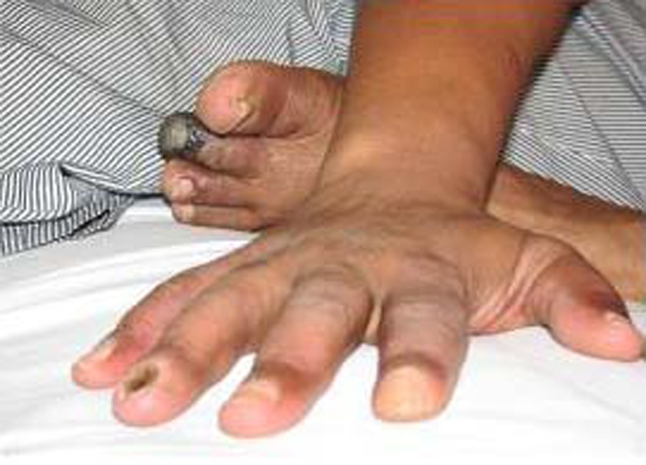
Scar lesions with substance loss of the second and fourth right fingers and first left toe.
The differential diagnoses were: systemic embolisation or thrombophilia, Buerger’s disease, vasculitis of large and medium-sized vessels, atherosclerotic arteriopathy (less likely in this age group) and systemic lupus erythematosus- related vasculitis.
The study for thrombophilia and immunology was negative (table 1). A possible source of systemic embolism was excluded by transthoracic echocardiogram and carotid-vertebral Doppler. The viral (HIV, hepatitis B virus and hepatitis C virus) and syphilitic serologies were negative. The thoracoabdominopelvic CT was unremarkable. The nailfold capillaroscopy was suggestive of systemic vascular disease, with active capillaritis. The angiography (abdominal, upper and lower limbs) revealed proximal occlusion of the left posterior tibial and interosseal arteries, with distal circulation by the anterior tibial artery (figure 4). A vascular surgery consult excluded the need for a revascularisation procedure (endovascular /surgery).
Table 1.
Relevant laboratory parameters.
| Parameter | Value |
|---|---|
| Haemoglobin | 15, 9 (13, 5–18 g/dl |
| Leucocytes/neutrophils | 13300 (4–10) mm3; 60, 7% (37–72) |
| C reactive protein | 2, 7 (<1) mg/dl |
| ESR | 22 (1–20) mm/1st h |
| Antithrombin III | 96% (80–120) |
| Protein C | 101% (70–140) |
| Protein S | 111, 5% (64–129) |
| Factor V Leiden | Negative |
| Homocysteine | 7, 61 (<15) µmol/l |
| Lupus anticoagulant | Negative: 1, 16 (0, 8–1, 2) R |
| Anticardiolipin | IgM-6 (<7) MPL; IgG-10 (<10) GPL |
| ANA | Negative (<1/160) |
| DNA ds | Negative (<1/10) |
| A-SSA | 0.1 (<40) U/ml |
| A-SSB | 0, 1 (<40) U/ml |
| A-SM/RNP | 0, 1 (<40) U/ml |
| A-SM | 0, 1 (<40) U/ml |
| A-Centromere | 0, 1 (<40) U/ml |
| A-SCL70 | 0, 1 (<40) U/ml |
| ANCA-C | 0, 1 (<25) U/ml |
| ANCA-P | 0, 1 (<25) U/ml |
| C3 | 161 (75–140) mg/dl |
| C4 | 38, 8 (10–34) mg/dl |
| C1q | 13, 24 (8–15) mg/dl |
| Circulating immunocomplexes | 1, 6 (<10, 8) µg/dl |
ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibodies; A-SCL, antiscleroderma; A-SSA, anti Sjögren’s syndrome A; A-SSB, anti Sjögren’s syndrome B; ESR, erythrocyte sedimentation rate; MPL, IgM phospholipid units; GPL, IgG phospholipid units; SM/RNP, Smith/Ribonucleoprotein.
Figure 4.
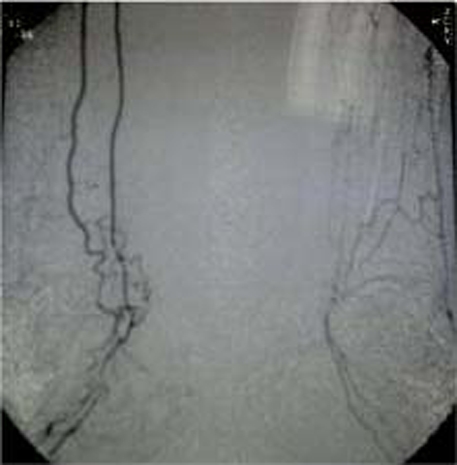
The angiography (abdominal, upper and lower limbs) revealed proximal occlusion of the left posterior tibial and interosseal arteries, with distal circulation by the anterior tibial artery.
Disarticulation of the second left toe was performed along with medical therapy with pentoxifylline and iloprost infusion, calcium antagonist (nifedipine), antiplatelet drug (aspirin), statin (atorvastatin), corticosteroid (prednisolone), low molecular weight heparin (later replaced by oral anticoagulation) and analgesic therapy (morphine, amitriptyline, gabapentin, paracetamol and ibuprofen).
Outcome and follow-up
The combination therapy: anticoagulation strategy, aspirin, prednisolone, pentoxifylline, nifedipine and atorvastatin revealed a long-term efficacy. Adherence to tobacco withdrawal was also crucial.
Discussion
Buerger’s disease or Tromboangiitis obliterans (TAO) is a non-atherosclerotic segmental inflammatory disease that most commonly affects the small and medium-sized arteries, veins and nerves of the arms and legs.2
It is most common in the Orient, Southeast Asia, India and the Middle East.3 In fact, all racial and ethnic groups appear to be susceptible. The recent decline in the incidence of TAO has been more apparent than real and has most likely been related to initial over-diagnosis of the disease (based on recognition of its status as a distinct entity), followed by underdiagnosis (based on scepticism concerning its status). Patients with TAO constitute only 4% to 5% of all those with ischemic peripheral vascular disease.4 More recently, prevalence has been estimated at 12, 6–20 cases per 100000 people in the United States.5
One hundred years after the original description by Leo Buerger, the aetiology of the disease remains unknown.6 However, use of or exposure to tobacco is central to the initiation and progression of the disease.2
TAO is more common in males (male-to-female ratio, 3:1); its incidence is believed to be increasing among women, and this trend is postulated to be due to the increased prevalence of smoking among women.5 It has been postulated that TAO is an ‘autoimmune’ reaction triggered by some constituents of tobacco.7 Patients with the disease show hypersensitivity to intradermally injected tobacco extracts, have increased cellular sensitivity to types I and III collagen, elevated serum antiendothelial cell antibody titres and impaired peripheral vasculature endothelium-dependent vasorelaxation. Increased prevalence of HLA-A9, HLA-A54 and HLA-B5 is observed in these patients, which suggests a genetic predisposition to the disease.5
The hypothesis that infectious microorganisms as a contribute to the pathophysiology of the disease was also studied.6 An increased prevalence of hepatitis B infection was recognised.6 A recent study examined a possible association with long-term infection of the gums.8 9
Less than 5% of TAO patients are non-smokers. These cases might be triggered by cold, frostbite, traumatism of extremities or even abuse of sympathicomimetic drugs.1 The use of drugs such as cocaine, cannabis and amphetamines may mimic TAO, showing a similar arteriographic pattern.10 11
The clinical criteria for TAO, edited by Olin in 2000 include: age under 45 years; current or recent history of tobacco use; presence of distal extremity ischemia, indicated by claudication, pain at rest, ischemic ulcers or gangrenes and documented by non-invasive vascular testing; exclusion of autoimmune diseases, hypercoagulable states and diabetes mellitus; exclusion of a proximal source of emboli by echocardiography or arteriography; consistent arteriographic findings in the clinically involved and non-involved limbs.12
Superficial thrombophlebitis and Raynaud’s phenomenon occurs in approximately 40% of patients with TAO.12 Migrating phlebitis (phlebitis saltans) in young patients is therefore highly suggestive of TAO and may parallel disease activity.1 2
TAO may begin with joint manifestations such as recurrent episodes of arthritis of large joints, with transient, migratory single-joint episodes accompanied by local signs of inflammation. The wrists and knees are the most frequently involved joints. The arthritis is non-erosive. Joint problems precede the diagnosis of TAO by about 10 years on average.1
TAO usually begins with ischemia of the distal small arteries and veins. As the disease progresses, it may involve more proximal arteries. Large arteries involvement is unusual and rarely occurs in the absence of small-vessel occlusive disease.2 However, it has been reported in many other vascular beds. There are case reports of cerebral, coronary, renal, mesenteric, pulmonary, iliac and aorta arteries involvement; even multiple-organ involvement may exist.2 12
Biopsy and tissue sample are rarely required to establish the diagnosis. However, in a few cases with unusual location, the diagnosis should be established only when histopathological examination identifies the acute-phase lesion.2 3 In all stages, the normal architecture of the vessels wall including the internal elastic lamina remains intact and these findings distinguish from atherosclerosis and other systemic vasculitis.3 The most frequent injury is the presence of typical giant cell granuloma at the periphery of the thrombus.6
Extensive arterial occlusion accompanied by the development of corkscrew collateral vessels is characteristic angiographic finding, but not pathognomonic. The disease is most often confined to the distal circulation and is almost always infra-popliteal in the lower extremities and distal to the brachial artery in the upper extremities.3
Currently, there is no specific treatment for TAO.13 Absolute discontinuation of tobacco use is the only strategy proven to prevent the progression of Buerger’s disease. Smoking as few as 1 or 2 cigarettes daily, using chewing tobacco, or even using nicotine replacements may maintain the disease activity.5 Selective cannabinoid receptor antagonists, such as rimonabant, which shows promise as a treatment for helping patients to stop smoking, open up interesting new perspectives for this disease strongly related to tobacco use.1
Local hygiene, as well as the treatment of fungal and bacterial infection in the extremity, should not be ignored.4 Calcium inhibitors are frequently recommended, although there is no proof that they are actually effective. Similarly, there is no clinical evidence of benefits with the use of vasodilators, thrombolytic agents, anticoagulants and corticosteroids.1 4 Non-steroidal anti-inflammatory drugs are the treatment of choice for superficial thrombosis.1
Prostaglandins, in particular the intravenous iloprost, represents one of the more valid treatments in the TAO.13 It has been shown to be effective in improving symptoms, accelerating resolution of distal extremity trophic changes, and reducing the amputation rate.5
Recent studies have suggested that vascular damage caused by endothelin-1 may trigger peripheral arterial occlusive disease. The anti-inflammatory, antifibrotic and selective vasodilatory properties of bosentan (endothelin-1 receptor antagonist) have been shown to alleviate pain at rest and reduce the size of ischaemic ulcers caused by damage to the microcirculation.14
Amputation of a limb or a segment of a limb must be postponed until after the patient has ceased smoking and gangrene has set in with clear demarcation. There is no medical evidence that cervical or lumbar sympathectomy will improve survival or decrease the amputation rate; nevertheless, by improving collateral circulation and increasing superficial blood flow to the skin, such a procedure may help heal the ischemic ulceration and thus be beneficial in selected cases. Bypass grafting has been successful in cases involving a femoro-popliteal segment.4
A promising new approach is on the edge with the use of gene transfer to induce therapeutic angiogenesis in TAO.1 The implantation of autologous bone marrow mononuclear cells or mesenchymal stem cells derived from human umbilical cord blood into ischemic limbs can restore limb function by increasing new collateral vessel formation.15–17
The clinical course of TAO is characterised by acute exacerbations separated by phases of remission that may last several years.1 A striking dichotomy is observed with regard to the prognosis, which is dependent upon whether absolute avoidance of tobacco is achieved: 94% avoid amputation; 43% may suffer an amputation if they continue to smoke.5
Learning points.
-
▶
Buerger’s disease or Tromboangiitis obliterans often poses a diagnosis challenge and requires a high degree of suspicion.
-
▶
It remains a poorly investigated disease. The importance of tobacco is consensual and its withdrawal is the cornerstone of therapy.
-
▶
The emergence of new vasodilators biological agents and gene-or cell-based therapy may provide significant tools for the management of this systemic vasculitis.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Puéchal X, Fiessinger JN. Thromboangiitis obliterans or Buerger’s disease: challenges for the rheumatologist. Rheumatology (Oxford) 2007;46:192–9 [DOI] [PubMed] [Google Scholar]
- 2.Olin JW. Thromboangiitis obliterans (Buerger´s disease)-review article. NEJM 2010;343:864–9 [DOI] [PubMed] [Google Scholar]
- 3.Lee KS, Paik CN, Chung WC, et al. Colon Ischemia Associated with Buerger’s Disease: Case Report and Review of the Literature. Gut Liver 2010;4:287–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansari A. Thromboangiitis obliterans: current perspectives and future directions. Tex Heart Inst J 1990;17:112–7 [PMC free article] [PubMed] [Google Scholar]
- 5.Hanly EJ. Buerger Disease (Tromboangiitis Obliterans). Emedicine (WebMD). 2009. Available from URL: http://emedicine.medscape.com/article/460027-overview (accessed 1 August 2011).
- 6.Quintas A, Albuquerque R. [Buerger’s disease: current concepts]. Rev Port Cir Cardiotorac Vasc 2008;15:33–40 [PubMed] [Google Scholar]
- 7.Vasculitis Center Doctors Buerger´s disease. Johns Hopkins Medicine (WebMD). 2011. Available from URL: http://www.hopkinsvasculitis.org/types-vasculitis/buergers-disease (accessed 1 August 2011).
- 8.Iwai T, Inoue Y, Umeda M, et al. Oral bacteria in the occluded arteries of patients with Buerger disease. J Vasc Surg 2005;42:107–15 [DOI] [PubMed] [Google Scholar]
- 9.Mayo Clinic staff. Buerger´s disease. Mayo Foundation for Medical Edication and Research 2010 (WebMD). 2011. Available from URL: http://www.mayoclinic.com/health/buergers-disease/DS00807 (accessed 1 August 2011).
- 10.Olin JW, Shih A. Thromboangiitis obliterans (Buerger’s disease). Curr Opin Rheumatol 2006;18:18–24 [DOI] [PubMed] [Google Scholar]
- 11.Roncon-Albuquerque R, Serrão P, Vale-Pereira R, et al. Plasma catecholamines in Buerger’s disease: effects of cigarette smoking and surgical sympathectomy. Eur J Vasc Endovasc Surg 2002;24:338–43 [DOI] [PubMed] [Google Scholar]
- 12.Arkkila PET Tromboangiitis Obliterans (Buerger´s disease). Orphanet Encyclopedia (WebMD). 2005. Available from URL: http://orpha.net/data/patho/GB/uk-buerger.pdf (accessed 1 August 2011).
- 13.Reny JL, Champion K, Emmerich J, et al. Maladie de Buerger. Sang Thrombose vaisseaux 2002;7:430–5 [Google Scholar]
- 14.De Haro J, Florez A, Fernandez JL, et al. Treatment of Buerger disease (Tromboangiitis Obliterans) with bosentan: a case report. BMJ Case Reports 2009. dói: 10.1136/bcr.08.2008.0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch AT. Critical limb ischemia and stem cell research: anchoring hope with informed adverse event reporting. Circulation 2006;114:2581–3 [DOI] [PubMed] [Google Scholar]
- 16.Kim DI, Kim MJ, Joh JH, et al. Angiogenesis facilitated by autologous whole bone marrow stem cell transplantation for Buerger’s disease. Stem Cells 2006;24:1194–200 [DOI] [PubMed] [Google Scholar]
- 17.Kim SW, Han H, Chae GT, et al. Successfull stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem cells 2006;24:1620–6 [DOI] [PubMed] [Google Scholar]


