Abstract
Neonatal alloimmune thrombocytopenia is a rare (1/1000–5000 births) life-threatening disorder, caused by fetomaternal incompatibility for a fetal human platelet alloantigen inherited from the father, with production of maternal alloantibodies against fetal platelets, leading to severe thrombocytopenia and potential bleeding. Intracranial haemorrhage is the most feared complication. This report presents the case of a term newborn infant, born from caesarean section after a normal pregnancy, presenting signs of skin bleeding with different ages. Obstetric history included a previous spontaneous abortion after amniocentesis. Severe thrombocytopenia (4×109/l platelets) was found and brain ultrasound showed multiple intracranial haemorrhages. Human platelet antigen (HPA) phenotyping showed maternal negative HPA-1a and paternal positive HPA-1a platelets. Strongly positive anti-HPA-1a and weakly positive anti-human leukocyte antigen class I alloantibodies were found in the mother. Multiple platelet transfusions, intravenous immunoglobulin and corticosteroid were given but favourable response was accomplished only after a compatible platelet transfusion. Brain MRI showed multiple subacute and chronic haemorrhages.
Background
Neonatal alloimmune thrombocytopenia (NAIT) occurs when fetal platelets contain an antigen that the mother lacks. The fetomaternal incompatibility for a platelet antigen is due to the passage of antigen-positive fetal platelets into the circulation of an antigen-negative mother, causing maternal immunisation with production of immunoglobulin G alloantibodies directed against the ‘foreign’ antigen. These alloantibodies cross the placenta bidding to foetal platelets and causing its destruction, resulting in severe foetal and neonatal thrombocytopenia.1 2
Human platelet antigens (HPA) have different forms and its frequency varies with ethnicity. HPA-1a antigen is the commonest among caucasians, while the homozygous antigen for HPA-1b represents only 2.9%.1 2
The incidence of NAIT is estimated to be 1/1000–5000 births. It often develops in the first pregnancy of an unknown at-risk couple. Most NAIT newborn infants appear healthy and have an unexpected severe thrombocytopenia (<50×109/l). Still, signs consistent with moderate to severe thrombocytopenia, including petechiae, bruising and bleeding, can be present. Although skin bleeding may occur in almost half of the cases, intracranial haemorrhage is the most serious complication, present in about 8–22% of affected newborns, occurring in utero in 50–80% of cases.1–4
Diagnosis of the first affected child include demonstration of thrombocytopenia in the newborn, maternal and paternal platelet antigen testing (phenotyping), antiplatelet alloantibody testing in the mother’s serum and, when available, molecular study (genotying) of maternal, paternal and child platelet antigens.1–3
NAIT treatment must be instituted promptly. Compatible HPA platelets or washed maternal platelets are preferred as they will not be destroyed by maternal alloantibodies. Adequate platelet counts should be assured in the first 3–4 days lowering the risk of intracranial haemorrhage. Intravenous immunoglobulin (IVIG) and methylprednisolone are used as adjunctive therapy.1 4
This case remembers the complexity of this disease, the importance of its rapid recognition and prompt management.
Case presentation
The authors present the case of a newborn term female, first birth from a second pregnancy of healthy non-consanguineous parents, presenting generalised bruising, suffusions and petechias in different stages of development at birth.
Family medical history was normal. Obstetric maternal antecedents included a spontaneous abortion 1 year earlier, soon after an amniocentesis, performed at 16 weeks of pregnancy, due to mother’s age (38 years old). The previous fetus had a normal female karyotype (46, XX) and phenotype on necropsy, also showing signs of intrauterine growth restriction and acute retroplacental haematoma.
The current pregnancy was uneventful. A female karyotype (46, XX) was obtained. Serological maternal investigation was irrelevant (negative hepatitis B virus, hepatitis C virus, HIV and venereal disease research laboratory; immune to toxoplasma, rubella and cytomegalovirus) and routine fetal ultrasounds were normal. There was no maternal history of haemorrhage nor thrombocytopenia (>230×109/l); mother’s blood group was A Rh D positive. She was born from caesarean section at 40 weeks gestation, due to fetopelvic incompatibility and abnormal cardiotocographic record, needing reanimation with bag-mask ventilation with subsequent recovery (Apgar scores: 4/8/9). Generalised bruising, suffusions and petechias in different stages of development were noted (figure 1). Hepatosplenomegaly was absent. Weight was adequate for gestational age (3330 g).
Figure 1.
Clinical presentation: generalised bruising, suffusions and petechias.
Investigations
Cord blood was obtained showing a severe isolated thrombocytopenia of 16×109/l platelets. Peripheral blood confirmed the thrombocytopenia (lowest value 4×109/l) and excluded a possible infectious cause (negative C-reactive protein and blood culture). Because NAIT was suspected, newborn cranial ultrasound and parental blood testing were performed. Cranial ultrasound (figure 2) in the first 2 h of life showed a right parieto-occipital hyperechoic and homolateral peri-sylvian lesions. Platelet HPA phenotyping in maternal and paternal blood showed negative HPA-1a and positive HPA-1a platelets, respectively; the mother had strongly positive anti-HPA-1a and weakly positive anti-HLA class I alloantibodies; the mother’s serum and father’s platelet crossmatch proved to be incompatible.
Figure 2.
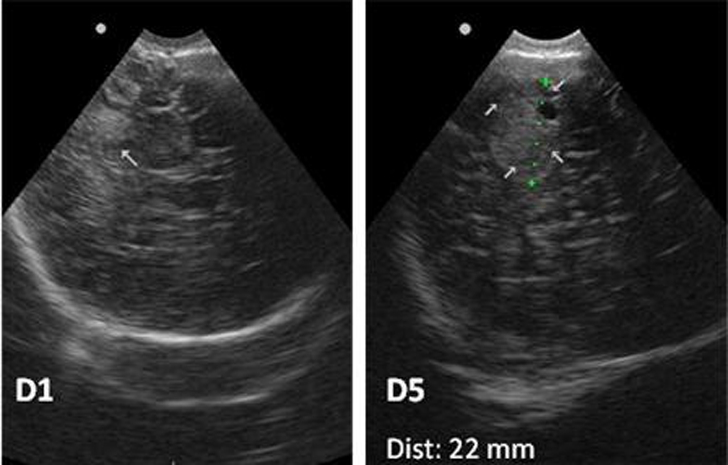
Transfontanelar ultrasound with hyperechogenic intracranial lesions (arrows) suggesting haemorrhage.
Treatment
Medical treatment was started in the first hours of life (figure 3). Due to the unavailability of compatible platelets (negative HPA-1a), two platelet transfusions (20 ml/kg/dose) from random donors were administered in day 1 with count normalisation. IVIG (1 g/kg/day for 2 days) and intravenous methylprednisolone (3 mg/kg/day for 5 days) were administered as adjunctive therapies. Despite this management and over the next 3 days, platelet count decreased (28×109/l platelets) leading to a third compatible platelet transfusion (negative HPA-1a donor) and IVIG administration.
Figure 3.
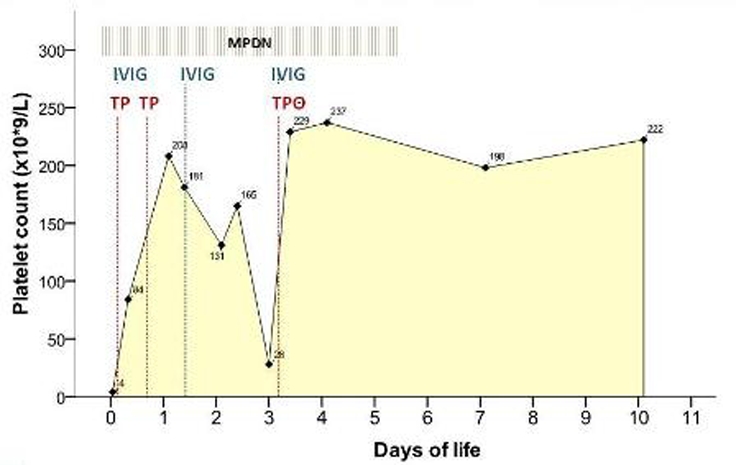
Treatment and platelet count response. TP-Random donor platelet transfusion; TPΘ-Platelet transfusion from a negative HPA-1a donor; IVIG-Intravenous immunoglobulin; MPDN-methylprednisolone.
Outcome and follow-up
Platelet count reached a normal plateau (>198×109/l platelets), signs of skin bleeding improved and cranial ultrasound was resemblant, at 5 days of life. Brain MRI on day 8 (figure 4) showed multiple subacute cerebral haemorrhages (1–4 weeks of development) in the right parietal lobe and in the posterior fossa, signs of chronic bleeding (haemosiderin deposits) in the occipital lobes and cerebellar hemispheres and vermis, these last also presenting marked atrophy.
Figure 4.
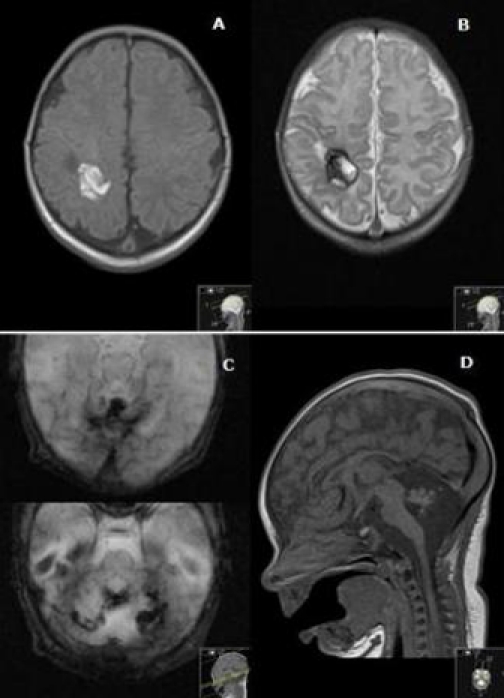
Brain MRI. Axial T1WI (A) and T2WI (B): right parietal haemorrhage with blood in early and late subacute stage. Axial T2 gradient echo (C) and sagittal T1WI (D): there are signs of chronic bleeding, with haemosiderin deposits (C) and marked atrophy (D) of the cerebellar hemispheres and vermis.
At day 11 of life, she was discharged home without clinical manifestations of neither bleeding nor focal deficits on neurological examination. Platelet count was normal (222×109/l platelets).
At 3 weeks life in the paediatric haematology consultation, molecular study was performed showing homozygous platelet alloantigen genotypes 1b/1b in the mother and 1a/1a in the father; the newborn was proved to be heterozygous HPA-1a/1b. Genetic counselling was offered.
Discussion
Any newborn infant with platelet count below 100×109/l should be considered abnormal and promptly studied for an underlying cause.3 NAIT is a rare severe condition. It must be suspected if clinical manifestations such as skin or gastrointestinal bleeding or intracranial haemorrhage on ultrasound and severe thrombocytopenia are present, in the absence of maternal thrombocytopenia. Because maternal screening is rarely performed, couples usually do not know they are at risk until they have delivered an affected child.1 3 5 The mother is asymptomatic, yet she or a sister may have a history of earlier affected pregnancies.4 In the previous case there was a history of spontaneous abortion 1 year earlier, after an amniocentesis at 16 weeks of pregnancy. This episode could have been related to foetal alloimmune thrombocytopenia. An explanation is that fetal platelet antigens are expressed since week 16 of gestation entering the maternal circulation. Antiplatelet antibodies can endure in maternal circulation for many years and additional exposure in subsequent pregnancies can be a further stimulus. Severity of NAIT tends to worsen with subsequent pregnancies, leading to an increased risk for intracranial haemorrhage.1 4 In our case, brain MRI showed that although undetected in fetal ultrasound, intracranial haemorrhage occurred in utero.
Infants with NAIT and intracranial haemorrhage have the poorest outcome. Mortality can range from 15% to 30% of these cases.4 6 Non-fatal cases usually are associated with neurologic sequelae including cerebral palsy, mental retardation, cortical blindness and seizures. When intracranial haemorrhage occurs in utero before week 20, abnormalities like porencephalic cysts, hydrocephaly, neuronal migrational disorders and superficial siderosis are often present.4 Due to the severe central nervous system abnormalities affecting the motor and coordination centers in our case, some compromise in the neurodevelopment is expected.
The treatment of severe NAIT with platelet transfusion takes into account the threshold of the platelet count. Term newborns with less than 30×109/l platelets and preterm infants with less than 50×109/l platelets should be transfused, especially if bleeding is present. Intravenous γ-globulin (1 g/kg/day for 1–4 days) is effective in 75% of cases but takes longer than does a platelet transfusion to achieve a safe platelet count. Intravenous methylprednisolone (1 mg/kg, q 8 h, 1–3 days) lacks on efficacy studies.1 4
Because of the high risk of recurrence of NAIT, all women with a history of NAIT must be counselled. Sisters of HPA-1a–negative women should also be tested.2 4 Attending the father’s homozygous genotype in the previous case, this couple will always produce HPA-1a/1b infants, who will be affected by this condition if antenatal management is not fulfilled. Although the severity of NAIT is often difficult to predict, it is known that it tends to worsen with subsequent pregnancies.4 Bussel et al. showed that a history of intracranial haemorrhage in a previously affected sibling is the only significant predictor of severity.7 For this reason, if this couple decides to proceed with a future pregnancy they must be closely monitored and treatment should be offered. In a couple with a previous affected infant, adequate therapy is maternal weekly IVIG from 20 weeks of gestation or earlier (12 weeks) if intracranial haemorrhage is present. Increase of the IVIG dose or daily prednisone may be used when a more intense prevention strategy is needed. Maternal anti-HPA-1a antibodies should be monitored. Antenatal management should avoid invasive procedures such as in utero platelet transfusions because of the high risk of fetal loss (5.5% per affected pregnancy). Elective caesarean delivery should be performed, minimising the haemorrhagic risks.2 4 8
Learning points.
-
▶
Suspect of NAIT in a well newborn who presents with bruising, petechial rash and/or suffusions and who is found to have isolated severe thrombocytopenia.
-
▶
Careful inquire about maternal personal and familial history and obstetric follow-up, including maternal platelet count.
-
▶
Treatment should include adequate platelet transfusion (HPA-1a/ HPA-5b negative platelets) and IVIG as earlier as possible to prevent bleeding complications. If adequate platelet transfusion is not available, random donor platelets should be used.
-
▶
Intracranial haemorrhage is the most serious complication with catastrophic neurologic sequelae.
-
▶
Genetic counselling should be offered to couples of affected children who want to have future pregnancies. Antenatal management is crucial in prevention of a poor outcome.
Acknowledgments
The authors thank Dr Manuela Benedito (haematology department), Dr Dolores Faria (neonatal intensive care unit) and all health professionals involved in the care of this patient.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Fernandes CJ. Neonatal Thrombocytopenia. http://www.uptodate.com/contents/neonatal-thrombocytopenia (accessed 15 Feb 2011).
- 2.Blanchsette VS, Johnson J, Rand M. The management of alloimmune neonatal thrombocytopenia. Baillieres Best Pract Res Clin Haematol 2000;13:365–90 [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz RL, Bussel JB, McFarland JG. Alloimmune thrombocytopenia: state of the art 2006. Am J Obstet Gynecol 2006;195:907–13 [DOI] [PubMed] [Google Scholar]
- 4.Arnold DM, Smith JW, Kelton JG. Diagnosis and management of neonatal alloimmune thrombocytopenia. Transfus Med Rev 2008;22:255–67 [DOI] [PubMed] [Google Scholar]
- 5.Mendes LR, Ferrão A, Malcata C, et al. trombocitopénia neonatal aloimune –apresentação clínica tardia. Acta Pediatr Port 2006;7:27–9 [Google Scholar]
- 6.Castro Conde JR, Martínez ED, Rodríguez RC, et al. CNS siderosis and dandy-walker variant after neonatal alloimmune thrombocytopenia. Pediatr Neurol 2005;32:346–9 [DOI] [PubMed] [Google Scholar]
- 7.Bussel JB, Zabusky MR, Berkowitz RL, et al. Fetal alloimmune thrombocytopenia. N Engl J Med 1997;337:22–6 [DOI] [PubMed] [Google Scholar]
- 8.Paidas MJ. Prenatal Management Of Neonatal Alloimmune Thrombocytopenia. http://www.uptodate.com/contents/prenatal-management-of-neonatal-alloimmune-thrombocytopenia (accessed 8 Feb 2011).



