Abstract
The essential trace element zinc (Zn) is widely required in cellular functions, and abnormal Zn homeostasis causes a variety of health problems that include growth retardation, immunodeficiency, hypogonadism, and neuronal and sensory dysfunctions. Zn homeostasis is regulated through Zn transporters, permeable channels, and metallothioneins. Recent studies highlight Zn’s dynamic activity and its role as a signaling mediator. Zn acts as an intracellular signaling molecule, capable of communicating between cells, converting extracellular stimuli to intracellular signals, and controlling intracellular events. We have proposed that intracellular Zn signaling falls into two classes, early and late Zn signaling. This review addresses recent findings regarding Zn signaling and its role in physiological processes and pathogenesis.
Keywords: Zinc, Signaling, Zinc transporter, Metallothionein, Disease
Introduction
The presence of zinc (Zn) was discovered in Aspergillus niger, the common bread mold, in the nineteenth century [1]. Zn was not recognized as indispensable for human life for almost another 100 years until the important discovery by Prasad et al. [2]. Although Zn salts and Zn-related compounds are normally colorless, unlike those of metals such as copper and iron, making a biological study of Zn more difficult, recent advances in life science research have contributed to unfolding its basic requirement for mammalian life [3], including the fact that Zn is pivotal for mammalian oocytogenesis, even before conception [4, 5].
The essential trace element Zn is a structural constituent in numerous proteins, including growth factors, cytokines, receptors, enzymes, and transcription factors belonging to cellular signaling pathways, and is essential for their biological activity [6, 7]. Emphasizing Zn’s physiological relevance to life, a human genome bioinformatics study revealed that approximately 10% of all proteins may bind with Zn [8]. The biological functions of these Zn-binding proteins would be maintained through cellular Zn levels, which are tightly controlled by Zn transporters and channels, and by Zn-sensing molecules such as metallothioneins (MTs) and metal-responsive-element-binding transcription factor-1 [9–14] (Fig. 1).
Fig. 1.
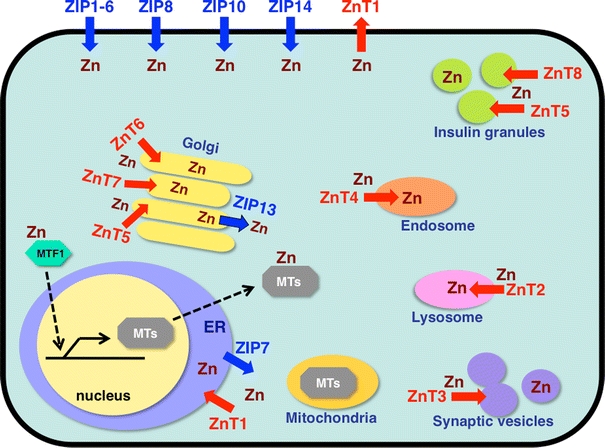
Subcellular localization of zinc (Zn) transporters and metallothioneins (MTs). Localization and potential functions of Zn transporters from the Slc39/Zrt/Irt-like protein (ZIP) (blue) and Slc30/ZnT (red) families, MT, and metal-responsive-element-binding transcription factor 1 (MTF1) within the cell, based on currently available information [30, 148–154]. Arrows show the predicted direction of Zn mobilization. ER endoplasmic reticulum
Although many studies have focused on Zn homeostasis and its biological relevance, recent advances in cell biology and chemistry have highlighted the existence and activity of free or labile Zn in cellular responses, particularly its neurotransmitter activity in synaptic vesicles [15, 16]. Dynamic changes in Zn levels in the brain correlating to physiological experiences and long-term memories have been documented [17, 18], suggesting that free Zn is closely involved in neurotransmitter functions. There is increasing evidence that Zn not only acts as a neurotransmitter to mediate intercellular communication, but also acts as an intracellular signaling molecule, much like calcium (Ca) [19]. Our observation that nuclear retention of the Zn-finger transcription factor Snail requires the Zn transporter Zrt/Irt-like protein (ZIP) 6/Liv1, which in the zebrafish gastrula organizer depends on signal transducers and activators of transcription 3 (STAT3) activation (Fig. 2a) [20], led to a hypothesis that Zn acts as an intracellular signaling molecule. In this case, intracellular Zn levels might change in response to extracellular stimuli through changes in Zn transporter expression, affecting the activation status of several intracellular signaling molecules, including Snail. There is growing evidence that Zn mediated by Zn transporters contributes to the regulation of intracellular signaling pathways (Fig. 2a–e), as we will discuss shortly.
Fig. 2.
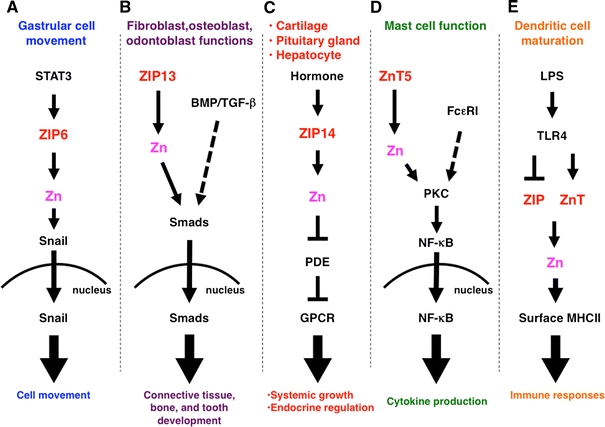
Roles of ZIP and ZnT Zn transporter family members in intracellular signaling. a The signal transducers and activators of transcription 3 (STAT3) downstream target ZIP6 is required for nuclear translocation of the Zn-finger transcription factor Snail, which regulates gastrular cell movement in zebrafish. b ZIP13 is required for the nuclear translocation of Smads in bone morphogenetic protein (BMP)/transforming growth factor beta (TGF-β) signaling, and is involved in tooth, bone, and connective tissue development. c ZIP14, which facilitates G protein-coupled receptor (GPCR) signaling by inhibiting hormone-stimulated phosphodiesterase (PDE) in the pituitary gland, liver, and cartilage, is required for endocrine reactions and systemic growth. d ZnT5 controls protein kinase C (PKC) translocation to the plasma membrane, leading to nuclear factor kappa B (NF-κB)-mediated cytokine production in mast cells under Fc epsilon receptor I (FcεRI) signaling. e Lipopolysaccharide (LPS) stimulation alters the expression of ZIP and ZnT family Zn transporters, resulting in downregulated intracellular Zn levels, followed by dendritic cell maturation and immune responses. TLR Toll-like receptor
We have proposed classifying intracellular Zn signals into transcription-independent early Zn signaling (EZS) and transcription-dependent late Zn signaling (LZS) [19] (Fig. 3). EZS occurs in the “Zn wave” phenomenon in mast cells, in which Zn levels change rapidly (within several minutes) upon extracellular stimulation [21]. In LZS, the intracellular Zn levels are altered several hours after extracellular stimulation, through changes in Zn transporter expression. Since many cytosolic proteins may have Zn-binding potential, both EZS and LZS are expected to be closely involved in a wide range of physiological responses, including development, immune functions, cancer progression, and hard and connective tissue disorders [19, 22–24].
Fig. 3.
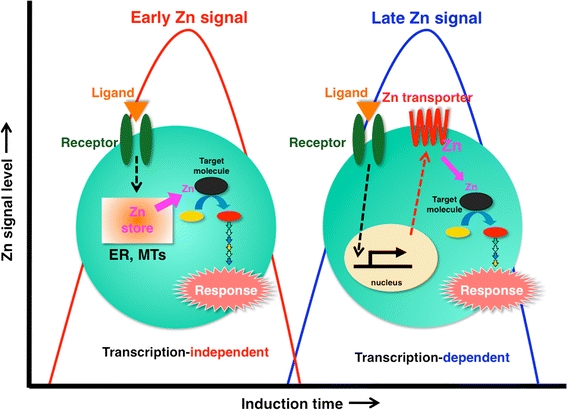
Early and late Zn signaling. Intracellular Zn signaling falls into two types: early Zn signaling (left), in which an extracellular stimulus directly induces elevated Zn levels within several minutes by releasing Zn from a Zn store such as ER or MTs, and late Zn signaling (right), which is induced several hours after stimulation and is dependent on a transcriptional change in Zn transporter expression. Zn waves in mast cells are an example of early Zn signaling (see Fig. 6)
Here, we review new findings on the role of Zn signaling in physiological processes and disease status, and discuss the impact of EZS and LZS on biological events.
Zn transporters and MTs in Zn homeostasis, health, and disease
Zn has wide-ranging effects on cellular functions [25, 26], and imbalances in its homeostasis cause various types of abnormalities in humans and in animal models [2, 15, 27]. The intracellular and extracellular Zn concentration and distribution is controlled by MTs [28], and by Zn transporters of the Slc39/ZIP and Slc30/ZnT families; these transporters increase and decrease, respectively, the cytosolic Zn level [29, 30] (Fig. 1).
Molecular and genetic advances have uncovered the biological significance of Zn transporters. Mice with a targeted disruption of ZIP1, ZIP2, or ZIP3 are sensitive to dietary Zn deficiency during pregnancy [31, 32]. Mutations in the human ZIP4 gene cause the inherited disorder acrodermatitis enteropathica [33, 34], in which the intestine’s ability to absorb dietary Zn is impaired. ZIP13-knockout (KO) mice suffer from disorganization in hard and connective tissues, including bone, teeth, skin, and eyes [23, 24]. ZIP13 is involved in bone morphogenetic protein (BMP)/transforming growth factor beta (TGF-β)-mediated Smad localization to the nucleus [23] (Fig. 2b). In humans, the loss of ZIP13 function causes the spondylocheiro dysplastic form of Ehlers–Danlos syndrome [23, 35]. ZIP14-KO mice exhibit retarded growth and impaired gluconeogenesis, and ZIP14 has been shown to regulate G protein-coupled receptor (GPCR) signaling, including that through the parathyroid hormone receptor 1, growth hormone releasing hormone receptor, and glucagon receptor, by inhibiting phosphodiesterase activity (Fig. 2c) [36]. The lethal milk mutant mice, which have a ZnT4 loss-of-function mutation, suffer from an inherited Zn deficiency in the milk [37]. A similar abnormality, in which a ZnT2 loss-of-function mutation reduces the concentration of Zn in the milk, has been found in a human case [38]. ZnT8 is an islet β-cell-specific Zn transporter that provides Zn to form insulin complexes [39]; its single-nucleotide polymorphism is associated with type 2 diabetes [40], and its deletion is accompanied by impaired insulin secretion [41]. ZnT5-KO mice suffer from growth retardation and osteogenic problems [42], and exhibit impaired cytokine production in mast cells [43] (Fig. 2d).
Molecular and genetic approaches using fruit flies have contributed to our understanding of Zn transporter roles. The Drosophila gene fear of intimacy, which shares similarities with mammalian ZIP6/Liv1 and ZIP10 [44], is essential for proper gonad formation, E-cadherin expression, and glial cell migration [45–47]. Zebrafish Liv1 controls the epithelial–mesenchymal transition under STAT3 activation [20] (Fig. 2a), suggesting that ZIP6 and/or ZIP10 may have critical roles in cell migration; this was also supported by studies using mammalian cells [48]. The Drosophila counterpart of ZIP7, Catsup, controls melanin synthesis [49]. Investigation of the Drosophila breathless mutant, which lacks functional fibroblast growth factor receptors (FGFRs), suggested that Catsup protein facilitates FGFR signaling by inhibiting FGFR protein downregulation [50]. A functional connection between ZIP7 and signaling was proposed by a study using cancer cells; ZIP7 positively regulates tyrosine kinases through Zn inhibition of protein tyrosine phosphatases [51]. These reports indicate that ZIP7’s importance in various biological events may be evolutionarily conserved.
MT molecules are also important in Zn homeostasis [28]; they will be only briefly mentioned here, since they are well described in other articles in this volume. MT was first discovered as a cadmium-binding protein in horse kidneys [52]. Their unique cysteine-rich structure, Zn-binding ability, and expression regulation have drawn many researchers to study MTs, and genetic advances using mouse models have enlarged our understanding of MT activity both in physiological and pathogenic processes such as inflammation. MTs protect against fibrinolytic disturbance and organ damage induced by the bacterial endotoxin lipopolysaccharide (LPS) and ozone (O3) [53]. MTs may protect against allergic inflammation through their antioxidant potential, and by suppressing inflammatory cytokines, including interleukin-1 beta [54]. MT defects may cause severe inflammatory responses in multiple sclerosis, Helicobacter-induced gastritis, and LPS-mediated lung injury [55–57], suggesting that MTs and Zn may coordinate to protect against various inflammatory conditions in mice [58]. Collectively, these reports strongly suggest that MTs and Zn transporters are linked with a wide variety of biological events, disorders, and regulatory functions.
Zn in allergy and immunology
Zn deficiency in humans accompanies many chronic diseases and is frequently a dietary problem. Zn homeostasis experiments using mouse models appear to be ideal for determining the impact of a single element nutritional deficiency on immune function, at both the cellular level and the molecular level [59]. Numerous results from animal models have confirmed that Zn deficiency induces thymic atrophy and lymphopenia, compromises cell- and antibody-mediated responses, and results in increased infection rates and durations [60–64].
Intracellular Zn levels and distribution have been investigated using Zn probes, which enable the imaging of distinct pools of Zn in allergy-related cells. For instance, mast cell granules fluoresce intensely with zinquin [65]. Airway epithelial cells are also rich in Zn [66]. In addition, Zn is found in the intracellular and extracellular matrix and accumulates in skin tissues following injury [67]. Furthermore, Zn accelerates wound healing and the reepithelialization process [68, 69]. It has been reported that Zn deficiency increases allergic eosinophilic inflammation, whereas dietary Zn supplementation attenuates its intensity [70]. Zn deficiency is a risk factor for developing asthma [71, 72]. These reports suggest that Zn is involved in the development of allergic disease. In addition, high levels of ZIP2 messenger RNA (mRNA) are found in the leukocytes of asthmatic infants [73]. However, the precise roles of Zn and Zn transporters in allergy-related cells are not well understood.
We found that Zn is involved in regulating allergic response in mast cells [74]. In these cells, Zn chelators inhibit histamine release, cytokine production, and lipid mediator secretion, and these inhibitory effects are rescued by Zn supplementation. Other metal chelators do not affect mast cell function. These results indicate that Zn is crucial for mast cell degranulation and for cytokine production. Similarly, depleting Zn with a Zn chelator, or with the clinically used heavy-metal chelator 2-3-dimercaptopropane-1-sulfonate, inhibits the mRNA expression of chemokines such as eotaxin in human lung cell lines [75]. Recently, Nishida et al. [43] reported that ZnT5 plays crucial roles in mast cell activation and mast cell-mediated allergic reactions (Fig. 2d). ZnT5 mRNA is highly expressed in mast cells, and its level is enhanced by Fc epsilon receptor I (FcεRI) stimulation, suggesting that ZnT5 is involved in FcεRI-mediated allergic reactions. ZnT5-KO mice have defects in mast cell delayed-type allergic reactions such as contact hypersensitivity, but not in immediate-type reactions such as anaphylaxis [43]. Consistent with this in vivo analysis, ZnT5 is required for FcεRI-mediated cytokine production but not for degranulation in mast cells. In ZnT5-KO mast cells, FcεRI-induced interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) mRNA are reduced. Furthermore, the FcεRI-induced translocation of protein kinase C (PKC) to the plasma membrane and the translocation of nuclear factor kappa B (NF-κB) to the nucleus are impaired in ZnT5-KO mast cells. Thus, ZnT5 is selectively needed for mast cell-mediated delayed-type allergic responses, and is a novel player in PKC/NF-κB signaling. This raises the question, presently unanswered, of how ZnT5 regulates the plasma membrane translocation of PKC. PKC contains Zn-binding motifs in its phorbol 12-myristate 13-acetate binding sites, and Zn is essential to its structure [76, 77]. Mutational analysis shows that PKC’s Zn-binding site is essential for PKC’s translocation to the plasma membrane (Fig. 4), suggesting a crucial link between ZnT5 and PKC activation.
Fig. 4.
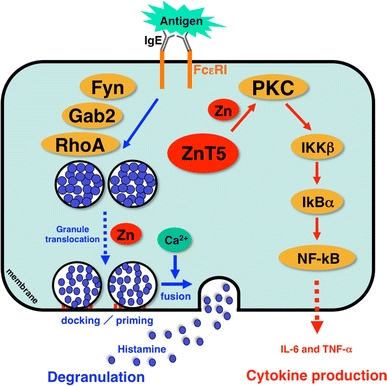
Zn and Zn transporters are indispensable for FcεRI-mediated mast cell activation. Zn is required for multiple steps of FcεRI-induced mast cell activation, including degranulation and cytokine production. Cytosolic Zn regulates the FcεRI-induced granule translocation process, which is mediated by a Fyn/Grb2-associated binder 2 (Gab2)/Ras homologue gene family member A (RhoA)-mediated calcium-independent pathway. Zn and ZnT5 are also required for the translocation of PKC to the plasma membrane and the subsequent nuclear translocation of NF-κB, which leads to the production of cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). IKKβ I-kappa B kinase beta, IkBα I-kappa B alpha
In addition to its role in allergy-related cells, Zn is involved in the control of both dendritic and T cells. The intracellular free Zn concentration decreases in mouse dendritic cells exposed to the endotoxin LPS, which is a Toll-like receptor (TLR) stimulant. Furthermore, artificially depleting intracellular Zn with a Zn chelator triggers dendritic cell maturation. On the other hand, artificially elevating the intracellular Zn level suppresses the dendritic cells’ ability to respond to LPS. LPS stimulation affects Zn transporter expression, effectively increasing (ZIP family) or decreasing (ZnT family) the level of intracellular Zn. Importantly, the ectopic expression of ZIP6, which is reduced by LPS stimulation, suppresses dendritic cell maturation and inhibits CD4+ T cell-stimulatory activity [78]. A similar Zn effect is observed in live animals; LPS injection reduces the intracellular free Zn and ZIP6 mRNA levels in dendritic cells, and treatment with Zn-depleting agents increases dendritic cell maturation [78]. These results show that extracellular stimuli, by affecting Zn transporter expression and thus changing the intracellular Zn levels, play a role in the process of dendritic cell maturation, an important step in T cell activation and immune response (Fig. 2e).
Zn is also involved in T cell function. It has been reported that Zn-supplying compounds such as polaprezinc and Z-103 suppress autoimmune disease models [79, 80], suggesting that Zn might suppress autoimmune disease by inhibiting T cell activation. However, the details of the mechanism behind this suppression are not understood. One of the important questions involved is how Zn affects T helper 17 (Th17) cells, since Th17 cell development is controlled by IL-6-induced STAT3 activation [81–85], and Th17 cells are important in autoimmune diseases such as collagen-induced arthritis (CIA), mutated IL-6 receptor and signal transducer glycoprotein 130 (gp130): Y759F gp130-induced arthritis, and experimental autoimmune encephalomyelitis (EAE) [86, 87]. CIA and EAE are mediated by antigen-specific Th17 cells, whose development is controlled under STAT3 activation. As expected, adding Zn to drinking water significantly suppresses both CIA and EAE development in animal models [88]. Zn suppresses Th17 cell development, which is dependent on the IL-6/STAT3 signaling pathway, during the induction of autoimmune diseases (Fig. 5). In fact, Zn directly inhibits STAT3’s tyrosine phosphorylation by Janus kinase without affecting the phosphorylation ability of Janus kinase proteins. STAT3 protein itself is unfolded by Zn binding, indicating that Zn directly binds STAT3 and inhibits its activation [88].
Fig. 5.
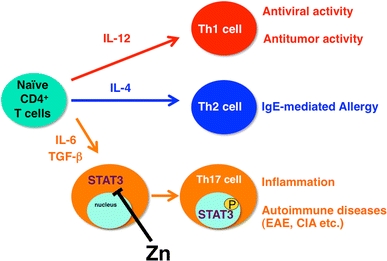
Zn suppresses T helper 17 (Th17) cell development by inhibiting STAT3 activation. Peripheral naive CD4+ T cell precursor cells can differentiate into three subsets of effector T cells (Th1, Th2, and Th17). The differentiation of these subsets is governed by selective cytokines, and each subset accomplishes specialized functions. Th17 cells, which are critical for the development of inflammation and autoimmune disease, are induced by IL-6 and tumor necrosis factor beta (TGF-β). Zn directly binds STAT3, inhibiting its activation by IL-6 and suppressing autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA). P tyrosine phosphorylation
It has also been reported that MTs are involved in asthma and ultraviolet B-induced contact hypersensitivity. Ovalbumin-induced airway inflammation is worse in the MT1 and MT2 double-KO (MT1/2-KO) mice than in wild-type mice. The cellular profile of the bronchoalveolar fluid, lung histology findings, and expression of proinflammatory molecules in the lungs were consistent with increased airway inflammation in MT1/2-KO mice [54]. In another allergy model, moderate daily ultraviolet B doses caused significantly more immunosuppression in MT1/2-KO mice than in wild-type mice [89]. These findings indicate that Zn transporters and Zn-related molecules are indispensably involved in allergic and immune responses and regulate a variety of signaling processes (Figs. 4, 5).
Zn signaling
As recent advances have revealed the existence of free or labile Zn in many physiological situations, Zn has been increasingly recognized as a potential signaling molecule [15, 16, 19].
Zn as a neurotransmitter, or the first messenger in cell–cell communication
An exchangeable Zn ion in the brain was first documented by Maske [90] in 1955. Zn is highly concentrated in synaptic vesicles and is released with glutamate in an activity-dependent manner [91]. Recently, Zn-imaging techniques using fluorescence sensor molecules, which allow the Zn concentration and distribution to be analyzed in real time, have been widely applied [92]. Microfluorescence imaging of Zn secretion showed that Zn is released from hippocampal mossy fiber terminal vesicles into the surrounding milieu upon exocytotic presynaptic stimulation [93–95]. It is then taken up into the cytoplasm of neighboring cells through gated Zn channels. The rapid influx of Zn through Ca-ion-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate/kainate channels triggers the generation of reactive oxygen species (ROS) and is potentially neurotoxic [96].
In the above scenario, Zn’s activity is similar to that of neurotransmitters, which are stored in membrane-enclosed synaptic vesicles, are released by exocytosis, and activate postsynaptic cells through transmitter-gated ion channels [97–99]. Since Zn modulates both the current response (mediated by excitatory and inhibitory neurotransmitter receptors) and the efficacy of transporter-driven neurotransmitter reuptake [100], synaptically released Zn has been proposed to function as an important regulator of synaptic transmission and plasticity [101, 102], or as an “atypical neurotransmitter” [103]. ZnT3 is highly expressed on synaptic vesicle membranes and is essential for loading Zn into synaptic vesicles.
Although ZnT3-KO mice show a loss of stainable Zn in synapses [104], they are behaviorally normal except for an enhanced susceptibility to kainite-induced seizures [105]. Recently, however, it was revealed that the ZnT3-KO mice exhibit age-dependent deficits in learning and memory that are not apparent in young animals. This age-dependent cognitive phenotype might be owing to its decreased level in the hippocampus [106]. In addition, extracellular-signal-regulated kinase 1 and 2 (Erk1/2) activation is reduced in the hippocampal mossy fibers in ZnT3-KO mice, suggesting that ZnT3 is involved in the presynaptic Erk1/2 signaling required for hippocampus-dependent memory [107]. Takeda et al. [17] reported that Zn chelation with clioquinol inhibits hippocampal long-term potentiation and cognitive behavior. This evidence suggests that synaptic Zn acts as a neurotransmitter for maintaining the synaptic environment.
Zn has been identified as an endogenous GPCR 39 (GPR39) agonist, and the concept that GPR39 is the Zn-sensing receptor in the brain is consistent with Zn’s role as a neurotransmitter or first messenger [108, 109]. Intercellular Zn communication mediated through the Zn-sensing GPR39 is also involved in regulating epithelial cell repair [110] and endocrine pancreatic function [111]. Also supporting Zn’s role in neurotransmission, Hosie et al. [112] identified two Zn-binding sites and characterized a third in the γ-aminobutyric acid receptor, using site-directed mutagenesis. In addition, Hirzel et al. [113] produced knock-in mice carrying the mutation D80A (a constructed Zn-binding site) in the glycine receptor alpha1 subunit gene to show that Zn modulates neurotransmission. It is also known that Zn inhibits N-methyl-d-aspartate receptor activity through a dual mechanism, a voltage-dependent channel block and a voltage-independent reduction in the probability of the channel opening [114–116]. Thus, it is likely that Zn acts as a neurotransmitter in addition to its other roles in the neural system.
Zn as an intracellular signaling molecule, or the second messenger
Several intracellular second messengers have been identified, including 3′,5′-cyclic adenosine monophosphate (cAMP), Ca2+, 3′,5′-cyclic guanosine monophosphate (cGMP), nitric oxide (NO), ROS, and lipid mediators [117]. Zn has also appeared as a potential intracellular signaling molecule in mitosis in starfish oocytes [118]. Recently, an essential role for Zn in arresting second metaphase exit has been reported. This suggests that Zn has the potential to act as an intracellular signaling molecule to regulate the developmental program in vertebrate fertilization [4, 5]. It has also been reported that Zn itself affects a variety of signaling molecules, including PKC, Ca/calmodulin-dependent protein kinase II, Erk1/2, cAMP-dependent protein kinase, protein tyrosine phosphatase, and caspase-3 [119–125]. In addition, Zn activates ion channels such as the transient receptor potential ankyrin 1 [126, 127], ATP-sensitive K+ [128], and large-conductance Ca-activated K+ [129] channels. Together, these findings suggest that Zn may function as an intracellular signaling molecule or second messenger, if extracellular stimuli such as cytokines and growth factors cause the intracellular Zn status to change, either independently, or in a manner dependent on the transcriptional changes of Zn transporters or MTs.
We have proposed that Zn signaling can be divided into EZS, which is directly and rapidly induced by an extracellular stimulus, and LZS, which depends on transcriptional changes to MTs, Zn transporters, and other Zn-related molecules [19] (Fig. 3). ZIP6/Liv1 is required for the epithelial–mesenchymal transition in the zebrafish gastrula organizer, because it is essential for the nuclear retention of the E-cadherin repressor Snail (Fig. 2a) [20]. Since ZIP6/Liv1 expression in the zebrafish organizer is dependent on STAT3 activation, any extracellular stimulus regulating STAT3 activation could change the intracellular Zn level by inducing changes in Zn transporters such as ZIP6/Liv1. TLR4 also alters the intracellular free Zn level, which it decreases by inducing changes in Zn transporter expression in dendritic cells (Fig. 2e) [78] and in pulmonary endothelial cells [130]. These observations support the idea that Zn acts as an intracellular second messenger, that is, as a molecule whose intracellular status is altered in response to an extracellular stimulus and that is capable of transducing the extracellular stimulus into an intracellular signaling event.
The role of Zn as a second messenger is further supported by our findings that ZIP13 is required for the BMP/TGF-β-induced nuclear localization of Smad proteins (Fig. 2b) [23], that ZIP14 is involved in GPCR-mediated signal transduction through cAMP basal level regulation (Fig. 2c) [36], and that FcεRI-stimulation-induced PKC activation is dependent on ZnT5 in mast cells (Fig. 2d) [43]. Another example showing that Zn transporters are involved in signaling pathways is that cation diffusion facilitator 1, a structural homologue of ZnT1 in Caenorhabditis elegans, might mediate Raf-1 activation by directly interacting with Raf-1 and removing Zn from the Raf-1 cysteine-rich domain [131]. Thus, extracellular stimuli can affect intracellular signaling pathways by changing the intracellular Zn status through changes in the expression of Zn transporters and other Zn-related molecules; this exemplifies the LZS type (Fig. 3) [19].
EZS, on the other hand, is exemplified by the transcription-independent increase in intracellular Zn level that occurs in mast cells some minutes after extracellular stimulation; we call this phenomenon a “Zn wave” (Fig. 6) [21]. The Zn wave originates from the region of the endoplasmic reticulum and depends on Ca2+ influx and Erk1/2 activation in mast cells (Fig. 6b). Since extracellular Zn is not involved in the Zn wave, and it is induced within several minutes of FcεRI stimulation, Zn here acts as an intracellular signaling molecule (Fig. 6). Using the Zn probe FluoZin-3, Haase et al. [132] revealed that the intracellular Zn level is elevated in peripheral blood mononuclear cells after phorbol 12-myristate 13-acetate stimulation, and in human leukocytes, especially monocytes, with physiological stimulation [133]. In addition, ZIP7 is reported to regulate intracellular Zn on the endoplasmic reticulum membrane [134]. It is also possible that transporters and channels other than ZIP or ZnT family members generate Zn waves in individual cell types with various types of stimulation. In any case, the precise molecular mechanisms generating the Zn wave are still elusive (Fig. 7a).
Fig. 6.
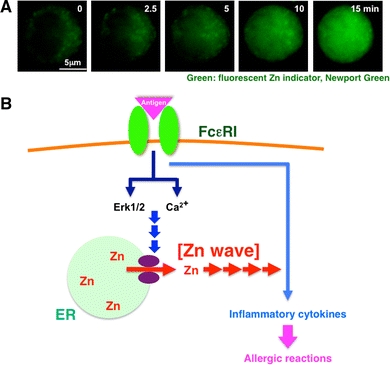
FcεRI increases intracellular free Zn levels, Zn wave: a type of early Zn signaling. a The intracellular Zn level increases within several minutes after antigen stimulation. To monitor the intracellular Zn level, mast cells were treated with the cell-permeable fluorescent Zn indicator Newport Green. Newport Green fluorescence remained steady in the cytoplasm, but gradually increased in the perinuclear and nuclear areas upon FcεRI stimulation. We named this phenomenon a “Zn wave.” b Early Zn signaling in mast cells. Extracellular antigen stimulation induces a Zn wave—a rapid alteration in intracellular Zn level—through the activation of Ca2+ and extracellular-signal-regulated kinase 1 and 2 (Erk1/2) signaling, which might positively affect the signal for inflammatory cytokine-mediated allergic reactions. This suggests that Zn has a role as an intracellular signaling molecule, transducing extracellular stimuli to physiological responses. Violet ovals Zn gatekeepers. (a Modified from Yamasaki et al. [21])
Fig. 7.
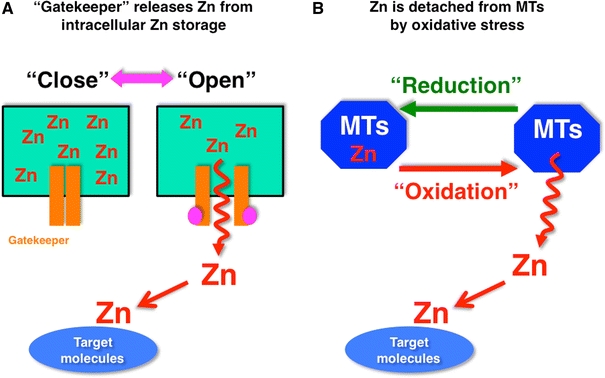
Model for Zn release for the generation of Zn signaling. a Labile Zn in intracellular Zn storage moves through “Zn gatekeepers” into the cytosol, and approaches target molecules. The Zn gatekeepers are closed in the steady state, but are opened by changes in activation status. b Most of the Zn in the cytosol is sequestered in MTs, and is detached from the MTs by intracellular oxidative stress. The rapid increase in intracellular Zn mediated by cellular events may act as an early Zn signaling to change the status of target molecules
Outside intracellular compartments, Zn-buffering proteins such as MTs are believed to be a major source of free Zn released into the cytosol space in response to an extracellular stimulus [135–137]. Zn is rapidly released from MTs by nitrosylation or by thiol ligand oxidation. Ultraviolet A irradiation can induce the generation of ROS, which might oxidize thiols in MTs and induce transient increases in intracellular free Zn level [138]. In pathological situations, N-methyl-d-aspartate receptor activation might induce endogenous NO synthesis and increase intracellular free Zn level by liberating Zn from MTs [139]. The liberation of intracellular Zn and the overactivation of potassium channels were proposed to be important components of nitrosative stress-induced neuronal death [140]. The liberated Zn induces its toxicity to neurons via activation of 12-lipoxygenase and mitogen-activated protein kinase [141]. The NO donor S-nitrosocysteine (SNOC) both increases free Zn level and inhibits LPS-induced tumor necrosis factor alpha and interleukin-1 beta release. This SNOC-induced increase in intracellular free Zn level may therefore mediate SNOC’s inhibitory effect via increased cGMP level [142]. In addition, cytokine stimulation sometimes induces NO production, thereby enhancing the intracellular free Zn level [143]. These reports indicate that Zn released from oxidative-reaction-stimulated MTs contributes to the EZS-type regulation of intracellular phenomena (Fig. 7b).
Conclusion and future prospects
As discussed in this review, Zn is clearly involved in regulating a wide variety of physiological responses. Although most Zn studies have focused on the general maintenance of its homeostasis, recent studies have shown that Zn’s functions are far more extensive than might be implied by its simple definition as an essential nutrient. The intracellular Zn status is dynamically altered in response to a variety of extracellular stimuli, and this change in Zn status is capable of transducing extracellular stimuli into intracellular signaling events. On the basis of the evidence accumulated, we have proposed that Zn acts as a signaling molecule in at least two modes: transcription-independent EZS and transcription-dependent LZS (Fig. 3) [19]. Several important questions remain to be resolved: What extracellular stimulus, in which cells or tissues, regulates the expression of the Zn transporters and channels involved in Zn signaling? How are these Zn transporters or channels activated in response to the extracellular stimulus? How does each Zn transporter specifically regulate the intracellular signaling pathway? How do Zn transporters transfer Zn into their target molecules? Is there any chaperon which transfers Zn from a given transporter to its specific target molecule? What are the target molecules and biological roles of Zn signaling? Another question is how to specifically monitor and visualize intracellular free Zn in vivo with high resolution; this will require the use of chemical compounds [92], multitracer detection devices [144], and two-photon and X-ray fluorescence microscopy [145, 146]. The development of drugs for Zn-related diseases is another emerging avenue of study. Modified Zn-containing pharmaceuticals may speed the discovery of Zn-signaling action points [147]. A deeper understanding of the early and late Zn signals and signaling dysfunctions will provide novel insights into Zn’s roles as a signaling molecule in mammalian health and diseases.
Acknowledgments
We thank Masami Kawamura, Ayumi Ito, and Mayumi Hara for their indispensable support. We also thank Mizuki Shimura and Ryoko Masuda for their secretarial assistance. This work was supported by KAKENHI and the JST-CREST program.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- BMP
Bone morphogenetic protein
- Ca
Calcium
- cAMP
3′,5′-Cyclic adenosine monophosphate
- cGMP
3′-5′-Cyclic guanosine monophosphate
- CIA
Collagen-induced arthritis
- EAE
Experimental autoimmune encephalomyelitis
- Erk1/2
Extracellular signal-regulated kinase 1 and 2
- EZS
Early zinc signaling
- FcεRI
Fc epsilon receptor I
- FGFR
Fibroblast growth factor receptor
- GPCR
G protein-coupled receptor
- GPR39
G protein-coupled receptor 39
- gp130
Glycoprotein 130
- IL-6
Interleukin-6
- KO
Knockout
- LPS
Lipopolysaccharide
- LZS
Late zinc signaling
- mRNA
Messenger RNA
- MT
Metallothionein
- MT1/2-KO
MT1 and MT2 double knockout
- NF-κB
Nuclear factor kappa B
- NO
Nitric oxide
- PKC
Protein kinase C
- ROS
Reactive oxygen species
- SNOC
S-nitrosocysteine
- STAT3
Signal transducers and activators of transcription 3
- TGF-β
Transforming growth factor beta
- TNF-α
Tumor necrosis factor alpha
- Th17
T helper 17
- TLR
Toll-like receptor
- Zn
Zinc
- ZIP
Zrt/Irt-like protein
Footnotes
This article is part of a JBIC special issue on metallothioneins.
References
- 1.Raulin J. Annales des sciences naturelles. Botanique et biologie végétale. 1869;11:93–345. [Google Scholar]
- 2.Prasad AS, Halsted JA, Nadimi M. Am J Med. 1961;31:532–546. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 3.Hershfinkel M, Aizenman E, Andrews G, Sekler I (2010) Sci Signal 3:mr2 [DOI] [PMC free article] [PubMed]
- 4.Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry AC. Development. 2010;137:2659–2669. doi: 10.1242/dev.049791. [DOI] [PubMed] [Google Scholar]
- 6.Vallee BL, Falchuk KH. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Prasad AS. Nutrition. 1995;11:93–99. [PubMed] [Google Scholar]
- 8.Andreini C, Banci L, Bertini I, Rosato A. J Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 9.Eide DJ. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 10.Palmiter RD, Huang L. Pflugers Arch. 2004;447:744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- 11.Vallee BL. Neurochem Int. 1995;27:23–33. doi: 10.1016/0197-0186(94)00165-q. [DOI] [PubMed] [Google Scholar]
- 12.Andrews GK. Biometals. 2001;14:223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- 13.Palmiter RD. Proc Natl Acad Sci USA. 2004;101:4918–4923. doi: 10.1073/pnas.0401022101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtlen P, Schaffner W. Bioessays. 2001;23:1010–1017. doi: 10.1002/bies.1146. [DOI] [PubMed] [Google Scholar]
- 15.Frederickson CJ, Koh JY, Bush AI. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 16.Sensi SL, Paoletti P, Bush AI, Sekler I. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 17.Takeda A, Takada S, Ando M, Itagaki K, Tamano H, Suzuki M, Iwaki H, Oku N. Neuroscience. 2010;171:443–450. doi: 10.1016/j.neuroscience.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Takeda A, Tamano H, Imano S, Oku N. Neuroscience. 2010;168:715–722. doi: 10.1016/j.neuroscience.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T. Adv Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita S, Miyagi C, Fukada T, Kagara N, Che YS, Hirano T. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami M, Hirano T. Cancer Sci. 2008;99:1515–1522. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, Idaira Y, Asada Y, Kitamura H, Yamasaki S, Hojyo S, Nakayama M, Ohara O, Koseki H, Dos Santos HG, Bonafe L, Ha-Vinh R, Zankl A, Unger S, Kraenzlin ME, Beckmann JS, Saito I, Rivolta C, Ikegawa S, Superti-Furga A, Hirano T. PLoS One. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukada T, Asada Y, Mishima K, Shimoda S, Saito I. J Oral Biosci. 2011;53:1–12. [Google Scholar]
- 25.Ibs KH, Rink L. J Nutr. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 26.Telford WG, Fraker PJ. J Cell Physiol. 1995;164:259–270. doi: 10.1002/jcp.1041640206. [DOI] [PubMed] [Google Scholar]
- 27.Rink L, Gabriel P. Proc Nutr Soc. 2000;59:541–552. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- 28.Blindauer CA, Leszczyszyn OI. Nat Prod Rep. 2010;27:720–741. doi: 10.1039/b906685n. [DOI] [PubMed] [Google Scholar]
- 29.Fukada T, Kambe T (2011) Metallomics. doi:10.1039/C1MT00011J [DOI] [PubMed]
- 30.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Mol Cell Biol. 2005;25:5607–5615. doi: 10.1128/MCB.25.13.5607-5615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Genesis. 2006;44:239–251. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 33.Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giunta C, Elcioglu NH, Albrecht B, Eich G, Chambaz C, Janecke AR, Yeowell H, Weis M, Eyre DR, Kraenzlin M, Steinmann B. Am J Hum Genet. 2008;82:1290–1305. doi: 10.1016/j.ajhg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin B-H, Koseki H, Hirano T. PLoS One. 2011;6:e18059. doi: 10.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang L, Gitschier J. Nat Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 38.Chowanadisai W, Lonnerdal B, Kelleher SL. J Biol Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 39.Chimienti F, Favier A, Seve M. Biometals. 2005;18:313–317. doi: 10.1007/s10534-005-3687-9. [DOI] [PubMed] [Google Scholar]
- 40.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 41.Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O’Brien RM. Biochem J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue K, Matsuda K, Itoh M, Kawaguchi H, Tomoike H, Aoyagi T, Nagai R, Hori M, Nakamura Y, Tanaka T. Hum Mol Genet. 2002;11:1775–1784. doi: 10.1093/hmg/11.15.1775. [DOI] [PubMed] [Google Scholar]
- 43.Nishida K, Hasegawa A, Nakae S, Oboki K, Saito H, Yamasaki S, Hirano T. J Exp Med. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kambe T, Suzuki T, Nagao M, Yamaguchi-Iwai Y. Genomics Proteomics Bioinformatics. 2006;4:1–9. doi: 10.1016/S1672-0229(06)60010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathews WR, Ong D, Milutinovich AB, Van Doren M. Development. 2006;133:1143–1153. doi: 10.1242/dev.02256. [DOI] [PubMed] [Google Scholar]
- 46.Pielage J, Kippert A, Zhu M, Klambt C. Dev Biol. 2004;275:245–257. doi: 10.1016/j.ydbio.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 47.Van Doren M, Mathews WR, Samuels M, Moore LA, Broihier HT, Lehmann R. Development. 2003;130:2355–2364. doi: 10.1242/dev.00454. [DOI] [PubMed] [Google Scholar]
- 48.Kagara N, Tanaka N, Noguchi S, Hirano T. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stathakis DG, Burton DY, McIvor WE, Krishnakumar S, Wright TR, O’Donnell JM. Genetics. 1999;153:361–382. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsouna A, Lawal HO, Izevbaye I, Hsu T, O’Donnell JM. Dev Biol. 2007;308:30–43. doi: 10.1016/j.ydbio.2007.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hogstrand C, Kille P, Nicholson RI, Taylor KM. Trends Mol Med. 2009;15:101–111. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Margoshes M, Vallee BL. J Am Chem Soc. 1957;79:4813–4814. [Google Scholar]
- 53.Inoue K, Takano H, Shimada A, Wada E, Yanagisawa R, Sakurai M, Satoh M, Yoshikawa T. FASEB J. 2006;20:533–535. doi: 10.1096/fj.05-3864fje. [DOI] [PubMed] [Google Scholar]
- 54.Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, Hiyoshi K, Sato M, Shimada A, Inoue M, Yoshikawa T. Exp Biol Med (Maywood) 2005;230:75–81. doi: 10.1177/153537020523000110. [DOI] [PubMed] [Google Scholar]
- 55.Penkowa M, Espejo C, Martinez-Caceres EM, Poulsen CB, Montalban X, Hidalgo J. J Neuroimmunol. 2001;119:248–260. doi: 10.1016/s0165-5728(01)00357-5. [DOI] [PubMed] [Google Scholar]
- 56.Tran CD, Huynh H, van den Berg M, van der Pas M, Campbell MA, Philcox JC, Coyle P, Rofe AM, Butler RN. Helicobacter. 2003;8:533–541. doi: 10.1046/j.1523-5378.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- 57.Takano H, Inoue K, Yanagisawa R, Sato M, Shimada A, Morita T, Sawada M, Nakamura K, Sanbongi C, Yoshikawa T. Thorax. 2004;59:1057–1062. doi: 10.1136/thx.2004.024232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue K, Takano H, Shimada A, Satoh M. Mediators Inflamm. 2009;2009:101659. doi: 10.1155/2009/101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prasad AS. Mol Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandes G, Nair M, Onoe K, Tanaka T, Floyd R, Good RA. Proc Natl Acad Sci USA. 1979;76:457–461. doi: 10.1073/pnas.76.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad AS. Am J Clin Nutr. 1991;53:403–412. doi: 10.1093/ajcn/53.2.403. [DOI] [PubMed] [Google Scholar]
- 62.Shankar AH, Prasad AS. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 63.Keen CL, Gershwin ME. Annu Rev Nutr. 1990;10:415–431. doi: 10.1146/annurev.nu.10.070190.002215. [DOI] [PubMed] [Google Scholar]
- 64.Fraker PJ, King LE. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 65.Ho LH, Ruffin RE, Murgia C, Li L, Krilis SA, Zalewski PD. J Immunol. 2004;172:7750–7760. doi: 10.4049/jimmunol.172.12.7750. [DOI] [PubMed] [Google Scholar]
- 66.Truong-Tran AQ, Ruffin RE, Zalewski PD. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1172–L1183. doi: 10.1152/ajplung.2000.279.6.L1172. [DOI] [PubMed] [Google Scholar]
- 67.Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren MS. Wound Repair Regen. 2007;15:2–16. doi: 10.1111/j.1524-475X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 68.Lansdown AB. Lancet. 1996;347:706–707. doi: 10.1016/s0140-6736(96)90072-0. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz JR, Marsh RG, Draelos ZD. Dermatol Surg. 2005;31:837–847. doi: 10.1111/j.1524-4725.2005.31729. [DOI] [PubMed] [Google Scholar]
- 70.Richter M, Bonneau R, Girard MA, Beaulieu C, Larivee P. Chest. 2003;123:446S. doi: 10.1378/chest.123.3_suppl.446s. [DOI] [PubMed] [Google Scholar]
- 71.Riccioni G, D’Orazio N. Expert Opin Investig Drugs. 2005;14:1145–1155. doi: 10.1517/13543784.14.9.1145. [DOI] [PubMed] [Google Scholar]
- 72.Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE. Pharmacol Ther. 2005;105:127–149. doi: 10.1016/j.pharmthera.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Xu TF, Wang XL, Yang JZ, Hu XY, Wu WF, Guo L, Kang LD, Zhang LY. Pediatr Pulmonol. 2009;44:763–767. doi: 10.1002/ppul.21052. [DOI] [PubMed] [Google Scholar]
- 74.Kabu K, Yamasaki S, Kamimura D, Ito Y, Hasegawa A, Sato E, Kitamura H, Nishida K, Hirano T. J Immunol. 2006;177:1296–1305. doi: 10.4049/jimmunol.177.2.1296. [DOI] [PubMed] [Google Scholar]
- 75.Richter M, Cantin AM, Beaulieu C, Cloutier A, Larivee P. Am J Physiol Lung Cell Mol Physiol. 2003;285:L719–L729. doi: 10.1152/ajplung.00406.2002. [DOI] [PubMed] [Google Scholar]
- 76.Corbalan-Garcia S, Gomez-Fernandez JC. Biochim Biophys Acta. 2006;1761:633–654. doi: 10.1016/j.bbalip.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 77.Bernal PJ, Bauer EM, Cao R, Maniar S, Mosher M, Chen J, Wang QJ, Glorioso JC, Pitt BR, Watkins SC, St Croix CM (2011) Am J Physiol Lung Cell Mol Physiol. doi:10.1152/ajplung.00328.2010 [DOI] [PMC free article] [PubMed]
- 78.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 79.Ohkawara T, Takeda H, Kato K, Miyashita K, Kato M, Iwanaga T, Asaka M. Scand J Gastroenterol. 2005;40:1321–1327. doi: 10.1080/00365520510023530. [DOI] [PubMed] [Google Scholar]
- 80.Tran CD, Ball JM, Sundar S, Coyle P, Howarth GS. Dig Dis Sci. 2007;52:2113–2121. doi: 10.1007/s10620-007-9765-9. [DOI] [PubMed] [Google Scholar]
- 81.Bettelli E, Korn T, Kuchroo VK. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirano T. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 83.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 84.Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, Murakami M, Hirano T. Int Immunol. 2007;19:695–702. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- 85.Veldhoen M, Stockinger B. Trends Immunol. 2006;27:358–361. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Bettelli E, Oukka M, Kuchroo VK. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 88.Kitabayashi C, Fukada T, Kanamoto M, Ohashi W, Hojyo S, Atsumi T, Ueda N, Azuma I, Hirota H, Murakami M, Hirano T. Int Immunol. 2010;22:375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 89.Reeve VE, Nishimura N, Bosnic M, Michalska AE, Choo KH. Immunology. 2000;100:399–404. doi: 10.1046/j.1365-2567.2000.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maske H. Klin Wochenschr. 1955;33:1058. doi: 10.1007/BF01467958. [DOI] [PubMed] [Google Scholar]
- 91.Assaf SY, Chung SH. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 92.Kikuchi K. Chem Soc Rev. 2010;39:2048–2053. doi: 10.1039/b819316a. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ. J Neurophysiol. 2001;86:2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- 94.Qian J, Noebels JL. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N, Ikegaya Y. J Cell Biol. 2002;158:215–220. doi: 10.1083/jcb.200204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiss JH, Sensi SL. Trends Neurosci. 2000;23:365–371. doi: 10.1016/s0166-2236(00)01610-6. [DOI] [PubMed] [Google Scholar]
- 97.Xie X, Smart TG. Pflugers Arch. 1994;427:481–486. doi: 10.1007/BF00374264. [DOI] [PubMed] [Google Scholar]
- 98.Hershfinkel M, Moran A, Grossman N, Sekler I. Proc Natl Acad Sci USA. 2001;98:11749–11754. doi: 10.1073/pnas.201193398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colvin RA, Fontaine CP, Laskowski M, Thomas D. Eur J Pharmacol. 2003;479:171–185. doi: 10.1016/j.ejphar.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 100.Smart TG, Hosie AM, Miller PS. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- 101.Lu YM, Taverna FA, Tu R, Ackerley CA, Wang YT, Roder J. Synapse. 2000;38:187–197. doi: 10.1002/1098-2396(200011)38:2<187::AID-SYN10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 102.Vogt K, Mellor J, Tong G, Nicoll R. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 103.Baranano DE, Ferris CD, Snyder SH. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- 104.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Proc Natl Acad Sci USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopantsev V, Wenzel HJ, Cole TB, Palmiter RD, Schwartzkroin PA. Neuroscience. 2003;116:237–248. doi: 10.1016/s0306-4522(02)00570-5. [DOI] [PubMed] [Google Scholar]
- 106.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. J Neurosci. 2010;30:1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sindreu C, Palmiter RD, Storm DR (2011) Proc Natl Acad Sci USA 108:3366–3370 [DOI] [PMC free article] [PubMed]
- 108.Yasuda S, Miyazaki T, Munechika K, Yamashita M, Ikeda Y, Kamizono A. J Recept Signal Transduct Res. 2007;27:235–246. doi: 10.1080/10799890701506147. [DOI] [PubMed] [Google Scholar]
- 109.Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, Hershfinkel M. J Neurosci. 2009;29:2890–2901. doi: 10.1523/JNEUROSCI.5093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharir H, Zinger A, Nevo A, Sekler I, Hershfinkel M. J Biol Chem. 2010;285:26097–26106. doi: 10.1074/jbc.M110.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holst B, Egerod KL, Jin C, Petersen PS, Ostergaard MV, Hald J, Sprinkel AM, Storling J, Mandrup-Poulsen T, Holst JJ, Thams P, Orskov C, Wierup N, Sundler F, Madsen OD, Schwartz TW. Endocrinology. 2009;150:2577–2585. doi: 10.1210/en.2008-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hosie AM, Dunne EL, Harvey RJ, Smart TG. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- 113.Hirzel K, Muller U, Latal AT, Hulsmann S, Grudzinska J, Seeliger MW, Betz H, Laube B. Neuron. 2006;52:679–690. doi: 10.1016/j.neuron.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 114.Mayer ML, Vyklicky L., Jr J Physiol. 1989;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Christine CW, Choi DW. J Neurosci. 1990;10:108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Legendre P, Westbrook GL. J Physiol. 1990;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gomperts BD, Tatham PER, Kramer IM. Signal transduction. San Diego: Academic Press; 2002. [Google Scholar]
- 118.Fujii T. Nature. 1954;174:1108–1109. doi: 10.1038/1741108a0. [DOI] [PubMed] [Google Scholar]
- 119.Hubbard SR, Bishop WR, Kirschmeier P, George SJ, Cramer SP, Hendrickson WA. Science. 1991;254:1776–1779. doi: 10.1126/science.1763327. [DOI] [PubMed] [Google Scholar]
- 120.Lengyel I, Fieuw-Makaroff S, Hall AL, Sim AT, Rostas JA, Dunkley PR. J Neurochem. 2000;75:594–605. doi: 10.1046/j.1471-4159.2000.0750594.x. [DOI] [PubMed] [Google Scholar]
- 121.Murakami K, Whiteley MK, Routtenberg A. J Biol Chem. 1987;262:13902–13906. [PubMed] [Google Scholar]
- 122.Park JA, Koh JY. J Neurochem. 1999;73:450–456. doi: 10.1046/j.1471-4159.1999.0730450.x. [DOI] [PubMed] [Google Scholar]
- 123.Brautigan DL, Bornstein P, Gallis B. J Biol Chem. 1981;256:6519–6522. [PubMed] [Google Scholar]
- 124.Haase H, Maret W. Biometals. 2005;18:333–338. doi: 10.1007/s10534-005-3707-9. [DOI] [PubMed] [Google Scholar]
- 125.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA. J Biol Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 126.Andersson DA, Gentry C, Moss S, Bevan S. Proc Natl Acad Sci USA. 2009;106:8374–8379. doi: 10.1073/pnas.0812675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Nat Chem Biol. 2009;5:183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prost AL, Bloc A, Hussy N, Derand R, Vivaudou M. J Physiol. 2004;559:157–167. doi: 10.1113/jphysiol.2004.065094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hou S, Vigeland LE, Zhang G, Xu R, Li M, Heinemann SH, Hoshi T. J Biol Chem. 2010;285:6434–6442. doi: 10.1074/jbc.M109.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thambiayya K, Wasserloos KJ, Huang Z, Kagan VE, St Croix CM, Pitt BR. Am J Physiol Lung Cell Mol Physiol. 2011;300:L624–L632. doi: 10.1152/ajplung.00376.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jirakulaporn T, Muslin AJ. J Biol Chem. 2004;279:27807–27815. doi: 10.1074/jbc.M401210200. [DOI] [PubMed] [Google Scholar]
- 132.Haase H, Hebel S, Engelhardt G, Rink L. Anal Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 133.Haase H, Ober-Blobaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 134.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 135.Maret W. J Nutr. 2000;130:1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 136.Maret W. Antioxid Redox Signal. 2006;8:1419–1441. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 137.Maret W, Li Y. Chem Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 138.Pirev E, Calles C, Schroeder P, Sies H, Kroncke KD. Free Radic Biol Med. 2008;45:86–91. doi: 10.1016/j.freeradbiomed.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 139.Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, Gotz T, Han J, Ellisman MH, Perkins GA, Lipton SA. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 140.Pal S, He K, Aizenman E. Pflugers Arch. 2004;448:296–303. doi: 10.1007/s00424-004-1256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Aizenman E, DeFranco DB, Rosenberg PA. Mol Med. 2007;13:350–355. doi: 10.2119/2007-00042.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.von Bulow V, Rink L, Haase H. J Immunol. 2005;175:4697–4705. doi: 10.4049/jimmunol.175.7.4697. [DOI] [PubMed] [Google Scholar]
- 143.Spahl DU, Berendji-Grun D, Suschek CV, Kolb-Bachofen V, Kroncke KD. Proc Natl Acad Sci USA. 2003;100:13952–13957. doi: 10.1073/pnas.2335190100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kanayama Y, Tsuji T, Enomoto S, Amano R. Biometals. 2005;18:553–565. doi: 10.1007/s10534-005-4775-6. [DOI] [PubMed] [Google Scholar]
- 145.Fahrni CJ. Curr Opin Chem Biol. 2007;11:121–127. doi: 10.1016/j.cbpa.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 146.Sumalekshmy S, Henary MM, Siegel N, Lawson PV, Wu Y, Schmidt K, Bredas JL, Perry JW, Fahrni CJ. J Am Chem Soc. 2007;129:11888–11889. doi: 10.1021/ja073240o. [DOI] [PubMed] [Google Scholar]
- 147.Sakurai H, Yoshikawa Y, Yasui H. Chem Soc Rev. 2008;37:2383–2392. doi: 10.1039/b710347f. [DOI] [PubMed] [Google Scholar]
- 148.Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Genomics. 2002;80:630–645. doi: 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- 149.Taylor KM, Nicholson RI. Biochim Biophys Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 150.Chimienti F, Devergnas S, Favier A, Seve M. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 151.Wang F, Kim BE, Petris MJ, Eide DJ. J Biol Chem. 2004;279:51433–51441. doi: 10.1074/jbc.M408361200. [DOI] [PubMed] [Google Scholar]
- 152.Huang L, Kirschke CP, Zhang Y, Yu YY. J Biol Chem. 2005;280:15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 153.Kelleher SL, Lonnerdal B. Am J Physiol Cell Physiol. 2005;288:C1042–C1047. doi: 10.1152/ajpcell.00471.2004. [DOI] [PubMed] [Google Scholar]
- 154.Taylor KM, Morgan HE, Johnson A, Nicholson RI. FEBS Lett. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]


