Abnormal hippocampal blood flow in ill Gulf War veterans at baseline and after cholinergic challenge persisted or worsened 11 years after initial testing and nearly 20 years after the 1991 Gulf War, suggesting chronic alteration of hippocampal blood flow.
Abstract
Purpose:
To determine, with arterial spin labeling (ASL) perfusion magnetic resonance (MR) imaging and physostigmine challenge, if abnormal hippocampal blood flow in ill Gulf War veterans persists 11 years after initial testing with single photon emission computed tomography and nearly 20 years after the 1991 Gulf War.
Materials and Methods:
The local institutional review board approved this HIPAA-compliant study. Veterans were screened for contraindications and gave written informed consent before the study. In a semiblinded retrospective protocol, veterans in three Gulf War illness groups—syndrome 1 (impaired cognition), syndrome 2 (confusion-ataxia), and syndrome 3 (central neuropathic pain)—and a control group received intravenous infusions of saline in an initial session and physostigmine in a second session, 48 hours later. Each infusion was followed by measurement of hippocampal regional cerebral blood flow (rCBF) with pulsed ASL. A mixed-effects linear model adjusted for age was used to test for differences in rCBF after the cholinergic challenge across the four groups.
Results:
Physostigmine significantly decreased hippocampal rCBF in control subjects (P < .0005) and veterans with syndrome 1 (P < .05) but significantly increased hippocampal rCBF in veterans with syndrome 2 (P < .005) and veterans with syndrome 3 (P < .002). The abnormal increase in rCBF was found to have progressed to the left hippocampus of the veterans with syndrome 2 and to both hippocampi of the veterans with syndrome 3.
Conclusion:
Chronic hippocampal perfusion dysfunction persists or worsens in veterans with certain Gulf War syndromes. ASL MR imaging examination of hippocampal rCBF in a cholinergic challenge experiment may be useful as a diagnostic test for this condition.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11101715/-/DC1
Introduction
Gulf War illness is a poorly understood chronic illness, featuring a variety of symptoms and complaints that include fatigue, generalized neuropathic pain, memory and concentration deficits, balance disturbances, and depression. It affects an estimated 25% of the 700 000 military personnel who were deployed to the 1991 Persian Gulf War (1). Epidemiologic studies have associated Gulf War illness with exposure to neurotoxic chemicals (2–4), including organophosphate pesticides, pyridostigmine bromide, and low-level sarin nerve gas, all of which are cholinergic stimulants that inhibit cholinesterase and can cause persistent brain changes (1,4). Animal experiments have demonstrated that chronic brain changes, including alteration of cholinergic receptors exacerbated by heat (5,6), can occur after exposure to these chemicals either alone or in synergistic combinations. A case definition of Gulf War illness, derived by principal-components factor analysis of symptoms and validated in additional veteran samples, classifies Gulf War illness into three primary variants: syndrome 1 (impaired cognition), syndrome 2 (confusion-ataxia), and syndrome 3 (central neuropathic pain) (2,3). Veterans whose condition fits one of these three Gulf War illness case definitions have lower N-acetylaspartate-to-creatine or choline-to-creatine ratios than control veterans at magnetic resonance (MR) spectroscopy of the basal ganglia and pons (7); high-frequency heart rate variability (8); and abnormalities in audiovestibular function (9,10) and neuropsychologic tests of verbal, perceptual-motor, and general brain function (9,11).
Symptoms such as memory loss, occasional confusion, and irritability and disorders in motion control suggest impairment of the hippocampus (2,11,12). In studies of rodents, administration of cholinesterase-inhibiting chemicals, similar to those to which Gulf War veterans were exposed, has been found to affect the hippocampi acutely (13,14) and to chronically impair hippocampal synaptic transmission (15). MR spectroscopy studies have revealed reduced N-acetylaspartate-to-creatine ratios in the hippocampi bilaterally in ill Gulf War veterans compared with control veterans (16).The short-acting cholinesterase inhibitor physostigmine, often used to test the functional integrity of the cholinergic system, reduces the breakdown rate of acetylcholine at the synaptic junction and prolongs acetylcholine action on postsynaptic cholinergic receptors.
A previous study, conducted in 1997 and 1998 and published by our group (17), measured regional cerebral blood flow (rCBF) with technetium 99m hexamethylpropyleneamine oxime single photon emission computed tomography (SPECT). Results indicated that, in response to intravenous challenge with physostigmine, rCBF decreased in control veterans but paradoxically increased not only in the right hippocampus of veterans with syndrome 2 but also in other deep brain structures of those with syndromes 1 and 3 (17).
In the current study, undertaken to gain insights into the history of the illness and to test the use of arterial spin labeling (ASL) in place of SPECT for measuring rCBF, we present additional data in the same group of patients, acquired 11 years after our initial study described in the preceding paragraph. More veterans from the same military unit were included in the current study to augment the sample size.
The aim of this study was to determine with ASL MR imaging and physostigmine challenge if abnormal hippocampal blood flow in ill Gulf War veterans persisted 11 years after initial testing with SPECT and nearly 20 years after the 1991 Gulf War. The hypothesis was that differences in baseline rCBF (lower in veterans with syndrome 2 than in control subjects in the right hippocampus) and in the effect of physostigmine stimulation on rCBF (increased in veterans with syndromes 2 and 3 and decreased in control subjects and veterans with syndrome 1 in the right hippocampus) would be found, as observed in the original 1997–1998 study (17).
Materials and Methods
Subjects
For this retrospective study, 34 members (16 control subjects, four veterans with syndrome 1, 10 veterans with syndrome 2, three veterans with syndrome 3, and one other) of the original sample from the 1998 study (17) returned for reevaluation, along with 23 new Gulf War veterans (seven with syndrome 1, seven with syndrome 2, and nine with syndrome 3), also from the original epidemiologic study of this battalion. Exclusion criteria were standard MR safety contraindications, including metal implants, pregnancy, severe claustrophobia, and obesity; contraindications to radioactive nuclear medicine imaging (for another part of the study), and marked organ failure. Nine subjects were excluded (Appendix E1 [online]). This left 48 individuals (13 control subjects [mean age ± standard deviation, 60 years ± 6], 11 veterans with syndrome 1 [mean age, 51 years ± 6], 13 veterans with syndrome 2 [mean age, 63 years ± 6], and 11 veterans with syndrome 3 [mean age, 57 years ± 6). The local institutional review board approved this Health Insurance Portability and Accountability Act–compliant study. Veterans were screened for contraindications and gave written informed consent before the study. Prior to the physostigmine challenge perfusion, the veterans, who were housed in and cared for at the Clinical and Translational Research Center during their week-long study period, stopped using medicines or caffeine-containing drinks known to affect cerebral blood flow or the effects of physostigmine treatment (18,19). Study subjects received no treatments as part of this investigation.
Study Protocol
A week-long clinical, neuroimaging, and neuropsychologic and psychiatric study protocol, including face-name associative memory and simple face recognition tests, as well as Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and Clinician-Administered Posttraumatic stress disorder (PTSD) Scale assessments for PTSD, was administered to each subject. Data were collected from June 22, 2008, to July 3, 2009. As part of this protocol, each veteran underwent a blinded hippocampus perfusion study in two sessions separated by 2 days: the first session with saline infusion and the second with physostigmine infusion. Subjects did not know which agent was given in which session. Perfusion studies were performed in early afternoon, when cholinergic effects are maximal.
Physostigmine Administration and Physiology Measurements
For infusions, an intravenous line with antecubital catheter was prepared and connected to a 500-mL bag hooked to an infusion pump (MRidium; Mallinckrodt-Covidien, Paris, Ky). To counteract the peripheral autonomic actions of physostigmine, 0.3 mg glycopyrrolate was injected intravenously over the 1 minute prior to physostigmine infusion. The physostigmine infusion rate was 1.0 mg/h; the volume infusion rate was 130 mL/h. Infusion lasted 25–27 minutes before and continued for 12 minutes during perfusion imaging, making the total infusion time nearly 40 minutes. Heart rate and diastolic and systolic blood pressures were recorded before infusion and after imaging.
MR Imaging Protocol
Studies were performed with a 3.0-T total imaging matrix whole-body imaging unit (Trio; Siemens, Malvern, Pa) with a body coil for transmission and 12-channel phased array head coil as receiver. OPTIMAL FAIR (orthogonally positioned tagging imaging method for arterial labeling with flow alternated inversion recovery) was used to minimize partial volume effects and within-section transit time differences (20). OPTIMAL FAIR is modified from the standard axial FAIR with Q2TIPS (quantitative imaging of perfusion using a single subtraction, second version with interleaved thin-section TI(1) periodic saturation) ASL sequence (21) by making the imaging plane obliquely coronal, perpendicular to the major feeding arteries and the longitudinal anatomic axis of the hippocampus (Fig 1).
Figure 1:
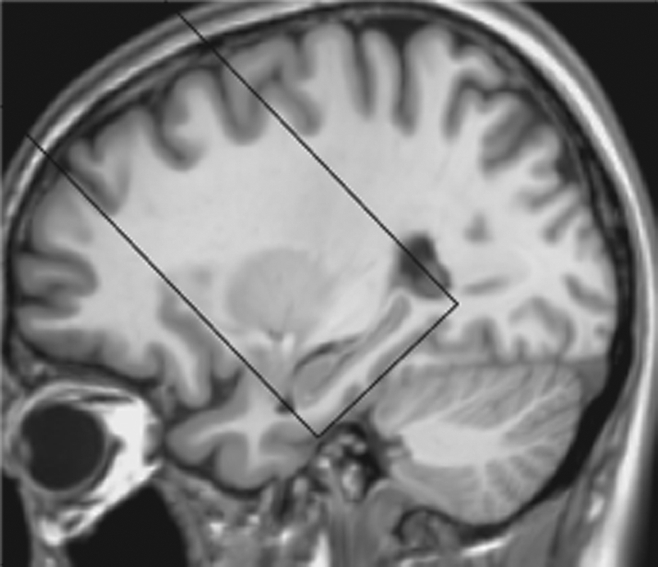
Image shows ASL perfusion imaging slab position. The oblique coronal ASL imaging sections were positioned to cover the head of the hippocampus, with the inferior edge of the imaging slab parallel to the longitudinal anteroposterior axis of the hippocampus and tangent to the gyrus of the temporal lobe, by using T1-weighted high-spatial-resolution anatomic images for accurate reference.
Auto-Align, Siemens’ coregistration tool, was used to ensure consistency of imaging section position across sessions. A gradient-echo scout acquisition, a 0.9 mm × 0.9 mm × 1.0-mm T1-weighted magnetization-prepared rapid acquisition gradient-echo (MP-RAGE) anatomic acquisition, hippocampus perfusion acquisitions, and a 0.7 × 0.7-mm2 in-plane gradient-echo acquisition with imaging section orientation and thickness matching that of the perfusion imaging slab were also performed.
Hippocampus perfusion acquisitions included 100 pairs of labeling and control ASL images and two M0 measurements acquired by using the same sequence with a repetition time of 8 seconds. Parameters were as follows: repetition time msec/echo time msec, 3000/14; field of view, 128 × 128 mm2; matrix size, 64 × 64; section thickness, 5.0 mm; intersection gap, 1.0 mm; number of imaging sections, 10; left-to-right phase encoding with 30% phase oversampling; 6/8 partial Fourier; integrated Parallel Acquisition Technique generalized autocalibrating partially parallel acquisitions factor of two with 24 reference lines; anteroposterior section acquisition order; an 148-mm selective inversion slab; a 328-mm spatially-confined inversion slab; temporal bolus width (TI1)/postbolus delay, 600/1200 msec; and inferior saturation pulse number/size/repetition interval, 48/20 mm/25 msec.
Motion Correction and rCBF Reconstruction
Image processing was performed with SPM2 (Functional Imaging Laboratory, University College London, London, England). Whenever the translational motion was larger than 1 mm or the rotation around any axis was larger than 1°, motion corrections were performed by using trilinear interpolation. Images with sharp, large motions (>2 mm translation and/or >2° rotation around any axis) were excluded from the analysis. After pair-wise subtraction and averaging, rCBF maps were calculated with Matlab 7.1 (MathWorks, Natick, Mass) scripts by applying a single-compartment model using the mean perfusion-weighted image and mean equilibrium magnetization (M0) image (22–24).
Voxel-based morphometry analysis of the high-spatial-resolution T1-weighted MP-RAGE anatomic images was performed with SPM8, with results spatially smoothed with an 8-mm isotropic Gaussian kernel, normalized to total intracranial volume, adjusted for subject age, and corrected for multiple comparisons with a spatial false discovery rate procedure.
rCBF Analysis
Hippocampus segmentations generated from the FMRIB Integrated Registration and Segmentation Tool of the FSL software package (25) were visually inspected and improved as necessary for individual subjects by manual adjustment of boundaries.
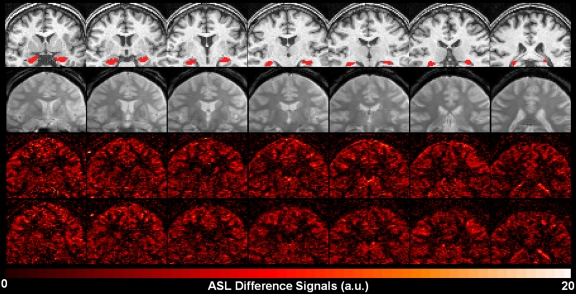
The high-spatial-resolution anatomic image and the corresponding hippocampus regions of interest (ROIs) were coregistered to the high-spatial-resolution gradient-echo image, then to the optimal reference image of the ASL series. To ensure that rCBF was only from the hippocampus, binary ROI masks were further constrained by excluding voxels with values interpolated between 0 to 1 and boundaries extending beyond the hippocampus. One subject’s segmented hippocampus ROIs and rCBF maps from the saline session overlaid on coregistered anatomic images illustrate typical results (Fig 2).
Figure 2:
Oblique coronal images in healthy veteran. Top row: coregistered T1-weighted high-spatial-resolution anatomic MP-RAGE images with overlaid hippocampus ROIs. Second row: proton density–weighted images from the saline session. Third row: perfusion-weighted images from the saline session. Bottom row: perfusion-weighted images from the physostigmine session.
Statistical Analysis
The primary hypothesis, based on prior studies (17), was that the change in rCBF in the hippocampus caused by physostigmine would be more negative in the controls than in the three syndrome groups. This was tested with a mixed-effects linear model for differences in rCBF between the two test sessions, the four clinical groups (with and without PTSD for the three syndromes), the two hemispheres, and their interactions, incorporating both within- and between-subject variance components (26,27), using the Mixed Procedure of SAS (version 9.2; SAS Institute, Cary, NC). The interaction between session and groups was the main test of the hypothesis. Since rCBF declines with age (28,29) and the age distribution differed among groups (see Subjects section, above), the model was adjusted for age.
To determine if the hippocampal rCBF data relate to memory performance, a Pearson correlation analysis was done on data from a face-name associative memory test reliant on hippocampus function, with a control task of a simple item (face) recognition test less reliant on hippocampus involvement, and the changes in hippocampus rCBF elicited by physostigmine challenge.
Results
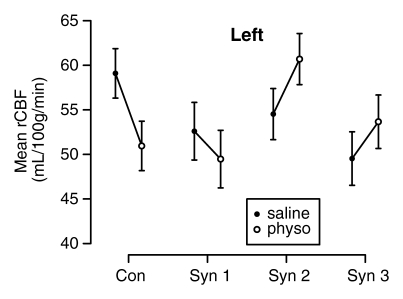
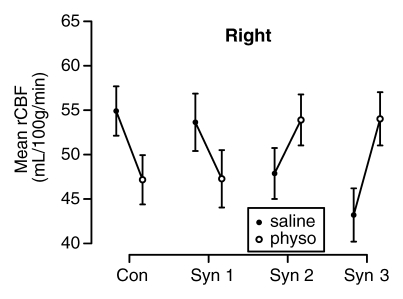
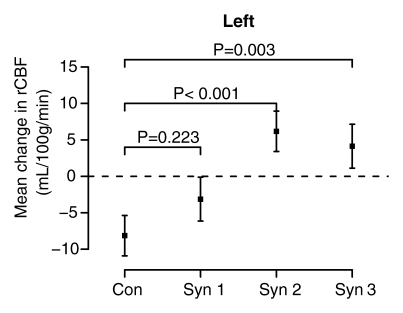
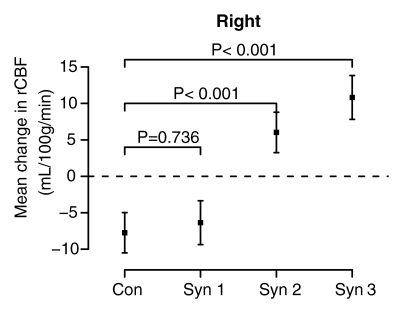
The mixed-effects linear model controlled for age strongly rejected the a priori null hypothesis of no difference in the effect of physostigmine stimulation on rCBF in the hippocampus across the four groups (session-by-group interaction, F = 14.87, df = 3;138, P < .0001) and found no difference in this result between the two hemispheres (session-by-group-by-side interaction, F = 0.97, df = 3;138, P = .41). In both hemispheres rCBF decreased significantly from session 1 (saline) to session 2 (physostigmine) in the control and syndrome 1 groups, but strongly increased in the syndrome 2 and 3 groups (Fig 3).
Figure 3a:
(a–d) Graphs show results of the cholinergic challenge experiment in Gulf War veterans with syndromes 1–3 (Syn 1, Syn2, and Syn 3, respectively) compared with those in a control group of well veterans (Con). (a, b) Graphs show group mean ASL-measured rCBF in the (a) left and (b) right hippocampus in session 1 (saline infusion) and session 2 (physostigmine [physo] infusion). (c, d) Graphs show group mean changes in ASL-measured rCBF in the (c) left and (d) right hippocampus with P values from contrasts in the mixed-effects linear model testing differences between syndrome groups and the control group. Error bars = standard errors of the mean.
Figure 3b:
(a–d) Graphs show results of the cholinergic challenge experiment in Gulf War veterans with syndromes 1–3 (Syn 1, Syn2, and Syn 3, respectively) compared with those in a control group of well veterans (Con). (a, b) Graphs show group mean ASL-measured rCBF in the (a) left and (b) right hippocampus in session 1 (saline infusion) and session 2 (physostigmine [physo] infusion). (c, d) Graphs show group mean changes in ASL-measured rCBF in the (c) left and (d) right hippocampus with P values from contrasts in the mixed-effects linear model testing differences between syndrome groups and the control group. Error bars = standard errors of the mean.
Figure 3c:
(a–d) Graphs show results of the cholinergic challenge experiment in Gulf War veterans with syndromes 1–3 (Syn 1, Syn2, and Syn 3, respectively) compared with those in a control group of well veterans (Con). (a, b) Graphs show group mean ASL-measured rCBF in the (a) left and (b) right hippocampus in session 1 (saline infusion) and session 2 (physostigmine [physo] infusion). (c, d) Graphs show group mean changes in ASL-measured rCBF in the (c) left and (d) right hippocampus with P values from contrasts in the mixed-effects linear model testing differences between syndrome groups and the control group. Error bars = standard errors of the mean.
Figure 3d:
(a–d) Graphs show results of the cholinergic challenge experiment in Gulf War veterans with syndromes 1–3 (Syn 1, Syn2, and Syn 3, respectively) compared with those in a control group of well veterans (Con). (a, b) Graphs show group mean ASL-measured rCBF in the (a) left and (b) right hippocampus in session 1 (saline infusion) and session 2 (physostigmine [physo] infusion). (c, d) Graphs show group mean changes in ASL-measured rCBF in the (c) left and (d) right hippocampus with P values from contrasts in the mixed-effects linear model testing differences between syndrome groups and the control group. Error bars = standard errors of the mean.
In addition, baseline hippocampal rCBF in session 1 (saline) was lower in all three syndrome groups than in the control group in both hemispheres. This difference was most pronounced in the syndrome 3 group in the left (P = .029) and right (P = .0055) hemispheres and in the syndrome 2 group in the right hemisphere (P = .057) (Fig 3).
Numeric values (means and standard errors) of the rCBF in the two infusion sessions for the four subject groups are summarized in the Table.
Estimated Group Mean Hippocampal rCBF for Saline and Physostigmine Sessions
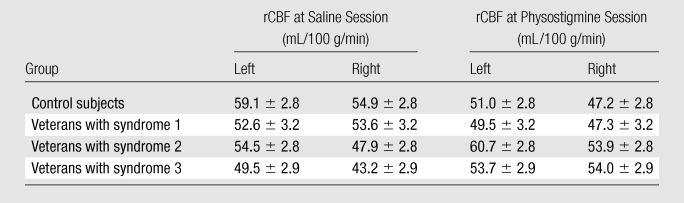
Note.—Data are means ± standard errors of the mean.
For the 46 subjects with both memory test and ASL data, hippocampal rCBF changes were uncorrelated with the control single item (face) recognition results (r = 0.08, P = .60 for left hippocampus and r = 0.01, P = .93 for right hippocampus), but the associative memory (face-name) test results were moderately correlated (r = 0.28, P = .06) with left hippocampus rCBF changes and significantly correlated (r = 0.32, P = .03) with right hippocampus rCBF changes.
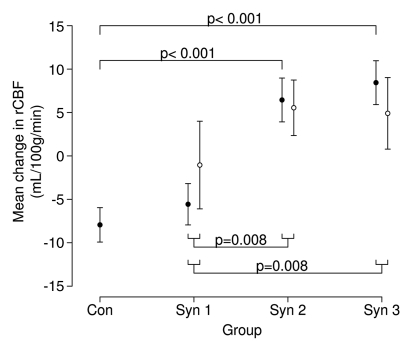
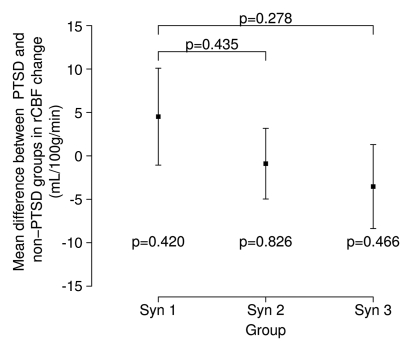
From Structured Clinical Interview and Clinician-Administered PTSD Scale assessments, diagnoses of PTSD were made in 21% of the 48 veterans who participated in this study: in no control subjects, in 18% of veterans with syndrome 1, in 38% of veterans with syndrome 2, and in 27% of veterans with syndrome 3. However, PTSD status did not contribute to group differences in hippocampal rCBF in response to physostigmine (Fig 4).
Figure 4a:
(a, b) Graphs show hippocampus rCBF changes elicited by physostigmine challenge in Gulf War veterans with syndromes 1–3 (Syn 1, Syn2, and Syn 3, respectively) with or without PTSD compared with a control group of well veterans (Con). (a) ○ = Subjects given a diagnosis of PTSD; ● = subjects without PTSD, with P values from contrasts in the mixed-effects linear model testing differences between syndrome groups without PTSD and the control group (top) and pooled differences between syndrome groups irrespective of PTSD status (bottom). (b) PTSD status does not affect the differences among the syndrome groups shown in a. Error bars = standard errors of the mean.
Figure 4b:
(a, b) Graphs show hippocampus rCBF changes elicited by physostigmine challenge in Gulf War veterans with syndromes 1–3 (Syn 1, Syn2, and Syn 3, respectively) with or without PTSD compared with a control group of well veterans (Con). (a) ○ = Subjects given a diagnosis of PTSD; ● = subjects without PTSD, with P values from contrasts in the mixed-effects linear model testing differences between syndrome groups without PTSD and the control group (top) and pooled differences between syndrome groups irrespective of PTSD status (bottom). (b) PTSD status does not affect the differences among the syndrome groups shown in a. Error bars = standard errors of the mean.
There were no obvious morphologic abnormalities or changes in MR imaging signal patterns in the brains of affected individuals (Appendix E2 [online]). Voxel-based morphometry indicated trends for syndrome-control differences (voxel-wise P < .001), but no statistically significant between-group regional volume differences survived after spatial FDR multiple comparisons corrections, although the syndrome 2 versus control subjects comparison in the brainstem approached significance (P = .08).
From the shortened physostigmine infusion and the premedication with glycopyrrolate, virtually no side effects resulted: One subject became nauseated during the saline infusion and one during the physostigmine infusion, but no subject vomited. The four groups did not differ significantly on changes from session 1 (saline) to session 2 (physostigmine) in heart rate (P = .98), systolic blood pressure (P = .58), or diastolic blood pressure (P = .24).
Discussion
In the current study we demonstrated that abnormal hippocampal blood flow in ill Gulf War veterans at baseline and after cholinergic challenge persists, and may have progressed, 11 years after initial testing and nearly 20 years after the 1991 Gulf War, suggesting chronic alteration of hippocampal blood flow. These findings replicate results of our initial SPECT study of largely the same group of veterans (17). In that study, only the veterans ill with syndrome 2, with the most severe symptoms (3,30), reacted to intravenous physostigmine challenge with a significant increase in rCBF in the hippocampus, compared with a decrease in rCBF in the control group, and this difference was statistically significant only in the right hippocampus; veterans with the milder syndrome 1 reacted similar to controls (17). In the current study, both syndrome 2 and 3 groups responded with statistically significant increased rCBF in both hippocampi, opposite to the control group. These findings suggest persistence over the 11-year period of the right hippocampal pathology in the syndrome 2 group, progression of the same pathologic process to the left hippocampus in the syndrome 2 group, and new involvement of both hippocampi in the syndrome 3 group.
The changes in hippocampus rCBF produced by physostigmine correlated significantly with performance on a face-name associative memory test dependent on hippocampal function. In this same construction battalion, correlations of functional brain activity in the thalamus and caudate with semantic memory performance have been noted in a recent functional MR imaging study (31), and an evoked response potential investigation has shown that ill veterans have impaired response inhibition associated with reduced amplitude in the P3 evoked response (32).
Causes other than the postulated exposure to neurotoxic chemicals might also contribute to or explain the differences in hippocampal rCBF found in this study. One of the most commonly hypothesized explanations for Gulf War illness is PTSD. However, in the current study, PTSD could not explain the observed differences in hippocampal rCBF; the major determinant of the abnormal responses was membership in the syndrome 2 or syndrome 3 group. Other potential contributing factors include chronic fatigue, exposures to smoke from oil well fires, hardened shell casings containing depleted uranium, and toxic biologic agents (33–37).
The clear difference in response to cholinergic challenge of the syndrome 1 group, which responded like the controls, and the syndrome 2 and 3 groups further emphasizes the prior findings indicating differences in brain pathology among the three syndrome groups. Abnormal response of rCBF to cholinergic challenge in the hippocampus appears to be an objective marker that distinguishes syndrome 1 from syndromes 2 and 3. A preliminary analysis indicates there was no group difference in cortical gray matter rCBF response to physostigmine, although group differences similar to those for the hippocampus were found in the amygdala and caudate (38).
The large difference between the syndrome 2 and 3 groups compared with the control and syndrome 1 groups suggests that a cholinergic challenge rCBF test might provide a useful component of a diagnostic protocol for Gulf War illness. Substitution of ASL for SPECT is an important innovation, because it avoids the radiation dose of two SPECT examinations and will allow both saline and physostigmine infusions to be assessed in the same session rather than on separate afternoons 2 days apart. We have developed a practical diagnostic adaptation of the protocol that can be completed in under 3 hours for testing in a larger sample of Gulf War veterans. The ability to administer the protocol without appreciable adverse effects (nausea, vomiting) of physostigmine (39) is also important.
The hippocampus is relatively small, and systematic differences in hippocampal volume among the groups could impact the rCBF measurements. Victims of the 1995 Tokyo subway sarin attack had reduced hippocampal volume and reduced volumes of insular cortex and surrounding white matter (40). Significantly smaller hippocampi and trends toward reduced gray matter volumes in frontal, parietal, and occipital cortices were recently found in veterans of the 1991 Gulf War exposed to sarin or cyclosarin, independent of syndrome classification (41). However, the current study showed no significant group differences in hippocampal volume. Furthermore, the possible confound of differential volume averaging due to individual or group hippocampus volume differences was mitigated by carefully choosing for analysis only those ASL voxels within the hippocampus.
Limitations of this study included inexact age matching of the subject groups, too few subjects to adequately power all the comparisons in higher order interactions, and lack of measurement of the effect of Gulf War illness and of the syndromes on arterial transit time, although arterial transit times were measured in similarly aged healthy controls. Since all groups but syndrome 1 were of similar age, effects of age-dependent delays in arterial delivery time should be minimal. Moreover, in each group the primary comparison was perfusion signal differences between physostigmine and saline infusions, which should reduce the sensitivity to transit time differences across groups. Addition of real-time prospective motion correction techniques, such as three-dimensional prospective acquisition correction (42), would increase the precision of the rCBF measurements in the most impaired veterans with chronic pain, tremors, and breathing difficulties, who tend to move more in the imaging unit. Finally, since this study replicated and extended a prior study in a single battalion of Gulf War veterans, the findings must be replicated again in a more widely representative sample of the Gulf War veteran population before they can be generalized.
Advances in Knowledge.
Abnormal hippocampal blood flow in ill Gulf War veterans at baseline and after cholinergic challenge persisted or worsened 11 years after initial testing and nearly 20 years after the 1991 Gulf War, suggesting chronic alteration of hippocampal blood flow.
Baseline and physostigmine challenge–related hippocampal perfusion measured by using arterial spin labeling (ASL) MR imaging can help differentiate among the three major variants of Gulf War syndrome.
Implications for Patient Care.
Quantitatively measuring hippocampal perfusion and perfusion response to physostigmine challenge can facilitate the diagnosis of Gulf War illness and its variants, will assist the investigation of pathologic mechanisms, and may be valuable in the evaluation of therapeutic efficacy.
Technologic advances can improve the clinical utility of the cholinergic challenge protocol by replacing single photon emission computed tomography with ASL MR imaging, which eliminates radiation exposure and can shorten the testing from two half-day sessions to a single 2-hour session, including two 15-minute ASL examinations.
Disclosures of Potential Conflicts of Interest: X.L. No potential conflicts of interest to disclose. J.S.S. No potential conflicts of interest to disclose. D.M.B. No potential conflicts of interest to disclose. J.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: received payment for expert testimony for plaintiffs and prosecution; is on the speakers bureau of Forrest Pharmaceuticals; receives royalties from Cambridge University Press. C.M.C. No potential conflicts of interest to disclose. M.M.B. No potential conflicts of interest to disclose. A.L.H. No potential conflicts of interest to disclose. T.N.O. No potential conflicts of interest to disclose. P.S.C. No potential conflicts of interest to disclose. R.W.B. No potential conflicts of interest to disclose. R.W.H. No potential conflicts of interest to disclose.
Supplementary Material
Acknowledgments
The authors acknowledge the important contributions of our 3.0-T imaging unit operators Victoria Vescovo Webster, MA, and Larry Steier, MBA; the research nurses Don Aultman, BSN, and Alice Cox, MS, who provided essential coordination and care of the research subjects; the management and administrative contributions of Kathy Norman, AA, Patricia Thompson, and Rick Thompson, MBA; the scientific advice of Michael Devous, PhD, and Hanzhang Lu, PhD; and the nursing and administrative staff of the National Institutes of Health—supported Clinical and Translational Research Center, who provided, cared for, and ran the related clinical research protocols on the research subjects during their 6–7-day hospital stay for the study.
Received September 10, 2010; revision requested October 20; revision received April 8, 2011; accepted April 22; final version accepted May 27.
Funding: This research was supported by the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research (grant UL1RR024982).
Abbreviations:
- ASL
- arterial spin labeling
- MP-RAGE
- magnetization-prepared rapid acquisition gradient echo
- PTSD
- posttraumatic stress disorder
- rCBF
- regional cerebral blood flow
- ROI
- region of interest
References
- 1.Binns JH, Barlow C, Bloom FE, et al. (Research Advisory Committee on Gulf War Veterans’ Illnesses). Gulf War Illness and the Health of Gulf War Veterans. Washington, DC: Department of Veterans Affairs http://www1.va.gov/rac-gwvi/ Published 2008. Accessed August 2, 2010.
- 2.Haley RW, Kurt TL, Hom J. Is there a Gulf War Syndrome? searching for syndromes by factor analysis of symptoms. JAMA 1997;277(3):215–222 [PubMed] [Google Scholar]
- 3.Haley RW, Luk GD, Petty F. Use of structural equation modeling to test the construct validity of a case definition of Gulf War syndrome: invariance over developmental and validation samples, service branches and publicity. Psychiatry Res 2001;102(2):175–200 [DOI] [PubMed] [Google Scholar]
- 4.Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A 2008;105(11):4295–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson RF, Barr EB, Blackwell WB, et al. Response of rats to low levels of sarin. Toxicol Appl Pharmacol 2002;184(2):67–76 [PubMed] [Google Scholar]
- 6.Henderson RF, Barr EB, Blackwell WB, et al. Response of F344 rats to inhalation of subclinical levels of sarin: exploring potential causes of Gulf War illness. Toxicol Ind Health 2001;17(5-10):294–297 [DOI] [PubMed] [Google Scholar]
- 7.Haley RW, Marshall WW, McDonald GG, Daugherty MA, Petty F, Fleckenstein JL. Brain abnormalities in Gulf War syndrome: evaluation with 1H MR spectroscopy. Radiology 2000;215(3):807–817 [DOI] [PubMed] [Google Scholar]
- 8.Haley RW, Vongpatanasin W, Wolfe GI, et al. Blunted circadian variation in autonomic regulation of sinus node function in veterans with Gulf War syndrome. Am J Med 2004;117(7):469–478 [DOI] [PubMed] [Google Scholar]
- 9.Haley RW, Hom J, Roland PS, et al. Evaluation of neurologic function in Gulf War veterans: A blinded case-control study. JAMA 1997;277(3):223–230 [PubMed] [Google Scholar]
- 10.Roland PS, Haley RW, Yellin W, Owens K, Shoup AG. Vestibular dysfunction in Gulf War syndrome. Otolaryngol Head Neck Surg 2000;122(3):319–329 [DOI] [PubMed] [Google Scholar]
- 11.Hom J, Haley RW, Kurt TL. Neuropsychological correlates of Gulf War syndrome. Arch Clin Neuropsychol 1997;12(6):531–544 [PubMed] [Google Scholar]
- 12.Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, Mass: MIT Press, 1993 [Google Scholar]
- 13.Prendergast MA, Terry AV, Jr, Buccafusco JJ. Chronic, low-level exposure to diisopropylfluorophosphate causes protracted impairment of spatial navigation learning. Psychopharmacology (Berl) 1997;129(2):183–191 [DOI] [PubMed] [Google Scholar]
- 14.Prendergast MA, Self RL, Smith KJ, et al. Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience 2007;146(1):330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speed H, Blaiss C, Powell C. Chronic exposure of adult mice to the pesticide, chlorpyrifos, results in enhanced emotional memory and altered hippocampal synaptic transmission. Presented at the 39th Annual Meeting of the Society for Neuroscience, October 2009 [Google Scholar]
- 16.Menon PM, Nasrallah HA, Reeves RR, Ali JA. Hippocampal dysfunction in Gulf War Syndrome: a proton MR spectroscopy study. Brain Res 2004;1009(1-2):189–194 [DOI] [PubMed] [Google Scholar]
- 17.Haley RW, Spence JS, Carmack PS, et al. Abnormal brain response to cholinergic challenge in chronic encephalopathy from the 1991 Gulf War. Psychiatry Res 2009;171(3):207–220 [DOI] [PubMed] [Google Scholar]
- 18.Cameron OG, Modell JG, Hariharan M. Caffeine and human cerebral blood flow: a positron emission tomography study. Life Sci 1990;47(13):1141–1146 [DOI] [PubMed] [Google Scholar]
- 19.Mathew RJ, Wilson WH. Substance abuse and cerebral blood flow. Am J Psychiatry 1991;148(3):292–305 [DOI] [PubMed] [Google Scholar]
- 20.Li X, Sarkar SN, Purdy DE, Haley RW, Briggs RW. Hippocampus perfusion studies using OPTIMAL FAIR [abstr]. In: Proceedings of the Eighteenth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2010; 275 [Google Scholar]
- 21.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med 1998;39(5):702–708 [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Licht DJ, Jahng GH, et al. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging 2003;18(4):404–413 [DOI] [PubMed] [Google Scholar]
- 23.Buxton RB. Quantifying CBF with arterial spin labeling. J Magn Reson Imaging 2005;22(6):723–726 [DOI] [PubMed] [Google Scholar]
- 24.Parkes LM. Quantification of cerebral perfusion using arterial spin labeling: two-compartment models. J Magn Reson Imaging 2005;22(6):732–736 [DOI] [PubMed] [Google Scholar]
- 25.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011;56(3):907–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Searle SR. Linear models. New York, NY: Wiley, 1971 [Google Scholar]
- 27.Milliken GA, Johnson DE. Analysis of Messy Data, Vol 1: Designed Experiments. 2nd ed. Boca Raton, Fla: Chapman & Hall/CRC, 1993 [Google Scholar]
- 28.Pantano P, Baron JC, Lebrun-Grandié P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 1984;15(4):635–641 [DOI] [PubMed] [Google Scholar]
- 29.Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology 1984;34(7):855–862 [DOI] [PubMed] [Google Scholar]
- 30.Haley RW, Maddrey AM, Gershenfeld HK. Severely reduced functional status in veterans fitting a case definition of Gulf War syndrome. Am J Public Health 2002;92(1):46–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calley CS, Kraut MA, Spence JS, Briggs RW, Haley RW, Hart J., Jr The neuroanatomic correlates of semantic memory deficits in patients with Gulf War illnesses: a pilot study. Brain Imaging Behav 2010;4(3-4):248–255 [DOI] [PubMed] [Google Scholar]
- 32.Tillman GD, Green TA, Ferree TC, et al. Impaired response inhibition in ill Gulf War veterans. J Neurol Sci 2010;297(1-2):1–5 [DOI] [PubMed] [Google Scholar]
- 33.Abou-Donia MB, Wilmarth KR, Jensen KF, Oehme FW, Kurt TL. Neurotoxicity resulting from coexposure to pyridostigmine bromide, DEET, and permethrin: implications of Gulf War chemical exposures. J Toxicol Environ Health 1996;48(1):35–56 [DOI] [PubMed] [Google Scholar]
- 34.Haley RW, Kurt TL. Self-reported exposure to neurotoxic chemical combinations in the Gulf War: a cross-sectional epidemiologic study. JAMA 1997;277(3):231–237 [PubMed] [Google Scholar]
- 35.Frost SD. Gulf War syndrome: proposed causes. Cleve Clin J Med 2000;67(1):17–20 [DOI] [PubMed] [Google Scholar]
- 36.Nicolson GL, Bruton DM, Jr, Nicolson NL. Chronic fatigue illness and Operation Desert Storm. J Occup Environ Med 1996;38(1):14–16 [DOI] [PubMed] [Google Scholar]
- 37.Nicolson GL, Nasralla M, Haier J, Nicolson NL. Gulf War illnesses: role of chemical, radiological, and biological exposures. In: Tapamainen H. ed. War and health. Helsinki, Finland: Zed, 2002; 431–446 [Google Scholar]
- 38.Liu PL, Aslan S, Li X, et al. Perfusion deficit to cholinergic challenge in veterans with Gulf War Illness. Neurotoxicology 2011;32(2):242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furey ML, Pietrini P, Alexander GE, et al. Time course of pharmacodynamic and pharmacokinetic effects of physostigmine assessed by functional brain imaging in humans. Pharmacol Biochem Behav 2000;66(3):475–481 [DOI] [PubMed] [Google Scholar]
- 40.Yamasue H, Abe O, Kasai K, et al. Human brain structural change related to acute single exposure to sarin. Ann Neurol 2007;61(1):37–46 [DOI] [PubMed] [Google Scholar]
- 41.Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in U.S. veterans. Neurotoxicology 2010;31(5):493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med 2000;44(3):457–465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.