Abstract
Cytoreductive conditioning regimes designed to allow for successful allogeneic hematopoietic stem cell transplantation (allo-HSCT) paradoxically are also detrimental to recovery of the immune system in general but lymphopoiesis in particular. Post-transplant immune depletion is particularly striking within the T cell compartment which is exquisitely sensitive to negative regulation, evidenced by the profound decline in thymic function with age. As a consequence, regeneration of the immune system remains a significant unmet clinical need. Over the past decade studies have revealed several promising therapeutic strategies to address ineffective lymphopoiesis and post-transplant immune deficiency. These include the use of cytokines such as IL-7, IL-12 and IL-15; growth factors and hormones like keratinocyte growth factor (KGF), insulin-like growth factor (IGF)-1 and growth hormone (GH); adoptive transfer of ex vivo-generated precursor T cells (preT) and sex steroid ablation (SSA). Moreover, recently several novel approaches have been proposed to generate whole thymii ex vivo using stem cell technologies and bioscaffolds. Increasingly, however, when transferred to the clinic, these strategies alone are not sufficient to restore thymopoiesis in all patients leading to the potential of combination strategies as a way to reign in non-responders. Synergistic enhancement in combination may be due to differential targets may therefore be effective in improving clinical outcomes in the transplant settings as well as in other lymphopenic states induced by high dose chemotherapy/radiation therapy or HIV, and may also be useful in improving responses to vaccination and augmenting anti-tumor immunotherapy.
Keywords: Immune regeneration, thymus, allogeneic hematopoietic stem cell transplantation
The importance for immune regeneration
Allogeneic hematopoietic stem cell transplant (allo-HSCT) is a potentially curative therapy for leukemia patients and others with hematological malignancies. Its use, however, is restricted by several major complications, including graft versus host disease (GVHD), malignant relapse and post-transplant immune deficiency. Following transplantation, recipients can suffer a period of prolonged immune deficiency, especially devastating to the T cell compartment, which leads to an increase in opportunistic infections and higher treatment-associated morbidity and mortality due to the extended period of time that it takes to recover from such insult (1). Furthermore, one of the most significant physiological problems underlying a broad spectrum of diseases is the well-recognised progressive decline in immune competence with age. This is manifest at many levels including increased opportunistic infections, incidence and burden of cancer and, somewhat paradoxically, autoimmune disease. There is also a markedly reduced capacity to respond to overt vaccines but of paramount importance is the very poor recovery from immune insults such as common cancer cytoreductive treatments of chemotherapy and radiotherapy, particularly when in combination with allo-HSCT. This delay in immune recovery can precipitate high morbidity, and often mortality, from opportunistic infections and may even facilitate cancer relapse. In fact, the risk of opportunistic infection in the post-transplant period is directly correlated to the recovery of T cells. The severity and duration of this immune deficiency is influenced by several factors such as previous chemo- or radiation- therapy, GVHD and donor/host incompatibility, however, there is a clear inverse relationship between transplant recipient age and the capacity to generate T lymphocytes. Therefore, strategies to enhance T cell recovery could significantly improve the outcome of allo-HSCT.
Optimal T cell development requires a functional thymus and many patients who could benefit from enhancing immune regeneration after allo-HSCT have poor thymic function due to their age or their exposure to chemo- and radiation therapy. Thymopoiesis is a complex process involving the cross-talk between developing thymocytes and the non-hematopoietic supporting stromal microenvironment (2). Moreover, thymic involution caused by age or exposure to chemo- and radiation therapy impacts on both developing thymocytes and the thymic stromal compartment (3). Consequently, regeneration of intrathymic hematopoietic and stromal compartments is of critical importance for sustained and T cell production following immunodepletion.
Immune Degeneration: aetiology and implications
Treatment with chemotherapy or radiation therapy results in the severe depletion of all hematopoietic cells of the immune system. Both alkylating chemotherapeutics and irradiation target highly proliferative cells (4–6), including developing and naïve lymphocytes making them particularly depleted following treatment (7). Delayed recovery following immunodepletion is associated with a high degree of morbidity and mortality (7–9). Lymphoid recovery is critically dependent on functioning primary immune organs. In particular CD4+ T cells are dependent on a functioning thymus for recovery (10). Compared to children, adults whose thymii have already involuted, are significantly impaired in their ability to recover following chemotherapy (11–13). Interestingly, while CD8+ T cell recovery in both young and aged patients is quite rapid (14), these are predominantly extrathymically derived by clonal expansion (15, 16). While delayed, full immune recovery is possible up until middle age, however, in older patients the peripheral naïve T cell receptor repertoire is never fully restored (17). Similarly, recovery of B cells and NK cells are also severely impaired in aged compared to young patients (17). Furthermore, much like the TCR repertoire, the B cell repertoire is severely diminished after chemotherapy and suffers a prolonged recovery (18).
Interestingly while quiescent HSCs are largely numerically spared from many chemotherapeutic and low-dose radiation regimes (19), their hematopoietic function appears to be significantly impaired (20, 21). Importantly this was also shown with human CD34+ HSCs (22) suggesting that the standard clinical practice of collecting autologous HSCs in remission, oftentimes after prolonged chemotherapy treatment, may lead to poorer transplant outcomes. Furthermore, immunodepletion from radiation or chemotherapy was shown to cause enhanced senescence in HSCs that was coupled with an upregulation in the cyclin-dependent kinase inhibitors p19Arf and p16ink4A (23, 24) mimicking some of the effects seen with age.
The profound involution of the thymus with age is one of the most widely studied effects of age on the immune system (25–27). While the proportion of thymocyte subsets remains unaffected with age, there is a profound decline in the supply of BM and intrathymic lymphoid progenitors (28, 29) and a significant disruption to the thymic architecture (30–34). This results in a decline in the emigration of naïve T cells into the periphery (35) and a consequent homeostatic expansion of pre-existing peripheral memory T cells (36, 37). Taken together, this results in a decline in the peripheral T cell receptor repertoire (38) and subsequently a reduced responsiveness to both new and previously encountered antigens (39, 40). Age-related thymic involution has profound implications for designing regenerative strategies after immunodepleting therapies as a functioning thymus is crucial to the recovery of T cells following immunodepletion (41).
Toolkit for Immune Regeneration
Previous studies have demonstrated several novel treatments with beneficial regenerative effects on the thymus, including the administration of growth factors such as keratinocyte growth factor (KGF), IL-7 and fms-like tyrosine kinase 3 ligand (Flt3L), withdrawal of sex steroids and adoptive transfer of in vitro generated precursor T (pre-T) cells (31, 34, 42–50). Administration of growth factors to aid in thymic regeneration specifically targets either intrathymic hematopoietic cells (IL-7 and Flt3L) or the stromal microenvironment (KGF). Rejuvenation of either compartment leads to a commensurate regeneration within the other, suggesting that absolute thymic renewal can be achieved by promoting either lymphoid or stromal expansion.
Cytokines and Hormones
Several exogenously administered cytokines and hormones have been nominated for their potential to regenerate lymphopoiesis. Keratinocyte Growth Factor (KGF), IL-7, Flt-3 Ligand (Flt3L) and Growth Hormone (GH) have all shown promise in their regenerative abilities (1, 51–53).
Exogenous administration of KGF has been found to increase thymic cellularity up to four fold in the aged and following radiation or chemotherapy (42, 54, 55) and significantly enhances response to plasmid tumor vaccines (56). Furthermore, KGF can actually protect TECs from GVHD mediated thymic damage (55) and KGF-induced thymopoiesis is mediated by proliferation and expansion of TECs (57). The pro-lymphopoietic cytokine IL-7, which has long been recognised for its role in steady-state lymphopoiesis (58, 59), has also been studied for its potential in enhancing immune regeneration. Several studies have demonstrated the beneficial effects of exogenous administration of IL7 which enhances thymopoiesis and recent thymic emigrants as well as aiding peripheral T cell function in aged mice or following allogeneic BMT (43, 60–63). The mechanism behind IL7 induced thymic regeneration lies in its ability to reverse age-related increases in apoptosis (64), while simultaneously enhancing the proliferation (43) of lymphocytes and lymphoid precursors. Administration of Flt3L enhances both thymic dependent and independent T cell reconstitution (48, 65). The effects of Flt3L are predominantly due to an expansion in Flt3+ progenitors in the BM (66). However, increases in T cell reconstitution can be at the expense of B-lymphopoiesis which is significantly declined with exogenous Flt3L administration and, in particular, its effects on early progenitors with both lymphoid and myeloid potential (67, 68). Use of growth hormone (GH) has also been proposed as a possible regenerative therapy. Treatment with exogenous GH regenerates the aged thymus (27, 69) and enhances HPC function in the BM (70). GH has also been shown to reverse irradiation-associated loss of BM function determined by colony formation (70).
Several other cytokines and growth factors have been evaluated for a beneficial role in regenerating the immune system. These include IGF-1, which promotes TEC expansion and enhances reconstitution following HSCT (71–73); IL-15, which predominantly promotes proliferation of circulating NK and T cells (74, 75); and IL-12, which stimulates thymic expression of IL-7 and enhances hematopoietic engraftment after transplant (76–78), although IL-12 and IL-15 have also been recently found to also act on regulatory lymphoid-tissue inducer cells and NK cells (79, 80).
Sex steroid withdrawal
As sex steroids have been implicated in the degeneration of BM and thymic lymphopoiesis (81, 82), sex steroid ablation (SSA), which can be achieved in a reversible chemical fashion (83, 84), has been investigated for its potential in enhancing the immune system. Studies have found that removal of sex steroids leads to reorganised thymic architecture, an enhanced ability to import circulating progenitors (85) and subsequently enhances thymopoiesis in aged mice and humans (31, 34, 45, 50, 85–89). The effects of SSA, however, are not restricted to the thymus with enhanced B-lymphopoiesis and lymphoid progenitors (29, 90–94)) also observed, as well as enhancing overall immune recovery following autologous (95) and allogeneic (44) BMT as well as cytoablative therapy (29, 50, 89). Taken together these studies indicate the wide-ranging implications on immune recovery following SSA and provide evidence for clinical application of SSA in treatments where immune depletion is an unavoidable side effect.
Precursor Cell Therapies and Artificial Organs
The length of time to recovery following HSCT is at least in part due to the length of time required to develop and mature from HSC to functional T cell. While early strategies seeking to fill the void in T cell development after transplant employed isolation and co-transplant of BM-derived lymphoid precursor cells (96), the advent of the robust OP9-DL1 in vitro T cell development system, in which large numbers of highly purified T cell precursors can be generated by incubating hematopoietic stem cells with the bone marrow stromal cell line OP9, transduced with the Notch ligand Delta-like 1 (OP9-DL1), has meant that even more mature T cell precursors can be used to offer a ready supply of T cell precursors well before they develop from HSCs. Adoptive transfer of OP9-DL1-derived T cell precursors into lethally irradiated allo-HSCT recipients caused significant increases in thymic cellularity and chimerism, as well as enhanced peripheral T and NK cell reconstitution compared with recipients of allogeneic hematopoietic stem cells (HSCs) only (46, 97). In addition to their significant benefit for immune regeneration following transplant, in vitro generated pre-T cells can also be genetically engineered for tumor-specificity and subsequently used for targeted tumor immunotherapy (47). However, the impact of pre-T cell treatment on the supporting stromal microenvironment will be of critical importance in promoting long-term thymic regeneration.
Consequently, in addition to providing a supply of T cell precursors, several groups are attempting to identify and isolate populations of thymic epithelial progenitor cells (TEPC) that could be used to directly enhance the function of the thymus by providing regenerative benefit to the supporting stromal microenvironment. While using different strategies TEPC have been successfully isolated from fetal thymii and coaxed into generating a new thymus in FoxN1−/− recipients (98–101), the identity and existence of a similar population in the adult thymus has thus far remained elusive (102, 103). Recently, however, in a glimpse of the possibilities and potential of iPS technologies, a recent study has directed the reprogramming of thymic epithelial cells into functional multipotent skin stem cells (104). A reverse of this approach, by reprogramming skin epithelium into TEPC, would offer an excellent opportunity to either regenerate the thymus by grafting TEPC directly or even ex vivo generation of a transplantable thymus.
There are currently several approaches being considered to rejuvenate immunity that do not rely on the endogenous thymus at all but rather concentrate on forming whole organs ex vivo that can be transplanted into patients as required (52, 105). These include the decellularization of an existing organ and using biomatrices in addition to vascularising chambers. One such strategy gaining momentum is to decellularize an existing organ, which removes all cellular compartments of the organ, leaving only the extracellular matrix components. Critically, this approach removes the significant immune barriers preventing xenogenic transplantation. This technique has so far been achieved only in heart, liver and lung (106–109). Another promising alternate approach for generating an ectopic thymus is the use of biomatrices that can be used to seed TEPC and mesenchymal elements to form an ex vivo generated thymus. One study, which used this approach in combination with implantable chambers that promote vascularization, found that this approach could be used to generate a functional thymus in vivo (110). However, at this stage these approaches still require TEPC to initiate thymic organogenesis highlighting the dependence of fetal tissue and the importance of discovering an adult TEPC.
Immune crosstalk: Rationale for combination strategies
The considerable crosstalk that occurs between developing thymocytes and the supporting microenvironment has been known for some time. However, while most of these interactions remain poorly understood, thymic crosstalk has been implicated in regulating Notch signalling (111), IL-7 expression (112, 113) and thymic organogenesis. Deficiency in specific lymphoid or stromal subsets has led to findings of commensurate deficiency in the corresponding compartment, for instance, presence of CD44−CD25+ TN2 thymocytes is critically required for cTEC organisation while single positive (CD4+ or CD8+) thymocytes are crucial for the maintenance of mTECs (114, 115). Moreover, while differentiation from TEC progenitors into mTEC or cTEC is not dependent on crosstalk with lymphoid progenitors (116, 117), development of the thymus itself is critically dependent on interactions with the earliest lymphoid progenitors in a highly specific window (118) and conditional deletion of TECs leads to profound loss in T cell production (119).
These studies suggest the significant contribution of each compartment to the development and maintenance of the other. Despite, or perhaps even because of it, this crosstalk between the thymic stroma and the hematopoietic compartment suggests that a combination of strategies targeting different compartments contributing towards thymopoiesis will lead to a greater regenerative boost than could be achieved with either strategies alone. In fact KGF has been proposed as an effective agent when used in conjunction with other regenerative therapies, successfully enhancing the sole regenerative benefits of preT cells (46), SSA (120) and temporary inhibition of p53 (121). Contrary to this, it is unlikely that IL-7 could be used effectively in combination with KGF or SSA as both of these strategies promote intrathymic production of IL-7 and studies in knockout mice demonstrate both of these regenerative strategies are dependent on IL-7 (42, 44).
The crosstalk between developing T cells and the supporting thymic stromal microenvironment is critical for normal thymus function and has been exploited to great effect for thymic regeneration. However, the fundamental relationship between the BM and the thymus, which is primarily predicated on the supply of lymphoid precursors, has been far less exploited as a means for promoting immune regeneration. While the precise identity of the circulating progenitor released from the BM to seed the thymus is unclear (122–126) their potential for improving immune reconstitution is not (96). Moreover, fundamental defects in HSC function, particularly in their ability to differentiate down the lymphoid lineage (127), may contribute towards some of the age-related changes observed in the thymus (94). Moreover, several strategies have been studied for their impact on BM recovery. These include parathyroid hormone (PTH), which can enhance HSC numbers (128) and retinoic acid (129), which accelerates B lymphopoiesis. However, at least in the aged setting, improving HSC function alone is not sufficient to restore thymopoiesis (130) and the reduced importation of progenitors is not enough to cause thymic involution (131) suggesting that these defects in HSC function merely contribute rather than cause age-related declines in lymphopoiesis. Nevertheless, strategies combining different aspects of immune renewal, from thymus-restricted KGF and preT cells to BM-restricted PTH and retinoic acids as well as more systemic therapies like IL7, Flt3L and SSA – aid the overall regeneration of the immune system. Taken together these findings lay the groundwork for the effective use of combination strategies for systemic immune renewal.
Concluding Remarks
These novel approaches to restore immune capacity through the translation of pre-clinical research will likely result in the development of new strategies to improve the outcome for a variety of patients who incur considerable morbidity and mortality from infections and relapse after transplant. Finally these strategies could also be used in a variety of clinical settings to overcome lymphocytopenia or to stimulate lymphocyte regeneration, including autologous HSCT, high dose chemotherapy, AIDS, vaccination, tolerance-induction or directed tumour therapies. Tying these strategies together, it stands to reason that using KGF to act directly on the thymic stroma, SSA to give an overall regenerative boost to the thymus and the supply of endogenous BM-derived precursors, IL7 to enhance precursor T cells and circulating mature T cells and finally, administering a ready-supply of ex vivo generated precursor T cells, will give the greatest clinical outcomes in modalities of immune depletion.
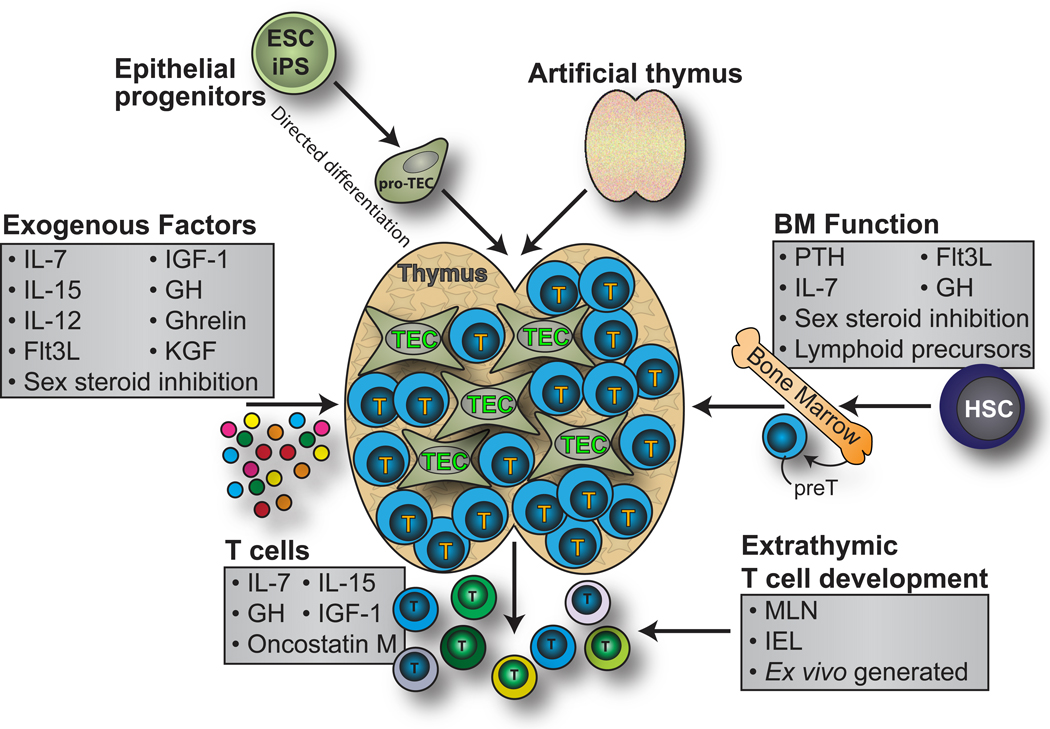
Figure 1. Strategies to enhance T cell immunity following allo-HSCT.
Several therapeutic strategies have been developed for boosting T cell reconstitution following immunodepletion. Exogenous cytokines and hormones such as IL-7, IL-12, IL-15, KGF, Flt3L, GH and IGF-1 have all been used to directly or indirectly boost T cell development in the thymus. T cell development prior to thymic involvement can also be targeted using factors such as PTH, administration of BM-derived lymphoid precursors or sex steroid inhibition. Several of these strategies, such as IL-7, IL-15, GH and IGF-1 also target peripheral lymphocytes mediating the expansion of pre-existing clones and leading to rapid, albeit limited, immune reconstitution. However, all of these therapies use the pre-existing thymic architecture, which can be critically damaged due to age or the conditioning regimes required for successful engraftment. One alternative solution would be to generate thymic tissue ex vivo using embryonic stem cell (or iPS)-derived TEPCs or even developing entire artificial organs using biomatrices or decellularization protocols.
Table 1.
Mechanisms underlying the most promising strategies for immune regeneration
| Therapy | Impact on immunity | References |
|---|---|---|
| IL-7 | Directly promotes expansion of lymphoid progenitors and peripheral T cells | (43, 49, 62, 64, 75, 132–135) |
| IL-12 | Enhances thymopoiesis by inducing expression of IL-7 and IL-2 | (77, 78) |
| IL-15 | Proliferation and expansion of circulating NK and T cells | (74, 75) |
| KGF | Promotes proliferation and expansion of thymic epithelial cells | (42, 54, 55, 57, 136) |
| Flt3L | Directly promotes expansion and differentiation of lymphoid progenitors | (48, 65, 66, 68, 137) |
| IGF-1 | Promotes expansion of thymic epithelial cells | (71–73) |
| GH/Ghrelin | Enhances thymopoiesis and BM hematopoietic function | (69, 70) |
| SSA | Impacts on proliferation of BM and intrathymic lymphoid progenitors. Also enhances TECs. | (29, 31, 34, 44, 45, 50, 89, 95, 138) |
| preT | Provides a ready-supply of T cell progenitors for thymopoiesis | (46, 47, 97, 139) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4:856–867. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 2.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 3.Chidgey A, Dudakov J, Seach N, Boyd R. Impact of niche aging on thymic regeneration and immune reconstitution. Semin Immunol. 2007;19:331–340. doi: 10.1016/j.smim.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Brock N. The history of the oxazaphosphorine cytostatics. Cancer. 1996;78:542–547. doi: 10.1002/(SICI)1097-0142(19960801)78:3<542::AID-CNCR23>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nature reviews. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 6.Trobaugh FE, Jr, Husseini S. Effects of radiation on hematopoietic tissue. Am J Med Technol. 1973;39:119–131. [PubMed] [Google Scholar]
- 7.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, McClure LL, Gress RE. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84:2221–2228. [PubMed] [Google Scholar]
- 8.Pizzo PA, Rubin M, Freifeld A, Walsh TJ. The child with cancer and infection. II. Nonbacterial infections. The Journal of pediatrics. 1991;119:845–857. doi: 10.1016/s0022-3476(05)83032-x. [DOI] [PubMed] [Google Scholar]
- 9.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells. 2000;18:10–18. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 10.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, Gress RE. Age-dependent incidence, time course, and consequences of thymic renewal in adults. The Journal of clinical investigation. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 12.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–425. [PubMed] [Google Scholar]
- 13.Weinberg K, Annett G, Kashyap A, Lenarsky C, Forman SJ, Parkman R. The effect of thymic function on immunocompetence following bone marrow transplantation. Biol Blood Marrow Transplant. 1995;1:18–23. [PubMed] [Google Scholar]
- 14.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Magrath IT, Wexler LH, Dimitrov DS, Gress RE. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–3707. [PubMed] [Google Scholar]
- 15.Fagnoni FF, Lozza L, Zibera C, Zambelli A, Ponchio L, Gibelli N, Oliviero B, Pavesi L, Gennari R, Vescovini R, Sansoni P, Da Prada G, Robustelli Della Cuna G. T-cell dynamics after high-dose chemotherapy in adults: elucidation of the elusive CD8+ subset reveals multiple homeostatic T-cell compartments with distinct implications for immune competence. Immunology. 2002;106:27–37. doi: 10.1046/j.1365-2567.2002.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitger A, Neu N, Kern H, Panzer-Grumayer ER, Greinix H, Nachbaur D, Niederwieser D, Fink FM. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90:850–857. [PubMed] [Google Scholar]
- 17.Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN, Dimopoulos MA. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. European journal of clinical investigation. 2005;35:380–387. doi: 10.1111/j.1365-2362.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Jin F, Freitas A, Szabo P, Weksler ME. Impaired regeneration of the peripheral B cell repertoire from bone marrow following lymphopenia in old mice. Eur J Immunol. 2001;31:500–505. doi: 10.1002/1521-4141(200102)31:2<500::aid-immu500>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Gardner RV, McKinnon E, Astle CM. Analysis of the stem cell sparing properties of cyclophosphamide. Eur J Haematol. 2001;67:14–22. doi: 10.1034/j.1600-0609.2001.067001014.x. [DOI] [PubMed] [Google Scholar]
- 20.Gardner RV, McKinnon E, Poretta C, Leiva L. Hemopoietic function after use of IL-1 with chemotherapy or irradiation. J. Immunol. 2003;171:1202–1206. doi: 10.4049/jimmunol.171.3.1202. [DOI] [PubMed] [Google Scholar]
- 21.Meng A, Wang Y, Brown SA, Van Zant G, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol. 2003;31:1348–1356. doi: 10.1016/j.exphem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Offner F, Kerre T, De Smedt M, Plum J. Bone marrow CD34 cells generate fewer T cells in vitro with increasing age and following chemotherapy. Br J Haematol. 1999;104:801–808. doi: 10.1046/j.1365-2141.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 23.Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–5419. [PubMed] [Google Scholar]
- 24.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- 26.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 27.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 28.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 29.Dudakov JA, Goldberg GL, Reiseger JJ, Chidgey AP, Boyd RL. Withdrawal of Sex Steroids Reverses Age- and Chemotherapy-Related Defects in Bone Marrow Lymphopoiesis. J. Immunol. 2009;182:6247–6260. doi: 10.4049/jimmunol.0802446. [DOI] [PubMed] [Google Scholar]
- 30.George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 32.Rodewald HR. The thymus in the age of retirement. Nature. 1998;396:630–631. doi: 10.1038/25251. [DOI] [PubMed] [Google Scholar]
- 33.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 34.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J. Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 35.Berzins SP, Uldrich AP, Sutherland JS, Gill J, Miller JF, Godfrey DI, Boyd RL. Thymic regeneration: teaching an old immune system new tricks. Trends Mol Med. 2002;8:469–476. doi: 10.1016/s1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
- 36.Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, Hayakawa K, Hardy RR, Weigle WO. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J. Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- 37.Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech. Ageing Dev. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- 38.Kurashima C, Utsuyama M, Kasai M, Ishijima SA, Konno A, Hirokawa K. The role of thymus in the aging of Th cell subpopulations and age-associated alteration of cytokine production by these cells. Int Immunol. 1995;7:97–104. doi: 10.1093/intimm/7.1.97. [DOI] [PubMed] [Google Scholar]
- 39.LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, Szabo P, Weksler ME, Nikolich-Zugich J. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J. Immunol. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 40.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205 doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 42.Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, Suh D, Muriglan SJ, Boyd RL, van den Brink MR. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alpdogan O, Muriglan SJ, Eng JM, Willis LM, Greenberg AS, Kappel BJ, van den Brink MR. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. The Journal of clinical investigation. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM, Hubbard VM, Kochman A, Willis LM, Greenberg AS, Tjoe KH, Sutherland JS, Chidgey A, van den Brink MRM, Boyd RL. Enhanced Immune Reconstitution by Sex Steroid Ablation following Allogeneic Hemopoietic Stem Cell Transplantation. J. Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, Samstein RM, Dudakov JA, Chidgey AP, Chen-Kiang S, Boyd RL, van den Brink MRM. Luteinizing Hormone-Releasing Hormone Enhances T Cell Recovery following Allogeneic Bone Marrow Transplantation. J. Immunol. 2009;182:5846–5854. doi: 10.4049/jimmunol.0801458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, Patel N, Chow A, Cabrera-Perez J, Radhakrishnan R, Diab A, Perales MA, Rizzuto G, Menet E, Pamer EG, Heller G, Zuniga-Pflucker JC, Alpdogan O, van den Brink MR. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 47.Zakrzewski JL, Suh D, Markley JC, Smith OM, King C, Goldberg GL, Jenq R, Holland AM, Grubin J, Cabrera-Perez J, Brentjens RJ, Lu SX, Rizzuto G, Sant'Angelo DB, Riviere I, Sadelain M, Heller G, Zuniga-Pflucker JC, Lu C, van den Brink MR. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol. 2008;26:453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fry TJ, Sinha M, Milliron M, Chu YW, Kapoor V, Gress RE, Thomas E, Mackall CL. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood. 2004;104:2794–2800. doi: 10.1182/blood-2003-11-3789. [DOI] [PubMed] [Google Scholar]
- 49.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 50.Dudakov JA, Goldberg GL, Reiseger JJ, Vlahos K, Chidgey AP, Boyd RL. Sex Steroid Ablation Enhances Hematopoietic Recovery following Cytotoxic Antineoplastic Therapy in Aged Mice. J. Immunol. 2009;183:7084–7094. doi: 10.4049/jimmunol.0900196. [DOI] [PubMed] [Google Scholar]
- 51.Holland AM, van den Brink MRM. Rejuvenation of the aging T cell compartment. Curr Opin Immunol. 2009;21:454–459. doi: 10.1016/j.coi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chidgey AP, Seach N, Dudakov J, Hammett MV, Boyd RL. Strategies for reconstituting and boosting T cell-based immunity following haematopoietic stem cell transplantation: pre-clinical and clinical approaches. Semin Immunopathol. 2008;30:457–477. doi: 10.1007/s00281-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg GL, Zakrzewski JL, Perales MA, van den Brink MR. Clinical strategies to enhance T cell reconstitution. Semin Immunol. 2007;19:289–296. doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min D, Panoskaltsis-Mortari A, Kuro OM, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossi S, Blazar BR, Farrell CL, Danilenko DM, Lacey DL, Weinberg KI, Krenger W, Hollander GA. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 56.Jenq RR, King CG, Volk C, Suh D, Smith OM, Rao UK, Yim NL, Holland AM, Lu SX, Zakrzewski JL, Goldberg GL, Diab A, Alpdogan O, Penack O, Na I-K, Kappel LW, Wolchok JD, Houghton AN, Perales M-A, van den Brink MRM. Keratinocyte growth factor enhances DNA plasmid tumor vaccine responses after murine allogeneic bone marrow transplantation. Blood. 2009;113:1574–1580. doi: 10.1182/blood-2008-05-155697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossi SW, Jeker LT, Ueno T, Kuse S, Keller MP, Zuklys S, Gudkov AV, Takahama Y, Krenger W, Blazar BR, Hollander GA. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109:3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasson SC, Zaunders JJ, Kelleher AD. The IL-7/IL-7 receptor axis: understanding its central role in T-cell homeostasis and the challenges facing its utilization as a novel therapy. Curr Drug Targets. 2006;7:1571–1582. doi: 10.2174/138945006779025365. [DOI] [PubMed] [Google Scholar]
- 59.Awong G, LaMotte-Mohs R, Zuniga-Pflucker JC. Key players for T-cell regeneration. Curr Opin Hematol. 2010;17:327–332. doi: 10.1097/MOH.0b013e3283395133. [DOI] [PubMed] [Google Scholar]
- 60.Lu H, Zhao Z, Kalina T, Gillespy T, 3rd, Liggitt D, Andrews RG, Maloney DG, Kiem HP, Storek J. Interleukin-7 improves reconstitution of antiviral CD4 T cells. Clin Immunol. 2005;114:30–41. doi: 10.1016/j.clim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol. Immunol. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [erratum appears in Mol Immunol 2002 Oct;39(5–6):379-80] [DOI] [PubMed] [Google Scholar]
- 62.Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, Hecht TT, Hill BJ, Komschlies K, Tomaszewski J, Franchini G, Mackall CL. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 63.Chu YW, Memon SA, Sharrow SO, Hakim FT, Eckhaus M, Lucas PJ, Gress RE. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood. 2004;104:1110–1119. doi: 10.1182/blood-2003-10-3635. [DOI] [PubMed] [Google Scholar]
- 64.Andrew D, Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J. Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 65.Kenins L, Gill JW, Boyd RL, Hollander GA, Wodnar-Filipowicz A. Intrathymic expression of Flt3 ligand enhances thymic recovery after irradiation. J Exp Med. 2008;205:523–531. doi: 10.1084/jem.20072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wils EJ, Braakman E, Verjans GM, Rombouts EJ, Broers AE, Niesters HG, Wagemaker G, Staal FJ, Lowenberg B, Spits H, Cornelissen JJ. Flt3 ligand expands lymphoid progenitors prior to recovery of thymopoiesis and accelerates T cell reconstitution after bone marrow transplantation. J. Immunol. 2007;178:3551–3557. doi: 10.4049/jimmunol.178.6.3551. [DOI] [PubMed] [Google Scholar]
- 67.Balciunaite G, Ceredig R, Massa S, Rolink AG. A B220+ CD117+ CD19-hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35:2019–2030. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 68.Ceredig R, Rauch M, Balciunaite G, Rolink AG. Increasing Flt3L availability alters composition of a novel bone marrow lymphoid progenitor compartment. Blood. 2006;108:1216–1222. doi: 10.1182/blood-2005-10-006643. [DOI] [PubMed] [Google Scholar]
- 69.Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlo-Stella C, Di Nicola M, Milani R, Longoni P, Milanesi M, Bifulco C, Stucchi C, Guidetti A, Cleris L, Formelli F, Garotta G, Gianni AM. Age- and irradiation-associated loss of bone marrow hematopoietic function in mice is reversed by recombinant human growth hormone. Exp Hematol. 2004;32:171–178. doi: 10.1016/j.exphem.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, O'Reilly RJ, van den Brink MR. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 72.Chu YW, Schmitz S, Choudhury B, Telford W, Kapoor V, Garfield S, Howe D, Gress RE. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood. 2008;112:2836–2846. doi: 10.1182/blood-2008-04-149435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taguchi T, Takenouchi H, Matsui J, Tang WR, Itagaki M, Shiozawa Y, Suzuki K, Sakaguchi S, Ktagiri YU, Takahashi T, Okita H, Fujimoto J, Kiyokawa N. Involvement of insulin-like growth factor-I and insulin-like growth factor binding proteins in pro-B-cell development. Exp. Hematol. 2006;34:508–518. doi: 10.1016/j.exphem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Alpdogan O, Eng JM, Muriglan SJ, Willis LM, Hubbard VM, Tjoe KH, Terwey TH, Kochman A, van den Brink MR. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005;105:865–873. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 75.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Chen J, Wang J, Li J, Wu Q, Chu Lim F, Yang P, Hsu HC, Curiel DT, Mountz JD. Enhancement of cytotoxic T-lymphocyte response in aged mice by a novel treatment with recombinant AdIL-12 and wild-type adenovirus in rapid succession. Mol. Ther. 2008;16:1500–1506. doi: 10.1038/mt.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen T, Burke KA, Zhan Y, Wang X, Shibata D, Zhao Y. IL-12 facilitates both the recovery of endogenous hematopoiesis and the engraftment of stem cells after ionizing radiation. Exp Hematol. 2007;35:203–213. doi: 10.1016/j.exphem.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Li L, Hsu HC, Stockard CR, Yang P, Zhou J, Wu Q, Grizzle WE, Mountz JD. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. J. Immunol. 2004;172:2909–2916. doi: 10.4049/jimmunol.172.5.2909. [DOI] [PubMed] [Google Scholar]
- 79.Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11:1030–1038. doi: 10.1038/ni.1947. [DOI] [PubMed] [Google Scholar]
- 80.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CAJ, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3,àíNKp46+ cell subsets from Id2-dependent precursors. The Journal of Experimental Medicine. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15131–15136. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 83.Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 84.Huirne JA, Lambalk CB. Gonadotropin-releasing-hormone-receptor antagonists. Lancet. 2001;358:1793–1803. doi: 10.1016/S0140-6736(01)06797-6. [DOI] [PubMed] [Google Scholar]
- 85.Williams KM, Lucas PJ, Bare CV, Wang J, Chu YW, Tayler E, Kapoor V, Gress RE. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008;112:3255–3263. doi: 10.1182/blood-2008-04-153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greenstein BD, Fitzpatrick FT, Kendall MD, Wheeler MJ. Regeneration of the thymus in old male rats treated with a stable analogue of LHRH. J Endocrinol. 1987;112:345–350. doi: 10.1677/joe.0.1120345. [DOI] [PubMed] [Google Scholar]
- 87.Olsen NJ, Watson MB, Henderson GS, Kovacs WJ. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology. 1991;129:2471–2476. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- 88.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 2004;173:6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 89.Goldberg GL, Dudakov JA, Reiseger JJ, Seach N, Ueno T, Vlahos K, Hammett MV, Young LF, Heng TSP, Boyd RL, Chidgey AP. Sex Steroid Ablation Enhances Immune Reconstitution Following Cytotoxic Antineoplastic Therapy in Young Mice. J. Immunol. 2010;184:6014–6024. doi: 10.4049/jimmunol.0802445. [DOI] [PubMed] [Google Scholar]
- 90.Erben RG, Eberle J, Stangassinger M. B lymphopoiesis is upregulated after orchiectomy and is correlated with estradiol but not testosterone serum levels in aged male rats. Horm. Metab. Res. 2001;33:491–498. doi: 10.1055/s-2001-16943. [DOI] [PubMed] [Google Scholar]
- 91.Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED. Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol. 2001;13:553–558. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- 92.Erben RG, Raith S, Eberle J, Stangassinger M. Ovariectomy augments B lymphopoiesis and generation of monocyte-macrophage precursors in rat bone marrow. Am J Physiol. 1998;274:E476–E483. doi: 10.1152/ajpendo.1998.274.3.e476. [DOI] [PubMed] [Google Scholar]
- 93.Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dudakov JA, Khong DMP, Boyd RL, Chidgey AP. Feeding the fire: the role of defective bone marrow function in exacerbating thymic involution. Trends Immunol. 2010;31:191–198. doi: 10.1016/j.it.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP, Boyd RL. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- 96.Arber C, BitMansour A, Sparer TE, Higgins JP, Mocarski ES, Weissman IL, Shizuru JA, Brown JM. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 97.Holland AM, Zakrzewski JL, Goldberg GL, Ghosh A, van den Brink MR. Adoptive precursor cell therapy to enhance immune reconstitution after hematopoietic stem cell transplantation in mouse and man. Semin Immunopathol. 2008;30:479–487. doi: 10.1007/s00281-008-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gill J, Malin M, Hollander GA, Boyd R. Generation of a complete thymic microenvironment by MTS24(+) thymic epithelial cells. Nat Immunol. 2002;3:635–642. doi: 10.1038/ni812. [DOI] [PubMed] [Google Scholar]
- 99.Bennett AR, Farley A, Blair NF, Gordon J, Sharp L, Blackburn CC. Identification and characterization of thymic epithelial progenitor cells. Immunity. 2002;16:803–814. doi: 10.1016/s1074-7613(02)00321-7. [DOI] [PubMed] [Google Scholar]
- 100.Depreter MG, Blair NF, Gaskell TL, Nowell CS, Davern K, Pagliocca A, Stenhouse FH, Farley AM, Fraser A, Vrana J, Robertson K, Morahan G, Tomlinson SR, Blackburn CC. Identification of Plet-1 as a specific marker of early thymic epithelial progenitor cells. Proc Natl Acad Sci U S A. 2008;105:961–966. doi: 10.1073/pnas.0711170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rossi SW, Chidgey AP, Parnell SM, Jenkinson WE, Scott HS, Boyd RL, Jenkinson EJ, Anderson G. Redefining epithelial progenitor potential in the developing thymus. Eur J Immunol. 2007;37:2411–2418. doi: 10.1002/eji.200737275. [DOI] [PubMed] [Google Scholar]
- 102.Jenkinson WE, Bacon A, White AJ, Anderson G, Jenkinson EJ. An epithelial progenitor pool regulates thymus growth. J. Immunol. 2008;181:6101–6108. doi: 10.4049/jimmunol.181.9.6101. [DOI] [PubMed] [Google Scholar]
- 103.Bleul CC, Corbeaux T, Reuter A, Fisch P, Monting JS, Boehm T. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441:992–996. doi: 10.1038/nature04850. [DOI] [PubMed] [Google Scholar]
- 104.Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y. Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature. 2010;466:978–982. doi: 10.1038/nature09269. [DOI] [PubMed] [Google Scholar]
- 105.Seach N, Layton D, Lim J, Chidgey A, Boyd R. Thymic generation and regeneration: a new paradigm for establishing clinical tolerance of stem cell-based therapies. Curr Opin Biotechnol. 2007;18:441–447. doi: 10.1016/j.copbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 107.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 109.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-Engineered Lungs for in Vivo Implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seach N, Mattesich M, Abberton K, Matsuda K, Tilkorn DJ, Rophael J, Boyd RL, Morrison WA. Vascularized tissue engineering mouse chamber model supports thymopoiesis of ectopic thymus tissue grafts. Tissue Eng Part C Methods. 2010;16:543–551. doi: 10.1089/ten.TEC.2009.0135. [DOI] [PubMed] [Google Scholar]
- 111.Fiorini E, Ferrero I, Merck E, Favre S, Pierres M, Luther SA, MacDonald HR. Cutting Edge: Thymic Crosstalk Regulates Delta-Like 4 Expression on Cortical Epithelial Cells. J. Immunol. 2008;181:8199–8203. doi: 10.4049/jimmunol.181.12.8199. [DOI] [PubMed] [Google Scholar]
- 112.Alves NL, Huntington ND, Mention J-J, Richard-Le Goff O, Di Santo JP. Cutting Edge: A Thymocyte-Thymic Epithelial Cell Cross-Talk Dynamically Regulates Intrathymic IL-7 Expression In Vivo. J. Immunol. 2010;184:5949–5953. doi: 10.4049/jimmunol.1000601. [DOI] [PubMed] [Google Scholar]
- 113.Alves NL, Goff OR, Huntington ND, Sousa AP, Ribeiro VS, Bordack A, Vives FL, Peduto L, Chidgey A, Cumano A, Boyd R, Eberl G, Di Santo JP. Characterization of the thymic IL-7 niche in vivo. Proc Natl Acad Sci U S A. 2009;106:1512–1517. doi: 10.1073/pnas.0809559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Ewijk W, Hollander G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583–1591. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 115.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol. 1991;21:1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 116.Klug DB, Carter C, Gimenez-Conti IB, Richie ER. Cutting edge: thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J. Immunol. 2002;169:2842–2845. doi: 10.4049/jimmunol.169.6.2842. [DOI] [PubMed] [Google Scholar]
- 117.Jenkinson WE, Rossi SW, Jenkinson EJ, Anderson G. Development of functional thymic epithelial cells occurs independently of lymphostromal interactions. Mech Dev. 2005;122:1294–1299. doi: 10.1016/j.mod.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 118.Hollander GA, Wang B, Nichogiannopoulou A, Platenburg PP, van Ewijk W, Burakoff SJ, Gutierrez-Ramos JC, Terhorst C. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373:350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- 119.Chen L, Xiao S, Manley NR. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood. 2009;113:567–574. doi: 10.1182/blood-2008-05-156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, Taylor PA, Boyd RL, Hollander GA, Blazar BR. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008;111:5734–5744. doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kelly RM, Goren EM, Taylor PA, Mueller SN, Stefanski HE, Osborn MJ, Scott HS, Komarova EA, Gudkov AV, Hollander GA, Blazar BR. Short-term inhibition of p53 combined with keratinocyte growth factor improves thymic epithelial cell recovery and enhances T-cell reconstitution after murine bone marrow transplantation. Blood. 2010;115:1088–1097. doi: 10.1182/blood-2009-05-223198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y, Kawamoto H. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 123.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 124.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 125.Serwold T, Richie Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin CH, Aifantis I, Scimone ML, von Andrian UH, Reizis B, von Boehmer H, Gounari F. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- 127.Geiger H, Rudolph KL. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30:360–365. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 129.Chen X, Esplin BL, Garrett KP, Welner RS, Webb CF, Kincade PW. Retinoids accelerate B lineage lymphoid differentiation. J. Immunol. 2008;180:138–145. doi: 10.4049/jimmunol.180.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mackall CL, Gress RE. Thymic aging and T-cell regeneration. Immunol Rev. 1997;160:91–102. doi: 10.1111/j.1600-065x.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 131.Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su D-M. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int. Immunol. 2007;19:1201–1211. doi: 10.1093/intimm/dxm095. [DOI] [PubMed] [Google Scholar]
- 132.Alpdogan O, Schmaltz C, Muriglan SJ, Kappel BJ, Perales MA, Rotolo JA, Halm JA, Rich BE, van den Brink MR. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 133.Jacobs SR, Michalek RD, Rathmell JC. IL-7 Is Essential for Homeostatic Control of T Cell Metabolism In Vivo. J. Immunol. 2010;184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Phillips JA, Brondstetter TI, English CA, Lee HE, Virts EL, Thoman ML. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J. Immunol. 2004;173:4867–4874. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- 135.van Lent AU, Dontje W, Nagasawa M, Siamari R, Bakker AQ, Pouw SM, Maijoor KA, Weijer K, Cornelissen JJ, Blom B, Di Santo JP, Spits H, Legrand N. IL-7 Enhances Thymic Human T Cell Development in "Human Immune System" Rag2-/-IL-2R{gamma}c−/− Mice without Affecting Peripheral T Cell Homeostasis. J. Immunol. 2009;183:7645–7655. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 136.Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, Rubin JS, Rudensky A, Farr AG. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–3278. doi: 10.1182/blood-2002-04-1036. [DOI] [PubMed] [Google Scholar]
- 137.Kenins L, Gill JW, Hollander GA, Wodnar-Filipowicz A. Flt3 ligand-receptor interaction is important for maintenance of early thymic progenitor numbers in steady-state thymopoiesis. Eur. J. Immunol. 2010;40:81–90. doi: 10.1002/eji.200839213. [DOI] [PubMed] [Google Scholar]
- 138.Sutherland JS, Spyroglou L, Muirhead JL, Heng TS, Prieto-Hinojosa A, Prince HM, Chidgey AP, Schwarer AP, Boyd RL. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res. 2008;14:1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]
- 139.Zakrzewski JL, Holland AM, van den Brink MR. Adoptive precursor cell therapy to enhance immune reconstitution after hematopoietic stem cell transplantation. J. Mol. Med. 2007;85:837–843. doi: 10.1007/s00109-007-0175-4. [DOI] [PubMed] [Google Scholar]