Abstract
Background
We report the determinants of serum levels of vitamin D in a UK melanoma case–control study benefitting from detailed exposure and genotyping data.
Methods
Sun exposure, supplemental vitamin D, and SNPs reported to be associated with serum levels were assessed as predictors of a single serum 25-hydroxyvitamin D3 measurement adjusted for season, age, sex, and body mass index.
Results
Adjusted analyses showed that vitamin D levels were sub-optimal especially in the sun-sensitive individuals (−2.61 nmol/L, p = 0.03) and for inheritance of a genetic variant in the GC gene coding for the vitamin D-binding protein (−5.79 for heterozygotes versus wild type, p = <0.0001). Higher levels were associated with sun exposure at the weekend in summer (+4.71 nmol/L per tertile, p = <0.0001), and on hot holidays (+4.17 nmol/L per tertile, p = <0.0001). In smoothed scatter plots, vitamin D levels of 60 nmol/L in the non-sun-sensitive individuals were achieved after an average 6 h/day summer weekend sun exposure but not in the sun-sensitive individuals. Users of supplements had levels on average 11.0 nmol/L higher, p = <0.0001, and achieved optimal levels irrespective of sun exposure.
Conclusions
Sun exposure was associated with increased vitamin D levels, but levels more than 60 nmol/L were reached on average only in individuals reporting lengthy exposure (≥12 h/weekend). The sun-sensitive individuals did not achieve optimal levels without supplementation, which therefore should be considered for the majority of populations living in a temperate climate and melanoma patients in particular. Inherited variation in genes such as GC is a strong factor, and carriers of variant alleles may therefore require higher levels of supplementation.
Electronic supplementary material
The online version of this article (doi:10.1007/s10552-011-9827-3) contains supplementary material, which is available to authorized users.
Keywords: Vitamin D, Sun exposure, Vitamin D-binding protein, NADSYN1, DHCR7, GC, Sun sensitivity, Supplementation, Insufficiency, CYP2R1
Introduction
Vitamin D is important to human health [1–3], but sub-optimal levels have been commonly reported [4] even in hot countries such as Israel [5]. Blood levels are determined in part by sun exposure and pigmentation, so that darker-skinned people tend to have lower levels compared with paler-skinned people living at the same latitude [6]. Recent evidence suggested, however, that within white-skinned populations, the very fair surprisingly have lower vitamin D levels, which may result from different behaviors in the sun [7, 8]. Vitamin D is fat soluble, and obesity is associated with its lower levels in blood [9]. In many populations levels change with age [9]. Most recently, genome-wide association studies reported that a SNP in the gene coding for the group-specific complement (vitamin D-binding protein, GC) is associated with serum levels, with additional probable involvement of genes involved in the production of the active form of vitamin D [10, 11].
The approach to supplementation or recommended sun exposure internationally remains controversial, especially for those at increased risk of melanoma [12, 13]. The benefits of sun exposure in terms of its effects on serum vitamin D levels must be weighed against increased melanoma risk. There are also concerns from studies on prognosis in breast cancer patients [14] and risk of cardiovascular disease [15] that the risk curve may be U-shaped: that there may be increased risk of disease progression associated with very high levels of serum vitamin D.
We reported that lower serum vitamin D levels at diagnosis are associated with thicker melanomas and poorer outcome [16], so that understanding the determinants of vitamin D levels in this population is important. In this paper, we report the relationship between serum vitamin D levels and variables postulated to determine those levels, such as reported sun exposure, phenotype, dietary supplementation and the following SNPs [10, 11]: rs2282679, in the GC gene coding for vitamin D-binding protein; rs10741657 in CYP2R1, the gene that encodes vitamin D 25-hydroxylase, a key enzyme in the conversion of vitamin D3 to an active vitamin D receptor ligand; and rs7944926 and rs382925, intronic SNPs in NADSYN1, which are in tight linkage disequilibrium (LD) with several SNPs in the adjoining DHCR7 (7-dehydrocholesterol reductase) gene (important to vitamin D metabolism in the skin).
Materials and methods
A total of 960 population-ascertained incident melanoma cases were recruited between September 2000 and December 2005 [8, 17] in a geographically defined region of the UK. Recruitment/blood sampling took place wherever possible 3–6 months after diagnosis. A total of 513 population-ascertained controls were randomly invited from individuals of the same sex and 5-year age group by the family doctor of cases, and 174 sibling controls participated, as described previously [18]. Studies were approved by the UK Multi-Centre Research Ethics Committee (MREC) and the Patient Advisory Group (PIAG). Informed consent was obtained from each participant.
Comprehensive sun exposure data were collected by questionnaire and subsequent telephone interview [17]. An initial postal questionnaire was completed by all participants (including a life-long residence calendar), and comprehensive sun exposure data were subsequently collected by telephone, based upon that residence calendar. Data were collected on weekday and weekend exposure (in sunny and colder weather), and holiday sun exposure (at low and higher latitudes) throughout life at 10-year intervals and in the last year. Sun exposure variables for this study were generated by using data collected on sun exposure in the most recent year, which were classified into thirds based upon their distribution in the population controls. For variables where more than one-third of the population controls reported no sun exposure, the data were classified into three groups: individuals with no sun exposure, individuals with less than or equal to the median sun exposure, and individuals with more than the median sun exposure. Sex, natural hair color at age 18 years, sunburn frequency, propensity to burn, ability to tan, skin color of inside upper arm and freckling as a child [19] were self-reported. A measure of deprivation (the Townsend score) was derived from the subject’s current postcode based on 2001 UK Census data [20]. Higher scores are indicative of residence in more deprived communities. Data on the intake of supplements containing vitamin D were collected from cases (but not controls) and were categorized as taking any regular supplementation or not. Eye color and freckling scores were determined by research nurses as described elsewhere [21].
25-Hydroxyvitamin D2/D3 levels were measured as described elsewhere [16] in a single serum sample from 880 (92%) cases, 129 (74%) sibling controls and a subset (194, 38%) of population controls, taken around the time of data collection. Controls were sampled in pre-defined time periods only (due to funding constraints). 25-Hydroxyvitamin D2 and D3 levels were summed and henceforth referred to as “serum vitamin D level”. The SNPs rs2282679, rs7944926, rs10741657, and rs3829251 were genotyped in germline DNA using the Taqman genotyping assays C__26407519_10, C__12043682_10, C__2958430_10 and C__27497388_10, respectively (Applied Biosystems, Foster City, USA). See Supplementary Data.
Statistical methods
Multiple linear least squares regression models of the determinants of vitamin D levels were fitted using the “lm” routine in R version 2.10.1, for the cases, the controls, and the cases and controls combined. Models were adjusted for season, BMI, sex, age, Townsend score and case–control status (where appropriate). The coefficient of determination (r 2) was calculated to measure the percentage of variance in seasonally adjusted vitamin D levels explained by each covariate, adjusted for the variables listed above. Supplementation data were available for the cases only; a separate multiple linear regression model was fitted in this subset that additionally adjusted for vitamin D supplementation.
LOESS curves were fitted to illustrate the complex effects of sun exposure, sun sensitivity, and supplementation on vitamin D levels and on the difference in adjusted vitamin D levels attributable to SNPs. Further details of the statistical methodology can be found in Supplementary Data.
Results
The descriptive characteristics of participants are summarized in Table 1 [8, 18, 22]. Cases had lower levels of unadjusted vitamin D (crude mean level of 53.5 nmol/L) than controls (crude mean levels of 57.3 and 60.1 nmol/L for population and sibling controls). There was a statistically significant difference between cases and sibling controls but not between cases and population controls (Table 2). Overall suboptimal levels (<60 nmol/L) were common, being observed in 63% of cases and 55% of controls (data not shown).
Table 1.
Mean vitamin D levels and distribution of age, sex, sensitivity, BMI, Townsend score, and sunscreen usage in cases, population controls, and sibling controls
| Risk factor | Cases | Population controls | Sibling controls | p value |
|---|---|---|---|---|
| Vitamin D (nmol/L) | ||||
| Mean (SD) | 53.5 (21.9) | 57.3 (19.4) | 60.1 (25.2) | 0.0008* |
| Mean vitamin D by season | ||||
| 1 (Jan–Mar) | 45.5 (19.4) | 52.3 (22.8) | 46.6 (19.4) | 0.3* |
| 2 | 50.8 (20.4) | 55.3 (16.2) | 61.8 (28.5) | 0.05* |
| 3 | 65.3 (21.1) | 61.0 (18.9) | 65.6 (25.3) | 0.6* |
| 4 | 52.0 (21.3) | 55.0 (20.2) | 64.7 (22.2) | 0.008* |
| Sex | ||||
| Male | 350 (39.8%) | 78 (40.2%) | 40 (31.0%) | 0.2** |
| Female | 530 (60.2%) | 116 (59.8%) | 89 (69.0%) | |
| Age at diagnosis/interview | ||||
| <40 | 196 (22.2%) | 19 (9.8%) | 25 (19.4%) | <0.0001*** |
| 40–50 | 186 (21.1%) | 27 (13.9%) | 38 (29.5%) | |
| 50–60 | 213 (24.2%) | 63 (32.5%) | 29 (22.5%) | |
| 60–70 | 195 (22.2%) | 42 (21.6%) | 30 (23.2%) | |
| >70 | 90 (10.2%) | 43 (22.2%) | 7 (5.4%) | |
| Sun sensitivity score | ||||
| Not sensitive | 384 (43.8%) | 111 (57.2%) | 72 (56.3%) | 0.0003** |
| Sensitive | 493 (56.2%) | 83 (42.8%) | 56 (43.8%) | |
| BMI | ||||
| <18.5 | 8 (0.9%) | 1 (0.5%) | 2 (1.6%) | 0.02*** |
| 18.5–25 | 345 (39.8%) | 98 (50.5%) | 54 (42.2%) | |
| 25–30 | 338 (39.0%) | 74 (38.1%) | 52 (40.6%) | |
| >30 | 175 (20.2%) | 21 (10.8%) | 20 (15.6%) | |
| Townsend | ||||
| 1 (lowest quartile) | 149 (17.2%) | 39 (20.4%) | 29 (23.2%) | 0.05** |
| 2 | 246 (28.5%) | 63 (33.0%) | 38 (30.4%) | |
| 3 | 243 (28.1%) | 59 (30.9%) | 33 (26.4%) | |
| 4 (highest quartile) | 226 (26.2%) | 30 (15.7%) | 25 (20.0%) | |
| Recent sunscreen usage | ||||
| None | 326 (38.2%) | 66 (34.0%) | 60 (46.9%) | 0.1** |
| SPF low | 133 (15.6%) | 26 (13.4%) | 13 (10.2%) | |
| SPF high | 395 (46.3%) | 102 (52.6%) | 55 (43.0%) |
Vitamin D levels are reported both stratified by season and overall. Sun sensitivity is a dichotomous measure generated using factor analysis of six correlated variables related to sun sensitivity (see Supplementary Data). Values are given as absolute numbers of individuals belonging to each class and the percentage of the total. For vitamin D levels, the mean and standard deviation for each group is given
* Differences between vitamin D levels in cases, population, and sibling controls tested using a Kruskal–Wallis test
** Differences between groups (cases, population, and sibling controls) tested using a chi-squared test
*** Differences between groups (cases, population, and sibling controls) tested using Fisher’s exact test
Table 2.
Predictors of blood vitamin D concentration (nmol/L) in multiple linear regression models for cases, controls, and both cases and controls combined
| Factor | Cases | Controls | Cases + controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Estimate | SE | p value | n | Estimate | SE | p value | Estimate | SE | p value | |
| Season | |||||||||||
| 1 (Jan to March-baseline) | 239 | 0 | 53 | 0 | 0 | ||||||
| 2 | 183 | 5.56 | 1.98 | 0.005 | 71 | 9.00 | 3.89 | 0.02 | 6.41 | 1.76 | 0.0003 |
| 3 | 231 | 19.74 | 1.86 | <0.0001 | 115 | 14.04 | 3.65 | 0.0001 | 18.0 | 1.64 | <0.0001 |
| 4 | 197 | 5.83 | 1.94 | 0.003 | 76 | 9.67 | 3.85 | 0.01 | 6.94 | 1.73 | <0.0001 |
| BMI | 850 | −0.50 | 0.15 | 0.0007 | 315 | −0.62 | 0.24 | 0.01 | −0.52 | 0.12 | <0.0001 |
| Sex | |||||||||||
| Female (baseline) | 512 | 0 | 197 | 0 | 0 | ||||||
| Male | 338 | 0.86 | 1.44 | 0.5 | 118 | −1.00 | 2.60 | 0.7 | 0.69 | 1.25 | 0.6 |
| Age (per year) | 850 | 0.22 | 0.05 | <0.0001 | 315 | 0.1 | 0.10 | 0.6 | 0.20 | 0.05 | <0.0001 |
| Townsend | 850 | −0.26 | 0.23 | 0.2 | 315 | −0.46 | 0.49 | 0.3 | −0.32 | 0.21 | 0.1 |
| Case–control status | |||||||||||
| Case | – | – | – | – | – | – | – | – | 0 (baseline) | ||
| Population control | – | – | – | – | 191 | 0 (baseline) | −0.33 | 1.69 | 0.9 | ||
| Sibling control | – | – | – | – | 124 | 4.87 | 2.59 | 0.06 | 5.73 | 1.98 | 0.004 |
| Supplementation status | |||||||||||
| No (baseline) | 552 | 0 | – | – | – | – | – | – | – | ||
| Yes | 246 | 10.6* | 1.48 | <0.0001 | – | – | – | – | – | – | – |
| Sun sensitivity index | |||||||||||
| Non-sun-sensitive (baseline) | 373 | 0 | 180 | 0 | 0 | ||||||
| Sun sensitive | 476 | −2.74* | 1.38 | 0.05 | 135 | −1.71* | 2.40 | 0.5 | −2.61* | 1.20 | 0.03 |
| Nevus number | |||||||||||
| 0–9 (baseline) | 78 | 0 | 68 | 0 | 0 | ||||||
| 10–24 | 170 | 5.39* | 2.74 | 0.05 | 95 | 4.35* | 3.31 | 0.2 | 5.36* | 2.09 | 0.01 |
| >24 | 601 | 3.80* | 2.41 | 0.1 | 131 | 8.89* | 3.11 | 0.004 | 5.29* | 1.84 | 0.004 |
| Freckling total (%) | |||||||||||
| 0–16.7 (baseline) | 228 | 0 | 104 | 0 | 0 | ||||||
| 16.7–41.7 | 274 | 2.75* | 1.77 | 0.1 | 88 | 6.23* | 2.99 | 0.04 | 3.60* | 1.53 | 0.02 |
| >41.7 | 336 | 4.44* | 1.70 | 0.009 | 97 | 10.94* | 2.91 | 0.0002 | 5.88* | 1.47 | <0.0001 |
| Freckling shoulders (%) | |||||||||||
| 0–10 (baseline) | 185 | 0 | 98 | 0 | 0 | ||||||
| 10–60 | 390 | 5.77* | 1.75 | 0.001 | 113 | 7.18* | 2.82 | 0.01 | 6.11* | 1.48 | <0.0001 |
| >60 | 265 | 7.56* | 1.88 | <0.0001 | 80 | 13.37* | 3.07 | <0.0001 | 8.87* | 1.60 | <0.0001 |
| Sunscreen usage | |||||||||||
| None (baseline) | 318 | 0 | 125 | 0 | 0 | ||||||
| SPF low | 126 | 3.67* | 2.10 | 0.08 | 39 | 11.75* | 3.80 | 0.002 | 5.72* | 1.85 | 0.002 |
| SPF high | 385 | 0.54* | 1.51 | 0.7 | 151 | 3.61* | 2.50 | 0.2 | 1.32* | 1.30 | 0.3 |
| Daily sun exposure (per tertile) | 822 | 2.22** | 0.86 | 0.01 | 308 | 2.94** | 1.50 | 0.05 | 2.48** | 0.75 | 0.0009 |
| Weekend sun exposure | |||||||||||
| Overall (per tertile) | 831 | 3.21** | 0.88 | 0.0002 | 310 | 5.98** | 1.49 | <0.0001 | 3.97** | 0.76 | <0.0001 |
| Cooler months (per tertile) | 831 | 2.39** | 0.83 | 0.004 | 311 | 3.19** | 1.32 | 0.02 | 2.72** | 0.70 | 0.0001 |
| Warmer months (per tertile) | 831 | 3.11** | 0.90 | 0.0006 | 310 | 7.27** | 1.55 | <0.0001 | 4.17** | 1.19 | <0.0001 |
| Holiday sun exposure | |||||||||||
| Overall (per tertile) | 834 | 3.21** | 0.88 | 0.0003 | 312 | 4.25** | 1.44 | 0.003 | 3.56** | 0.75 | <0.0001 |
| Lower than 45o (per tertile) | 834 | 4.70** | 0.82 | <0.0001 | 312 | 3.99** | 1.39 | 0.004 | 4.47** | 1.18 | <0.0001 |
| rs2282679 (GC) | |||||||||||
| TT (baseline) | 426 | 0 | 158 | 0 | 0 | ||||||
| GT | 335 | −5.58* | 1.43 | 0.0001 | 138 | −5.85* | 2.44 | 0.01 | −5.79* | 1.24 | <0.0001 |
| GG | 85 | −12.57* | 2.34 | <0.0001 | 15 | −2.09* | 5.65 | 0.7 | −10.80* | 2.18 | <0.0001 |
| rs7944926 (NADSYN1) | |||||||||||
| GG (baseline) | 493 | 0 | 178 | 0 | 0 | ||||||
| AG | 300 | −3.65* | 1.46 | 0.01 | 120 | 0.86* | 2.47 | 0.7 | −2.50* | 1.26 | 0.05 |
| AA | 47 | −4.60* | 3.05 | 0.1 | 12 | −10.9* | 6.23 | 0.08 | −6.00* | 2.76 | 0.03 |
| rs10741657 (CYP2R1) | |||||||||||
| GG (baseline) | 287 | 0 | 125 | 0 | 0 | ||||||
| AG | 383 | −3.47* | 1.56 | 0.03 | 139 | 5.05* | 2.56 | 0.04 | −0.84* | 1.34 | 0.5 |
| AA | 154 | 0.66* | 2.00 | 0.7 | 45 | 9.03* | 3.60 | 0.01 | 3.21* | 1.76 | 0.07 |
Season, age, sex, BMI, case–control status (where appropriate), and Townsend score were included together in the multivariable model described in the top part of the table
* Corrected for season, age, sex, case–control status (where appropriate), BMI, and Townsend score. Adjusted estimates used as a baseline the estimated vitamin D level of a 54 year old case woman with a BMI score of 25, living in an area with a Townsend score of 0 (neither deprived nor affluent), whose blood was sampled in winter. In the control group only, the baseline was calculated using the estimated vitamin D level of a population control instead of a case
** Corrected for sun sensitivity status in addition to season, age, sex, case–control status (where appropriate), BMI, and Townsend score
Mean vitamin D levels are reported in Supplementary Table 2. Vitamin D levels varied with season (Table 2). Higher BMI was associated with lower serum vitamin D levels (adjusted estimate 0.52 units lower per unit of BMI, p = <0.0001). There was no effect of sex on vitamin D levels, but levels increased with age at diagnosis or interview both overall and in cases. Table 2 also shows vitamin D levels according to reported sun exposures for cases and controls separately and for all pooled, adjusted for the above factors. In the data from cases, we also adjusted for reported vitamin D supplementation. In most instances, little difference was seen in levels between cases and controls, so that we report special cases where differences were seen below. The strongest association with vitamin D levels overall was with holiday exposure at low latitudes (subjects adjusted mean levels increased by 9.1 units between the lowest and highest group of exposure). There was also strong association with average weekend exposure in recent warmer months, with weaker correlations with daily exposure and average holiday exposure. As reported previously [8], individuals with greater sun sensitivity overall had lower vitamin D levels (Table 2) and increased freckling on the shoulders (presumed to be a marker of greater habitual sun exposure in the fair-skinned) was associated with higher levels. Indeed, a strong positive association between freckling and higher reported levels of sun exposure is seen (Table S1).
Use of low sun protection factor (SPF) sunscreen compared with no use of sunscreen was associated with higher serum levels in the total data set (adjusted estimate 5.72, p = 0.002, Table 2), although there was no evidence of an effect from high SPF sunscreen use.
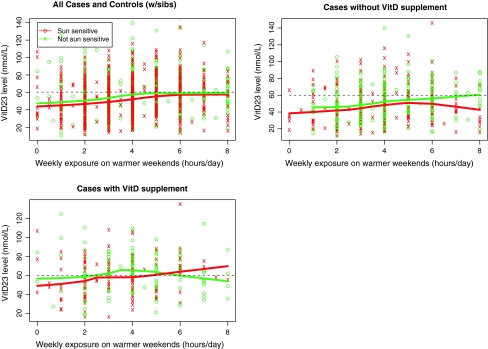
To investigate the association between reported sun exposure and vitamin D levels, we plotted reported recent weekend sun exposure in warmer months against the recorded single vitamin D measurement and fitted a locally weighted scatterplot smoothing (LOESS) curve for sun-sensitive individuals and a separate LOESS curve for non-sun-sensitive individuals (Fig. 1). It can be seen that in cases and controls considered together, the LOESS curve describing the trend in vitamin D levels increased to a plateau of just under 60 nmol/L in individuals reporting an average of 5 h per day of weekend sun exposure for non-sun-sensitive phenotypes. For individuals with sun-sensitive phenotypes, a lower plateau was reached in individuals reporting an average of 6 h per day of weekend sun exposure. In melanoma cases not taking supplements, the plateau level of 60 nmol/L was reached after a higher (6 h) average duration of exposure in those with non-sun-sensitive phenotypes but not at all in the sun-sensitive individuals. In those taking supplements, the plateau of 60 nmol/L was reached irrespective of reported sun exposure.
Fig. 1.
Influence of sensitivity, supplementation and sun exposure on vitamin D levels. For each subject recent weekend sun exposure in warmer months is plotted against serum vitamin D levels by skin sensitivity. Individuals who are sun-sensitive are in red, individuals who are not sun-sensitive are in green. LOESS curves are plotted to show how vitamin D levels vary with exposure for each group. In the second panel, only cases who have not taken vitamin D supplements are shown and in the third panel only cases who have taken vitamin D supplements are shown
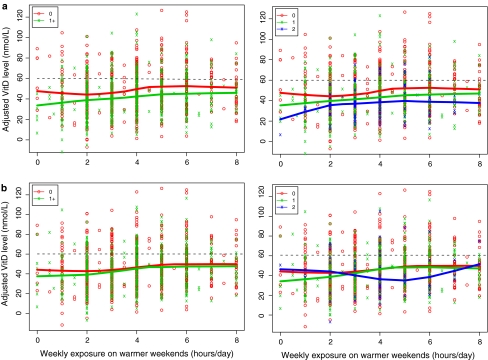
We looked at the effects of inherited variation in SNPs in three genes reported to be associated with vitamin D levels. Serum vitamin D levels were an estimated 5.79 units lower in those carrying 1 copy of rs2282679 (p = <0.0001) and 10.8 units lower in those carrying two copies of the minor allele (p = <0.0001) compared with homozygotes for the common allele (Table 2). Figure 2 illustrates the relationship between genotype, average hours of weekend sun exposure in warmer months and serum levels, and it is seen that the levels were related to number of minor alleles inherited. Inheritance of the minor allele of rs7944926 was also associated with lower serum levels, although this only reached the 5% significance level (adjusted estimates 2.50 lower, p = 0.05, for 1 copy and 6.0 lower, p = 0.03, for two copies, compared with no copies). Inheritance of the minor allele of rs3829251, which is in strong LD with rs7944926 (r 2 = 0.49, D′ = 0.97), showed a similar pattern of association (data not shown). There was no clear evidence of association between rs10741657 and serum levels in cases, or cases and controls combined (adjusted estimate −0.84, p = 0.5, for 1 copy of the minor allele, and 3.2, p = 0.07, for 2 copies, compared with none). However, there was some evidence of an association in the controls (adjusted estimate 5.05, p = 0.04 for 1 copy, 9.03, p = 0.01 for two copies compared with none).
Fig. 2.
Influence of SNP genotype and sun exposure on vitamin D levels. For each subject, recent weekend sun exposure in warmer months is plotted against adjusted serum vitamin D levels for the cases by presence of rare alleles for two SNPs under a dominant model (0, 1+) or as three distinct genotypes (0, 1, 2); a rs2282679, b rs7944926. LOESS curves are plotted to show how vitamin D levels vary with exposure for each group. Vitamin D levels adjusted by BMI, age, season the sample was taken, sex, case–control status, vitamin D supplementation, and Townsend score
The presence of red hair and a tendency to burn in the sun is largely a result of inherited variation in the gene coding for the melanocortin 1 receptor (MC1R). No relationship was seen, however, between MC1R genotype and serum vitamin D levels: for inheritance of two “R” variants which predict red hair most strongly or one “R” variant and one “r” variant, the estimate of the effect on serum levels was 0.87, p = 0.7 (data not shown), compared to inheritance of two wild-type alleles. No relationship was seen for other combinations of “R” or “r” alleles.
We looked at the proportion of the variance in serum vitamin D levels explained by the factors studied (Table 3) because of potential clinical relevance of the results. Since we only have supplementation data available for the cases, our analysis is based largely on this subset of the data although we show data additionally for controls. The factors that explained most of the variance in blood serum levels in analyses adjusted for age, sex, gender, Townsend score, season and case–control status were a measure of total sun exposure summating weekend, daily and holiday sun exposure (5.2%), dietary supplementation (6%) and inherited genotype of the SNP coding for the vitamin D-binding protein (4%). Of the different types of sun exposure investigated, average holiday exposure at lower latitudes explained the highest proportion (3.7%) of the variance. On average, participants who were homozygous for the variant allele in the gene coding for the vitamin D-binding protein (rs2282679) had mean seasonally adjusted serum vitamin D levels of 11.8 nmol/L lower than those wild type for this gene (Table 4). When the data were stratified by exposures shown to have a marked effect on seasonally adjusted vitamin D levels, genotype for this gene appeared to be most strongly associated with supplementation. When participants were supplementing and were wild type, their blood levels were 18.8 nmol/L on average higher than those who were homozygous for the rare variant. In those reporting on average of more than 5 h in the sun on warm weekends, there was a mean difference of 14.7 nmol/L in levels for homozygotes.
Table 3.
Proportion of variation explained (r 2) by environmental and genetic determinants for seasonally adjusted levels of vitamin D
| Factor | Seasonally adjusted only r 2 (%) | Adjusted r 2 (%) | Adjusted also with supplementation r 2 (%) | Seasonally adjusted only r 2 (%) | Adjusted r 2 (%) | Seasonally adjusted only r 2 (%) | Adjusted r 2 (%) |
|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases + controls | |||||
| BMI | 1.0 | – | – | 2.1 | – | 1.3 | – |
| Sex | 0.06 | – | – | 0.2 | – | 0.02 | – |
| Age (per year) | 2.1 | – | – | 0.03 | – | 1.4 | – |
| Townsend | 0.5 | – | – | 0.3 | – | 0.6 | – |
| Case–control status | – | – | – | 1.0 | – | 0.7 | – |
| Supplementation | 7.0 | 6.0 | – | – | – | – | |
| Sun sensitivity index | 0.6 | 0.5 | 0.9 | 0.3 | 0.2 | 0.6 | 0.4 |
| Nevus number | 0.4 | 0.5 | 0.4 | 3.2 | 2.8 | 0.3 | 0.8 |
| Freckling total (%) | 0.9 | 0.8 | 0.9 | 4.0 | 4.8 | 1.3 | 1.4 |
| Freckling shoulders (%) | 1.9 | 2.0 | 1.9 | 5.7 | 6.2 | 2.4 | 2.7 |
| Sunscreen usage | 0.5 | 0.4 | 0.3 | 3.5 | 3.0 | 0.9 | 0.8 |
| Sun exposure** | – | 5.2 | 5.4 | – | 8.7 | – | 6.0 |
| Daily sun exposure* | 0.4 | 0.8 | 1.0 | 1.3 | 1.2 | 0.6 | 1.0 |
| Weekend sun exposure* | |||||||
| Overall* | 2.3 | 1.7 | 2.1 | 5.1 | 5.1 | 3.2 | 2.4 |
| Weekend exposure cooler months* | 1.4 | 1.1 | 1.1 | 1.7 | 1.9 | 1.7 | 1.4 |
| Weekend exposure warmer months* | 2.0 | 1.5 | 2.0 | 6.7 | 6.7 | 3.1 | 2.5 |
| Holiday exposure* | |||||||
| Overall* | 2.0 | 1.6 | 1.8 | 2.3 | 2.7 | 2.3 | 2.0 |
| Holiday exposure lower than 45o* | 3.6 | 3.7 | 3.6 | 2.4 | 2.5 | 3.2 | 3.3 |
| rs_2282679 | 3.9 | 4.0 | 4.9 | 2.6 | 1.8 | 3.3 | 3.1 |
| rs_7944926 | 0.6 | 0.9 | 1.0 | 1.2 | 1.1 | 0.5 | 0.6 |
| rs_10741657 | 0.7 | 0.9 | 0.8 | 2.3 | 2.4 | 0.4 | 0.5 |
Adjusted models were corrected for age, sex, case–control status, vitamin D supplementation(where appropriate), BMI, and Townsend score
* Corrected for sun sensitivity status in addition to season, age, sex, vitamin D supplementation, BMI, and Townsend score in multivariable models
** Model includes daily sun exposure, weekend sun exposure in warmer months, weekend sun exposure in cooler months, holiday sun exposure and holiday sun exposure at low latitudes and is also corrected for sun sensitivity
Table 4.
Mean seasonally adjusted vitamin D levels stratified by rs2282679 (GC protein) genotype
| Mean vitamin D level (SD) | TT | GT | GG |
|---|---|---|---|
| 49.6 (21.4) | 43.7 (20.2) | 37.8 (16.8) | |
| Weekend exposure in warmer months (h/day) | |||
| ≤3 | 44.7 (20.8) | 40.0 (19.5) | 34.7 (14.4) |
| 3–5 | 51.3 (20.8) | 45.6 (20.5) | 40.2 (18.7) |
| >5 | 54.2 (22.4) | 48.1 (19.6) | 39.5 (18.7) |
| Holiday exposure <45N (h) | |||
| 0 | 47.2 (21.1) | 37.9 (18.0) | 35.5 (14.8) |
| 0–87.5 | 49.8 (21.3) | 45.8 (20.0) | 41.5 (17.8) |
| >87.5 | 52.8 (21.7) | 52.3 (20.8) | 36.7 (18.9) |
| Supplementation | |||
| No | 44.9 (20.1) | 38.9 (19.0) | 33.9 (14.5) |
| Yes | 56.7 (20.3) | 52.4 (19.1) | 37.9 (16.4) |
| Sensitivity | |||
| Non-sun-sensitive | 50.8 (21.7) | 45.6 (20.4) | 41.7 (17.1) |
| Sun-sensitive | 48.5 (21.2) | 41.9 (19.9) | 33.9 (15.8) |
Inheritance of less common variants in rs 2282679 (vitamin D-binding protein) is associated with lower levels of vitamin D. Here we show the effects of exposures moderating seasonally adjusted vitamin D level and the differences in levels achieved as a result of those exposures by genotype. The adjusted estimate assumes that blood was drawn in winter
Discussion
Vitamin D is recognized to be important for health overall [23, 24] and cancer prevention [3]. A recent meta-analysis found an association between increased vitamin D intake and decreased breast cancer risk [25]. Rhee et al. [26] also recently reviewed studies of colorectal cancer and concluded that prospective studies showed fairly uniform reduction in risk in relation to higher vitamin D levels. We have previously reported that serum vitamin D levels in UK melanoma patients are low and that low vitamin D levels are associated with both thicker tumors at diagnosis and survival even when stratified for thickness [16]. It is important therefore to understand the determinants of serum vitamin D levels in cancer patients.
A strength of this study is that uniquely we report detailed sun exposure data in conjunction with measured phenotypes of relevance to behaviors in the sun, dietary supplementation and inherited SNPs postulated to influence vitamin D levels. The main weaknesses of the study are a lack of supplementation data and serum vitamin D measures in all of the controls. The data were collected from melanoma patients and healthy individuals, and therefore, the conclusions should be extrapolated to other populations with caution.
The relationship between sun exposure and melanoma risk is complex, in that sunburn and sunny holidays are associated with increased risk of melanoma [27, 28], yet occupational exposure appears to be associated with a reduced risk [27]. Our recent observation that regular weekend sun exposure was protective for melanoma [29] is supportive of the view that vitamin D could have a role in the prevention of melanoma. The observation reported above that there was a statistically significant lower level of vitamin D in cases at diagnosis than in their sibling controls might be supportive of that view, but the data are based upon sampling after diagnosis so must be interpreted with caution.
Table 3 shows the proportion of variance in levels explained by genotype and phenotype and examination of the differences between cases and controls. It can be seen that in controls, a greater proportion is explained by phenotypic variables such as nevus number than in cases, and a greater proportion by weekend sun exposure than in cases. We previously reported that sunburn (which was most associated with sunny holidays) was a risk factor for melanoma, yet weekend sun exposure was protective [29]. So the difference in the effect of weekend sun exposure on vitamin D levels between cases and controls was expected. The significance of the other differences including nevus count is, however, difficult to interpret especially as we cannot allow for the effects of supplementation in the controls, and the total number of controls was much lower than cases, so that the relatively small differences between cases and controls could simply be a function of sample size.
It is recognized that blood levels of vitamin D are sub-optimal in many populations, even surprisingly in Australia [30]. Our data show that in the UK, obesity (BMI > 30) is associated with lower levels of vitamin D, as is widely reported. We also showed that increasing age was associated with higher levels, although older age groups in other studies from around the world have been reported to have lower levels of vitamin D [31]. Indeed higher levels of supplementation are commonly recommended for older age groups [32]. Some proportion of the reported lower vitamin D levels in the elderly in other studies may be related to reduced mobility and therefore reduced access to sun exposure [4], and we would not have identified this sub-population in our study of essentially mobile recruits. A previous UK study in healthy female twins also showed increased levels with age, although this did not reach statistical significance [7]. In this study, therefore, we have shown that in the UK individuals who are not housebound do not appear to show a reduction in vitamin D levels with age, and indeed, there was evidence of the opposite.
Sun exposure explained the greatest proportion of the variance in levels in cases as expected. In a large study called “Expolis” [33], in which time spent outdoors was estimated in randomly selected people living in seven European countries, the mean and median times per day outdoors was 1.68 and 1.38 h, respectively. This suggests that on average, the duration spent outside is less than that shown in our study to be associated with optimal levels of vitamin D (around 6 h/day weekend exposure in warmer months). Our data are therefore consistent with published data suggesting that vitamin D levels are consistently low in many studies worldwide, that is, our data are supportive of the view that the majority of the cases and controls in this study did not have sufficient sun exposure to result in optimal levels in the blood. It is of note moreover that for sun-sensitive people, there was a weaker relationship between weekend sun exposure and vitamin D levels (Fig. 1), and that overall sun sensitivity was associated with lower vitamin D levels which is consistent with previous studies [7, 34]. The lower levels in the most sun-sensitive individuals are postulated to be behavioral, and indeed a lower proportion of the variance in levels was associated with holiday sun exposure (1.6% compared with 2.6% in the non-sensitive, data not shown). Although sunny holidays, particularly at low latitudes, were associated with higher vitamin D levels in controls, overall the association was weaker. One interpretation of the data presented in Table 3 is that melanoma patients achieved more vitamin D synthesis as a result of holiday sun exposure than controls, who achieved more as regular weekend sun exposure, and this indeed may reflect the etiological relationship between holiday sun exposure and melanoma risk.
We found no evidence that regular use of high SPF sunscreen reduces vitamin D levels as reported by other studies (reviewed by Springbett [35]). Springbett’s conclusion was that although sunscreens have the potential to reduce vitamin D synthesis, in practice they do not. In the overall analysis, however, use of low SPF sunscreen did seem to be associated with higher levels. We postulated that the higher vitamin D levels might have resulted from participants using low SPF sunscreen in conjunction with sun-seeking behaviors. We see evidence to support this in our data; the median hours/year of sun exposure on holiday increased from 70 in those who used no sunscreen or high SPF sunscreen to 84 in low SPF users (data not shown). In the case-only comparison, however, this effect disappeared so that the significance of this effect remains unclear.
A strong predictor of serum levels was inheritance of the SNP in the gene coding for vitamin D-binding protein, (GC) rs2282679. The data suggest an additive effect on levels, and Fig. 2 shows evidence that sun exposure increases levels consistently, but the genotype influences blood levels reached. This supports evidence in similar previous studies suggesting an association between variants in the gene coding for vitamin D-binding protein and serum vitamin D levels [10, 11, 36–39]. Recent individual genome-wide association studies have associated these SNPs with serum vitamin D levels [10, 11], and our results provide independent confirmation that variation in the NADSYN1/DHCR7 region may influence vitamin D levels. We showed no relationship between the inheritance of variation at CYP2R1 rs10741657 and vitamin D levels in cases; however, we did see some evidence of association in controls. SNPs at this locus have been inconsistently associated with serum levels [10, 11, 40]. Carriers of two minor alleles of rs2282679 (8% in this study overall) had levels on average 11.8 nmol/L lower than wild-type homozygotes. This suggests that people will vary in how readily they respond to sun exposure and will need different levels of supplementation to obtain optimal serum levels.
The optimal level of vitamin D remains unclear, but levels of around 60–75 nmol/L are associated with a plateau in parathyroid hormone level [41], with reduced risk of cardiovascular disease [15] and optimal survival from breast cancer [14]. Taking a level of at least 60 nmol/L as optimal, our data suggest that these levels are only achieved, on average, by those with sun-sensitive phenotypes, when they take supplements. It is hypothesized that these very fair-skinned people were unable to sustain enough sun exposure to synthesize sufficient vitamin D while protecting themselves from sunburn. Optimal levels were reached in a much higher proportion of individuals, irrespective of phenotype, if they took supplements rather than relying on sun exposure alone. There was moreover little evidence that supplement takers had excessively high levels even if they had high sun exposure (Fig. 1).
In summary, we have shown that regular weekend and holiday sun exposure is associated with higher blood levels of vitamin D although optimal levels appeared to occur as a result of weekend exposure only when the participants reported exposure in the order of 6 h/day, so that overall the majority of participants in the study had sub-optimal levels. Photobiology studies have suggested that comparatively little sun exposure is sufficient to synthesize enough vitamin D, but the high prevalence of measured insufficiency in other studies suggests that in practice, this is an underestimate and this study would support that theory. Diffey recently also argued that the nature of casual sun exposure is insufficient to maintain adequate vitamin D in the modern world [42], and a UK photobiology study recently concluded that supplementation would have to be considered if year-round levels of vitamin D in excess of 60 nmol/L were required [43]. Pros and cons of sun exposure versus supplementation have been argued and were eloquently discussed by Lucas and Ponsonby [44], but in this study supplementation was associated with higher blood levels irrespective of sun exposure, and our data support its use especially in sun-sensitive individuals and melanoma patients. The marked relationship between inherited variation in the gene coding for vitamin D-binding protein, high BMI and blood levels suggests that some individuals will find it more difficult to achieve optimal levels than others and argues for measurement of serum levels after supplementation in the deficient.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The collection of samples in the Melanoma Cohort Study was funded by Cancer Research UK (project grant C8216/A6129, program awards C588/A4994 and C588/A10589) and Centre Award (C37059/A11941) and by the NIH (R01 CA83115). Recruitment was facilitated by the UK National Cancer Research Network. Julian H. Barth and Helen P. Field from the Department of Clinical Biochemistry at Leeds Teaching Hospitals Trust carried out the measurement of serum vitamin D. Patricia Mack, Elaine Fitzgibbon and Kate Gamble collected data for the studies. Paul King carried out data entry. Dr. Amy Downing of NYCRIS provided cancer registry data. The following recruited patients to the studies: Mr. J. Ausobsky, Yorkshire Clinic; Dr. A. Carmichael, Mr. M. Coady, Dr. S. Shehade, Mr. H. Siddiqui, Mr. K. Allison, Mr. K. Erdinger, Mr. Ramanathan, Mr. Toby Muir, James Cook University Hospital; Dr. A. S. Highet, Mr. K. R. Mannur, Mr. M. Telfer, Dr. K. Thomson, Mr. G. Miller, Mr. J. M. Hayward, Mr. J. Taylor, Mr. A. Coatesworth, Dr. A. E. Myatt, Dr. J. Schofield, Dr. Callum Lyon, Dr. J. M. Stainforth, York District Hospital & Scarborough Hospital; Dr. Alison Layton, Harrogate District Hospital; Dr. Anthony Maraveyas, Dr. S. Walton, Dr. N. Alexander, Mr. Alistair Platt, Mr. N. B. Hart, Mr. P. M. O’Hare, Mr. P. Stanley, Mr. M. Riaz, Mr. Ramakrishnan, Castle Hill Hospital and Princess Royal Hospitals Hull; Dr. D. Seukeran, Friarage Hospital; Dr. Bruce Pollock, Dr. E. D. A. Potts, Dr. S. Clark, Dr. S. MacDonald Hull, Mr. Le Roux Fourie, Miss. O M B Austin, Mr. S. Southern, Mr. O. M. Fenton, Mr. S. Majumder, Mr. A. R. Phipps, Pinderfields Hospital; Dr. D. Cowan, Dr. H. Hempel, Dr. J. Holder, Dr. M. Cheesbrough, Mr. D. Sutton, Huddersfield Royal Infirmary, Dr. I. Barbar, Dr. H. Galvin, Calderdale Royal Hospital, Dr. J. A. A. Langtry, Dr. S. Natarajan, Dr. Verlangi, Sunderland Royal Hospital; Mr. P. Baguley, Middlesbrough General Hospital; Mr. R. B. Berry, Mr. R. Debono, Mr. M. Erdmann, Mr. N. McLean, Mr. S. Rao, University Hospital North Durham; Mr. S. L. Knight, Mr. C. Fenn, Dr. M. Marples, Mr. Mark Liddington, Mr. S. Kay, Mr. H. Peach, Mr. A. Batchelor, Dr. M. Cronk, Dr. R. Sheehan-Dare, Dr. M. Goodfield, Dr. P. Patel, Dr. V. Goulden, Dr. A. Humphreys, Mr. K. Horgan, Mr. Chips Browning, Dr. Graeme Stables, Dr. S. Sommer and Dr. S. M. Wilkinson, Leeds Teaching Hospitals Trust; Dr. A. Wright, Mr. S. Al Ghazal, Mr. S. F. Worrall, Mr. R. M. Antrum, Mr. Ivan Foo, Mr. D. Watt, Dr. K. London, Dr. D. J. Barker, Prof D. T. Sharpe, Mr. M. Timmons, Bradford Royal Infirmary and Airedale Hospital; Dr. G. P. Ford, Dr. G. Taylor, Dr. M. Shah, Dewsbury and District Hospital. These pathologists also assisted: Dr. A. Clarke, York District Hospital; Dr. A. Gledhill, Harrogate District Hospital; Dr. P. A. Burgess, Dr. A. Roy, Hull Royal Infirmary; Dr. S. Edwards, Dr. A. Boon and Dr. Will Merchant, Leeds Teaching Hospitals NHS Trust; Dr. S. Nagara, and Dr. H. Cochrane, Sunderland Royal Hospital; Dr. P. Barrett, University Hospital North Durham; Dr. D. Henderson, Friarage Hospital; Dr. J. J. O’Dowd Airedale General Hospital; Dr. P. Batman, Bradford Royal Infirmary; Dr. P. W. Gudgeon and Dr. U. Raja and Dr. I. W. C. MacDonald, Dewsbury/Pontefract; Dr. G. D. H. Thomas, Huddersfield Royal Infirmary; Dr. A. Padwell, Calderdale.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- OR
Odds ratio
- CI
Confidence intervals
- SNP
Single-nucleotide polymorphism
References
- 1.Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009;4:1515–1522. doi: 10.2215/CJN.02260409. [DOI] [PubMed] [Google Scholar]
- 2.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102:156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burleigh E, Potter J. Vitamin D deficiency in outpatients—a Scottish perspective. Scott Med J. 2006;51:27–31. doi: 10.1258/rsmsmj.51.2.27. [DOI] [PubMed] [Google Scholar]
- 5.Azizi E, Pavlotsky F, Vered I, Kudish AI. Occupational exposure to solar UVB and seasonal monitoring of serum levels of 25-hydroxy vitamin D3: a case-control study. Photochem Photobiol. 2009;85:1240–1244. doi: 10.1111/j.1751-1097.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 6.Dawson-Hughes B. Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr. 2004;80:1763S–1766S. doi: 10.1093/ajcn/80.6.1763S. [DOI] [PubMed] [Google Scholar]
- 7.Glass D, Lens M, Swaminathan R, Spector TD, Bataille V. Pigmentation and vitamin D metabolism in Caucasians: low vitamin D serum levels in fair skin types in the UK. PLoS One. 2009;4:e6477. doi: 10.1371/journal.pone.0006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randerson-Moor JA, Taylor JC, Elliott F, et al. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer. 2009;45:3271–3281. doi: 10.1016/j.ejca.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adami S, Bertoldo F, Braga V, et al. 25-Hydroxy vitamin D levels in healthy premenopausal women: association with bone turnover markers and bone mineral density. Bone. 2009;45:423–426. doi: 10.1016/j.bone.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downing A, Newton-Bishop JA, Forman D. Recent trends in cutaneous malignant melanoma in the Yorkshire region of England; incidence, mortality and survival in relation to stage of disease, 1993–2003. Br J Cancer. 2006;95:91–95. doi: 10.1038/sj.bjc.6603216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchrest BA. Sun exposure and vitamin D sufficiency. Am J Clin Nutr. 2008;88:570S–577S. doi: 10.1093/ajcn/88.2.570S. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton-Bishop JA, Beswick S, Randerson-Moor J, et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falchi M, Bataille V, Hayward NK, et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat Genet. 2009;41:915–919. doi: 10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton-Bishop JA, Chang YM, Elliott F, et al. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur J Cancer. 2011;47:732–741. doi: 10.1016/j.ejca.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TK, Rivers JK, Gallagher RP. Site-specific protective effect of broad-spectrum sunscreen on nevus development among white schoolchildren in a randomized trial. J Am Acad Dermatol. 2005;52:786–792. doi: 10.1016/j.jaad.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the North. London, New York: Croome Helm; 1988. [Google Scholar]
- 21.Newton-Bishop JA, Chang YM, Iles MM. Melanocytic nevi, nevus genes, and melanoma risk in a large case-control study in the United Kingdom. Cancer Epidemiol Biomarkers Prev. 2010;19:2043–2054. doi: 10.1158/1055-9965.EPI-10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Downing A, Yu XQ, Newton-Bishop J, Forman D. Trends in prognostic factors and survival from cutaneous melanoma in Yorkshire, UK and New South Wales, Australia between 1993 and 2003. Int J Cancer. 2008;123:861–866. doi: 10.1002/ijc.23495. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 24.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11:7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121:469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 26.Rhee HV, Coebergh JW, Vries ED. Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur J Cancer Prev. 2009;18:458–475. doi: 10.1097/CEJ.0b013e32832f9bb1. [DOI] [PubMed] [Google Scholar]
- 27.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Chang YM, Barrett JH, Bishop DT, et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol. 2009;38:814–830. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton-Bishop JA, Chang YM, Elliott F, et al. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur J Cancer. 2011;47:732–741. doi: 10.1016/j.ejca.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Mei IA, Ponsonby AL, Engelsen O, et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115:1132–1139. doi: 10.1289/ehp.9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 32.Huotari A, Herzig KH. Vitamin D and living in northern latitudes—an endemic risk area for vitamin D deficiency. Int J Circumpolar Health. 2008;67:164–178. doi: 10.3402/ijch.v67i2-3.18258. [DOI] [PubMed] [Google Scholar]
- 33.Rotko T, Oglesby L, Kunzli N, Jantunen MJ. Population sampling in European air pollution exposure study, EXPOLIS: comparisons between the cities and representativeness of the samples. J Expo Anal Environ Epidemiol. 2000;10:355–364. doi: 10.1038/sj.jea.7500101. [DOI] [PubMed] [Google Scholar]
- 34.Malvy DJ, Guinot C, Preziosi P, et al. Relationship between vitamin D status and skin phototype in general adult population. Photochem Photobiol. 2000;71:466–469. doi: 10.1562/0031-8655(2000)071<0466:RBVDSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Springbett P, Buglass S, Young AR. Photoprotection and vitamin D status. J Photochem Photobiol B. 2010;101:160–168. doi: 10.1016/j.jphotobiol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Ahn J, Albanes D, Berndt SI, et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis. 2009;30:769–776. doi: 10.1093/carcin/bgp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y, van Meurs JBJ, Arp P, et al. Vitamin D binding protein genotype and osteoporosis. Calcified Tissue Int. 2009;85:85–93. doi: 10.1007/s00223-009-9251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos-Lopez E, Bruck P, Jansen T, Herwig J, Badenhoop K. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes-Metab Res. 2007;23:631–636. doi: 10.1002/dmrr.719. [DOI] [PubMed] [Google Scholar]
- 41.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Diffey BL. Is casual exposure to summer sunlight effective at maintaining adequate vitamin D status? Photodermatol Photoimmunol Photomed. 2010;26:172–176. doi: 10.1111/j.1600-0781.2010.00518.x. [DOI] [PubMed] [Google Scholar]
- 43.Webb AR, Kift R, Durkin MT, et al. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br J Dermatol. 2010;163:1050–1055. doi: 10.1111/j.1365-2133.2010.09975.x. [DOI] [PubMed] [Google Scholar]
- 44.Lucas RM, Ponsonby AL. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006;92:140–149. doi: 10.1016/j.pbiomolbio.2006.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.