To the Editor
Impaired insulin secretion by the pancreatic beta cell and a reduction in beta cell mass are central to the pathogenesis of type 1 and type 2 diabetes. An ideal approach for treating individuals with diabetes is to develop modalities that increase beta cell numbers by inducing their proliferation in situ. Much evidence demonstrates that, in rodents, beta cells exhibit robust proliferative capacity both in vitro and in vivo. Induction of mouse beta cell neogenesis/proliferation has been observed under beta cell stress conditions such as inflammation, obesity, or following continuous glucose infusion1-2. However, given the inherent difficulties of human studies, there are few and often conflicting reports on the proliferative capacity of human beta cells3. Indeed, in vitro conditions that induce rodent beta cell proliferation often fail to induce human beta cell proliferation4, and in vivo human data are often obtained from autopsy specimens3.
To address these limitations, we developed a new strain of immunodeficient hyperglycemic mice based on NOD-Rag1null IL2rγnull (NRG) mice expressing the mutant Ins2Akita (Akita) allele. Mice heterozygous for the Ins2Akita mutation spontaneously develop a non-immune mediated hyperglycemia due to misfolding of insulin-2 protein, induction of endoplasmic reticulum stress, and beta cell apoptosis5. Similar to immunocompetent mice bearing the Ins2Akita allele, NOD-Rag1null IL2rγnull Ins2Akita (NRG-Akita) mice become hyperglycemic at 3-5 weeks6. However, normoglycemia can be restored in these mice by transplantation of optimal numbers (4000 islet equivalents, IEQs) of human islets. In this report, we transplanted sub-optimal numbers (1500 IEQs) of human islets into NRG-Akita mice to investigate the in vivo proliferative capacity of human beta cells in response to hyperglycemia.
Materials and Methods
Mice
NOD-Rag1null IL2rγnull Ins2+/Akita (abbreviated as NRG-Akita) mice were maintained by mating NOD-Rag1null IL2rγnull Ins2+/Akita males with NOD-Rag1null IL2rγnull females (expressing wildtype Ins2 alleles). Offspring were genotyped for the Ins2Akita locus; NRG-Akita and NRG littermates were used as islet recipients.
Human Islets and Islet Transplantation
Human islets from adult donors were obtained from the JDRF Islet Isolation Centers or the NIH Integrated Islet Distribution Program. On the day of receipt, human islets (1500 IEQs) were transplanted under the subrenal capsule of NRG-Akita or NRG mice of either sex at 3-4 weeks of age, a time period at which NRG-Akita mice are first developing hyperglycemia6.
Bromodeoxyuridine Treatment and Immunofluorescence Staining
Mice were given drinking water containing 0.8 mg/ml of bromodeoxyuridine (BrdU) for 7 days prior to recovery of the human islet graft. Islet grafts were recovered 2-4 weeks following transplantation, and the proliferative capacity of human beta cells in the graft was determined by BrdU incorporation of insulin-expressing cells. Insulin+ and insulin+BrdU+ cells were visualized using a fluorescence microscope (Zeiss, New York, NY); cell counts were performed with MetaMorph software (Molecular Devices, Downington, PA).
Results
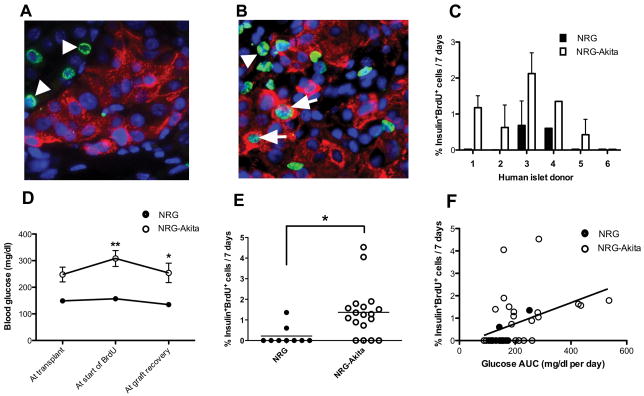
Human islets from 6 different donors were transplanted into both NRG-Akita and NRG mice. Insulin-positive staining was readily detectable in all human islet grafts, but very few insulin+BrdU+ cells were observed in islet grafts recovered from normoglycemic NRG mice (Fig. 1A). In contrast, insulin+BrdU+ cells from the same individual donors transplanted into hyperglycemic NRG-Akita mice were much more abundant (Fig. 1B; arrows). As expected, BrdU-positive cells were also detectable within the kidney tissue surrounding the islet graft (Fig. 1A, B; arrowheads).
FIGURE 1.
A, Human islets from the same donor engrafted in a (A) normoglycemic NRG or (B) hyperglycemic NRG-Akita mouse; insulin (red), BrdU (green); nuclei are blue (DAPI). Arrows point to insulin+BrdU+ cells; arrowheads to insulin−BrdU+ cells. C, Human beta cell proliferation by individual islet donor in grafts from NRG (n=11) and NRG-Akita (n=24) mice; mean ± s.e.m; P < 0.05, paired t test. D, Blood glucose levels of NRG and NRG-Akita mice at transplant, start of BrdU treatment, and graft recovery (7 days of BrdU); *P < 0.05, **P < 0.01. E, Percent human beta cell proliferation in islet grafts from the NRG and NRG-Akita mice; *P=0.0132. F, Pearson correlation of percent proliferating beta cells in human islet grafts with average daily glucose AUC (area under the curve) of all engrafted mice (n=35); *P=0.0121.
The islet grafts were first evaluated for each individual donor. Five of the 6 donors showed increased beta cell proliferation when engrafted in NRG-Akita as compared to NRG mice (P < 0.05, Fig. 1C). In contrast, donor #6 islets showed no detectable beta cell proliferation and, intriguingly, all NRG-Akita recipient mice engrafted with donor #6 islets became normoglycemic (data not shown). Upon exclusion of mice engrafted with islets from donor #6, NRG-Akita mice had significantly higher blood glucose levels than similarly engrafted NRG mice, both at the start of BrdU treatment and at graft recovery (Fig. 1D). These data verify that blood glucose levels of the engrafted NRG-Akita mice remained elevated, i.e., that the number of human islets transplanted was sub-optimal. The percent of proliferating beta cells in human islet grafts from hyperglycemic NRG-Akita mice was significantly greater than those from euglycemic NRG mice (Fig. 1E). The average proliferation of human beta cells in islet grafts from NRG-Akita mice was 1.37%, a ∼6-fold increase from that observed in NRG mice (0.22%). In addition, human beta cell proliferation showed a significant correlation with the blood glycemic levels of the NRG and NRG-Akita engrafted mice (Fig. 1F).
Discussion
Although mouse islets have been demonstrated to proliferate in response to inductive stimuli such as inflammation and hyperglycemia, the proliferative capacity of adult human beta cells is generally believed to be much less robust7. Consistent with this, we observed that human beta cell proliferation in islet grafts from normoglycemic NRG mice was minimal (∼0.2%). By comparison, we found that islet grafts from the same human donors transplanted into hyperglycemic NRG-Akita recipients exhibited a ∼6-fold increase (to ∼1.4%) in beta cell proliferation. Collectively, these data demonstrate that adult human beta cells can be induced to proliferate in vivo in response to hyperglycemia.
A similar 5 to 10-fold increase in beta cell proliferation in response to hyperglycemia has recently been reported in diabetic compared to non-diabetic mouse strains8. However, the rate of proliferation in mouse beta cells was much higher (∼10-fold) than we observed in human beta cells exposed to hyperglycemia. An earlier study by Tyrberg et al9 found an increase in human beta cell proliferation in vitro in response to glucose, although no difference in human beta cell proliferation was observed in vivo in islets transplanted into hyperglycemic compared to normoglycemic athymic mice. The reason for this difference from our in vivo findings is unknown, but it is worth noting that the high levels of NK cell activity that are present in athymic mice, as compared to NRG mice, may inhibit human beta cell function or proliferation10. Thus, NRG-Akita mice provide a unique model system for investigation of hyperglycemia-mediated signaling pathways that lead to the proliferation and expansion of pre-existing human beta cells. Such studies constitute one approach to understand the mechanisms that underlie the proliferation of human beta cells for transplantation, and may lead to potential therapeutics to facilitate and expand the endogenous beta cells of individuals with diabetes.
Acknowledgments
This work was supported by NIH Grants AI46629, HL077642, DK66636, DK69603, DK68854, DK72473, DK32520, DK20593, the VA Research Service, and grants from the Juvenile Diabetes Research Foundation, the Helmsley Foundation and the Brehm Foundation.
Contributor Information
Philip diIorio, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Agata Jurczyk, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Chaoxing Yang, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Waldemar J. Racki, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Michael A. Brehm, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Mark A. Atkinson, Department of Pathology, University of Florida, Gainesville, Florida.
Alvin C. Powers, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee.
Leonard D. Shultz, The Jackson Laboratory, Bar Harbor, ME 04609, USA.
Dale L. Greiner, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
Rita Bortell, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
References
- 1.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 2.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler PC, Meier JJ, Butler AE, et al. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 4.Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka M, Kayo T, Ikeda T, et al. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 6.Brehm MA, Bortell R, DiIorio P, et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgnull Ins2Akita mice. Diabetes. 2010;59:2265–2270. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuit FC, Drucker DJ. Beta-cell replication by loosening the brakes of glucagon-like peptide-1 receptor signaling. Diabetes. 2008;57:529–531. doi: 10.2337/db07-1578. [DOI] [PubMed] [Google Scholar]
- 8.Pechhold K, Zhu X, Harrison VS, et al. Dynamic changes in pancreatic endocrine cell abundance, distribution, and function in antigen-induced and spontaneous autoimmune diabetes. Diabetes. 2009;58:1175–1184. doi: 10.2337/db08-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyrberg B, Eizirik DL, Hellerstrom C, et al. Human pancreatic beta-cell deoxyribonucleic acid-synthesis in islet grafts decreases with increasing organ donor age but increases in response to glucose stimulation in vitro. Endocrinology. 1996;137:5694–5699. doi: 10.1210/endo.137.12.8940401. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura N, Woda BA, Tafuri A, Greiner DL, Reynolds CW, Ortaldo J, Chick W, Handler ES, Mordes JP, Rossini AA. Intrinsic cytotoxicity of natural killer cells to pancreatic islets in vitro. Diabetes. 1990;39:836–842. doi: 10.2337/diab.39.7.836. [DOI] [PubMed] [Google Scholar]