Abstract
Subtilosin A is a 35-amino acid long cyclical peptide produced by Bacillus amyloliquefaciens that has potent antimicrobial activity against a variety of human pathogens, including the bacterial vaginosis-related Gardnerella vaginalis. The specific mode of action of subtilosin against G. vaginalis was elucidated by studying its effects on the proton motive force’s (PMF) components: transmembrane electric potential (ΔΨ), transmembrane pH gradient (ΔpH), and intracellular ATP levels. The addition of subtilosin to G. vaginalis cells caused an immediate and total depletion of the ΔpH, but had no effect on the ΔΨ. Subtilosin also triggered an instant but partial efflux of intracellular ATP that was twofold higher than that of the positive control bacteriocin, nisin. Taken together, these data suggest that subtilosin inhibits G. vaginalis growth by creating transient pores in the cells’ cytoplasmic membrane, leading to an efflux of intracellular ions and ATP and eventually cell death.
Keywords: Bacteriocin, Subtilosin, Mode of action, Vaginal pathogen
Introduction
Bacterial vaginosis (BV) is a common condition characterized by an imbalance in the vaginal microflora, where healthy lactobacilli are replaced by a proliferation of facultative and anaerobic microorganisms, most notably Gardnerella vaginalis and Prevotella, Peptostreptococcus, Porphyromonas, and Mobiluncus spp. [9, 10, 22, 33]. It has been estimated that between 10 and 30% of women in North America are afflicted by this ailment, frequently prompting them to seek medical attention [33]. Although BV often remains asymptomatic [1], the unrestricted growth of these organisms has been demonstrated to have pathogenic effects, particularly in pregnant women. BV is associated with the development of pelvic inflammatory disease [14], as well as a variety of pregnancy-related complications, including low fetal birth weight [18], pre-term births with an elevated risk of infant death [28], intra-amniotic infections leading to fetal brain damage [11, 27], and spontaneous abortion [8, 26]. Also of great concern is the well-established connection between BV infection and sexually transmitted diseases (STDs). Bacterial vaginosis, and G. vaginalis in particular, has been shown to increase the probability of contracting HIV and to stimulate its proliferation in multiple cell lines [15, 16, 29, 37].
BV is typically treated by administering the antibiotics metronidazole and clindamycin orally or intravaginally. Although effective, these drugs do not specifically target the pathogens involved in BV, causing widespread inhibition of the healthy vaginal microbiota. In turn, this leads to a high (~20%) recurrence rate of BV shortly after cessation of treatment [40], often with newly arisen developed antibiotic resistances [3, 21, 23]. As such, it is critical that new treatments target the pathogens without affecting the host’s healthy vaginal microflora.
Bacteriocins are ribosomally synthesized peptides produced by bacteria that have antimicrobial activity against organisms closely related to the producer species [7]. Bacteriocins have garnered much attention for their use as safe, natural food preservatives, as well as their potential in medical applications. One bacteriocin, subtilosin A, has strong potential for inclusion in alternative BV therapies. Produced by both Bacillus subtilis [2, 34] and Bacillus amyloliquefaciens [35], subtilosin A (commonly referred to as subtilosin) has a cyclical, cross-linked structure unique among characterized bacteriocins. It has demonstrated antimicrobial activity against a wide variety of human pathogens [30], including G. vaginalis [35], and was recently shown to possess potent spermicidal activity while remaining completely nontoxic to human vaginal epithelial cells and healthy vaginal lactobacilli [30, 35, 36]. However, the inclusion of subtilosin in products aimed at BV prophylaxis or treatment requires a more detailed understanding of its specific mechanism of action against G. vaginalis, one of the primary pathogens involved in BV.
The primary mechanism of action for many bacteriocins is permeabilization of their target cell’s cytoplasmic membrane. Electrostatic interactions between the membrane and bacteriocin provide a temporary linkage that allows the hydrophobic peptide to insert itself into the membrane [31]. In many cases, these transient pores produce leakage of intracellular ions, amino acids, and other low molecular weight molecules and eventually cause a total depletion of the cell’s transmembrane ion potential (ΔpH) [5]. The disruption of this component of the proton motive force (PMF) may then lead to either a subsequent degradation of or efflux of intracellular ATP and/or disruption of the transmembrane electric potential (ΔΨ) [5, 6]. In vitro studies conducted by Thennarasu et al. [38] demonstrated that at high concentrations, subtilosin is able to bind to lipid bilayers, although this binding is modulated by lipid composition. Due to its cyclical, cross-linked structure and uncommon net anionic charge [24], subtilosin can partially penetrate the hydrophobic core of lipid bilayers, disrupting their structure and thereby causing membrane permeabilization. Based on this knowledge, we hypothesized that subtilosin would selectively inhibit the growth of G. vaginalis by depleting cells’ ATP levels and by dissipating one or more components of the proton motive force (PMF).
Materials and Methods
Bacterial Strains and Growth Conditions
Stock cultures of G. vaginalis ATCC 14018 were kept at −80 °C in BHI broth (Difco, Sparks, MD) supplemented with 3% horse serum (JRH Biosciences, Lenexa, KS) and 15% glycerol. Cultures of G. vaginalis were grown anaerobically in BHI broth + 3% horse serum at 37 °C without shaking. B. amyloliquefaciens cultures were grown overnight in MRS broth (Difco) at 37 °C without shaking. The initial cultures were subcultured multiple times before use in experimental testing.
Preparation of Antimicrobial Solutions
The partially purified preparation of subtilosin was prepared as previously described [35]. The purity of this preparation was confirmed via PAGE analysis, with a single protein band evident on the gel. Nisin (Sigma–Aldrich, St. Louis, MO; 100 AU/mL) was prepared according to the protocol given by Turovskiy et al. [39].
ATP Efflux Assay
The effect of subtilosin on ATP depletion in G. vaginalis cells was assessed by the previously established bioluminescence method [13] and modifications of Turovskiy et al. [39] using an ATP Bioluminescent Assay Kit (Sigma–Aldrich) and a Luminoskan™ single-tube luminometer (Labsystems, Helsinki, Finland). This kit correlates ATP release with relative fluorescence as a result of oxidation of the D-luciferin molecule by the firefly luciferase enzyme in the presence of ATP and Mg2+.
G. vaginalis cells were grown overnight in 15-mL BHI broth supplemented with 3% horse serum to an OD660 ≈ 0.6. Once they reached the appropriate growth stage, cells were centrifuged for 15 min at 4500 g (Hermle Z400K; LabNet, Woodbridge, NJ) at room temperature and then washed once with 50 mmol/L MES buffer (pH 6.5). The cells were then maintained at room temperature for 5 min prior to an energization period, in which the cells were resuspended in half their original volume of 50 mmol/L MES buffer (pH 6.5) with 0.2% glucose and held at room temperature for 20 min. Following energization, the cells were collected by centrifugation under the aforementioned conditions and resuspended in half their original volume in 50 mmol/L MES buffer (pH 6.5). This suspension was aliquoted in 100-μL volumes into sterile 1.5-mL microcentrifuge tubes, to which 20 μL of the appropriate treatment was added. Subtilosin was used at a final concentration of 2 μg/mL, while the positive control (bacteriocin nisin) reached a final concentration of 1.5 μg/mL, as per Winkowski et al. [41]. Subtilosin diluent (ddH2O) and nisin diluent (0.02 M hydrochloric acid, pH 1.7) were used as negative controls. Each sample then remained at room temperature for 5 min prior to recording bioluminescent measurements.
The total ATP concentration in G. vaginalis cells was measured by combining 20 μL of the final cell suspension with 4.9 mL of ice-cold ddH2O and 80 μL of DMSO. DMSO was chosen for its known ability to completely lyse bacterial cells, thus releasing all intracellular ATP. The data obtained for the negative controls were extremely uniform, allowing all other results to be normalized to their average and expressed as a percentage value.
Effect of Subtilosin on Proton Motive Force (PMF) in G. vaginalis
ΔΨ Dissipation Assay
The ability of subtilosin to affect the transmembrane electric potential (ΔΨ) of G. vaginalis cells was assessed according to the protocol given by Sims et al. [32] and the modifications outlined by Turovskiy et al. [39].
Briefly, G. vaginalis cells were grown as previously described to an OD600 of 0.6, harvested, then washed once, and resuspended in 1/100 of their original volume of fresh medium. The ΔΨ of the cells was monitored as a function of the fluorescent intensity of the probe 3,3′-dipropylthia-dicarbocyanine iodide [DiSC3 (5)] (Molecular Probes, Eugene, OR) at 22 °C using a PerkinElmer LS-50B spectrofluorometer (PerkinElmer Life and Analytical Science, Inc., Boston, MA) with a slit width of 10 nm and excitation and emission wavelengths of 643 and 666 nm, respectively. Initially, 5 μL of probe was added to 2 mL of fresh BHI broth supplemented with 3% horse serum in quartz cuvettes (10 mm light path) at a final concentration of 5 μmol/L. This was followed by addition of 20 μL of cell suspension, which caused an immediate decrease in fluorescence. Once the signal had equilibrated, the cells were exposed to 2 μL of 5 mM nigericin (Sigma) in order to convert the ΔpH into ΔΨ. After stabilization of the signal, subtilosin (2 μg/mL), the positive control nisin (1.5 μg/mL), or the negative control nisin diluent (HCl at pH 1.7) was added. Finally, any remaining ΔΨ was dissipated by the addition of 2 μL of 2 mmol/L valinomycin (Sigma).
ΔpH Dissipation Assay
The ability of subtilosin to affect the transmembrane pH gradient (ΔpH) of G. vaginalis cells was analyzed according to the protocol given by Molenaar et al. [25] and the modifications described by Turovskiy et al. [39].
Initially, G. vaginalis cells were grown overnight to an OD600 of 0.6, harvested, then washed twice, and resuspended in a hundredth of their original volume of 50 mmol/L potassium phosphate buffer (PPB, pH 6.0). The cells were then exposed for 5 min to the pH sensitive probe BCECF-AM (MP Biomedicals, Inc., Solon, OH) at ambient temperature to allow the probe to diffuse into the cytoplasm. Following exposure, the cells were washed twice with 1 mL of 50 mmol/L PBS (pH 6.0) and resuspended in 200 μL of the same. To measure dissipation of the transmembrane pH gradient, quartz cuvettes containing 2 mL of PPB (pH 7.0) were treated with 10 μL of the cell suspension. Fluorescence was read using a PerkinElmer LS-50B spectrofluorometer with slit widths of 5 nm for excitation and 15 nm for emission, and wavelengths of 502 and 525 nm, respectively. After signal stabilization, the cells were energized with 4 μL of 2.2 mmol/L glucose; the fluorescence subsequently rises as a result of an increase in intracellular pH. After again allowing for the signal to even out, 2 μL of 5 μmol/L valinomycin was added to convert the ΔΨ component of the PMF into ΔpH. The cells were then treated with either subtilosin (2 μg/mL), the positive control nisin (1.5 μg/mL), or the negative control nisin diluent (HCl at pH 1.7). Two microliter of 2 μmol/L nigericin was added to dissipate any remaining ΔpH.
Results
Subtilosin Causes an Efflux of ATP from G. vaginalis Cells
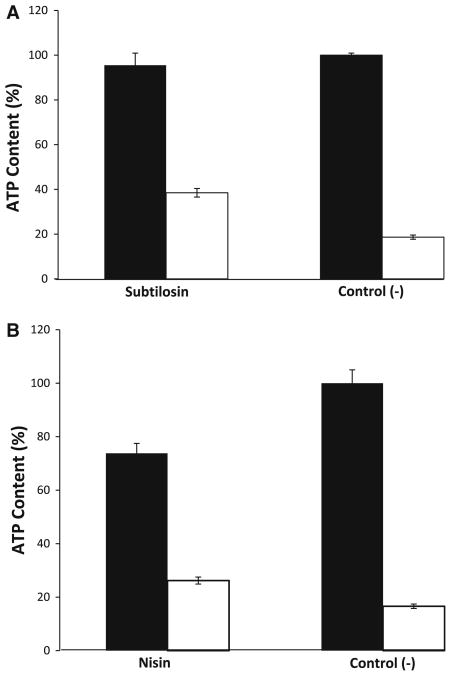
The effect of subtilosin on intracellular ATP levels in G. vaginalis cells was assessed as a function of bioluminescence, via the oxidation of the luminescent D-luciferin molecule by luciferase in the presence of extracellular ATP and Mg2+. Subtilosin caused an efflux of intracellular ATP, denoted by the increased intensity of the luminescence in Fig. 1a. On the other hand, the positive control (nisin) did not cause an efflux of ATP but instead triggered internal hydrolysis of the molecule, evidenced by a decrease in the luminescence in the total ATP sample (Fig. 1b). It was not possible to determine the effect of exposure to subtilosin and the controls past the single 5-min time point as the fastidiously anaerobic G. vaginalis cells poorly tolerated prolonged aerobic conditions (data not shown).
Fig. 1.
Subtilosin causes an efflux of intracellular ATP from G. vaginalis cells. Closed bars represent the total ATP content (intracellular + extracellular), while open bars represent extracellular ATP. Subtilosin (a) caused an efflux of ATP approximately 1.5-fold higher than that of nisin (b) and 2-fold higher than the negative control. Total ATP levels for nisin (b) were 20% lower than that of subtilosin (a) and both negative controls (a, b), indicating intracellular hydrolysis of ATP
Subtilosin has no Effect on G. vaginalis Transmembrane Electrical Potential (ΔΨ)
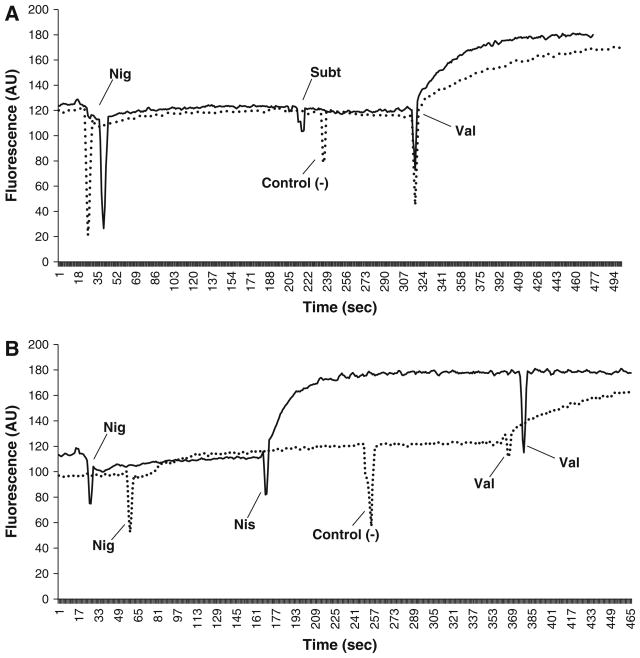
The ability of subtilosin to dissipate the transmembrane electrical potential (ΔΨ) in G. vaginalis cells was observed using the fluorescent probe 3,3′-dipropylthiadicarbocyanine iodide [DiSC3 (5)]. The ionophore nigericin (a K+/H+ exchanger) was added to the G. vaginalis cells in growth medium in order to convert the ΔpH to ΔΨ. The addition of nisin caused an instantaneous increase in the fluorescent signal of the probe as a result of the cellular membrane being depolarized by the bacteriocin (Fig. 2b). Subsequent introduction of the ionophore valinomycin had little effect, indicating nisin caused a complete collapse of this PMF component. However, the addition of subtilosin or the negative controls (nisin diluent and ddH2O) did not cause an elevation in the probe’s fluorescence, signifying they had no effect on the ΔΨ (Fig. 2a, b). For both nisin diluent and subtilosin, subsequent addition of valinomycin fully depleted the ΔΨ, resulting in a fluorescence increase comparable to that seen after the addition of nisin (Fig. 2a, b). Unlike the positive control nisin, which caused a complete dissipation of the ΔΨ, subtilosin does not cause G. vaginalis cell damage by depleting this component of the PMF.
Fig. 2.
Subtilosin has no effect on transmembrane electric potential (ΔΨ) in G. vaginalis cells. Two μmol/L of nigericin (Nig) was used to convert the ΔpH portion of the PMF into ΔΨ (a, b). Subtilosin (Sub, a) caused no fluctuation in the fluorescent signal, indicating it has no effect on the ΔΨ. Addition of nisin (Nis, b) caused a corresponding spike in fluorescence, due to release of the DiSC3 (5) probe and dissipation of the ΔΨ. In both cases, the negative controls (control (−)) subtilosin diluent (a) and nisin diluent (b) had no effect on the ΔΨ. Two μmol/L of valinomycin (Val) was used to dissipate any remaining ΔΨ (a, b). There was no increase in fluorescence in the nisin sample (b), demonstrating that nisin caused a total depletion of the ΔΨ portion of the PMF
Subtilosin Causes an Immediate Depletion of the Transmembrane pH Gradient (ΔpH)
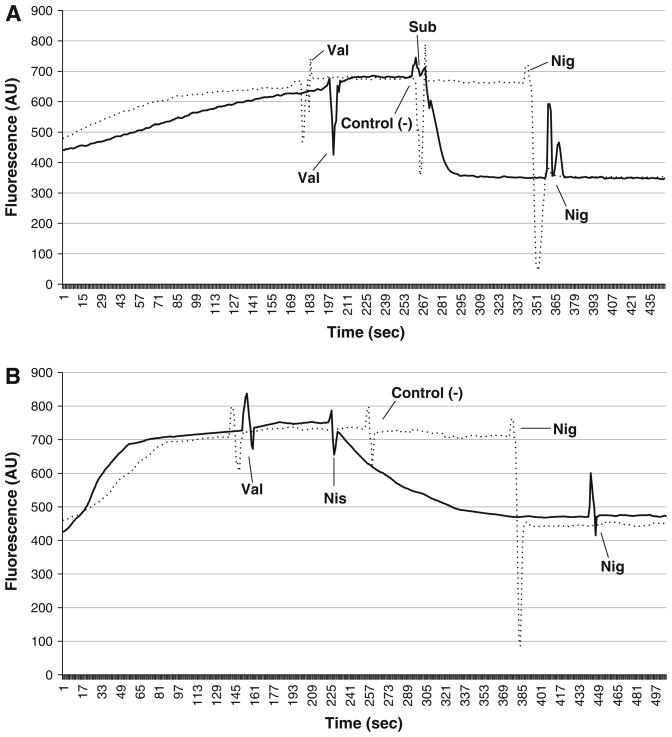
Subtilosin had an immediate impact on the transmembrane pH gradient (ΔpH). Prior to exposure to subtilosin and the controls, the ionophore valinomycin was introduced to convert all ΔΨ to ΔpH. Addition of subtilosin caused an instant drop in the signal intensity of the pH dependent, fluorescent probe BCECF-AM, indicating an immediate intracellular decrease in pH in the G. vaginalis cells (Fig. 3a). Nisin also caused a decrease in the fluorescent signal, although at a slower, more gradual rate (Fig. 3b). Since the assay buffer was designed to have a pH lower than the intracellular pH of G. vaginalis cells [39], the decrease in intracellular pH is due to a depletion of the ΔpH. Adding nigericin to deplete any remaining ΔpH did not cause a further drop in fluorescence for either sample, indicating both nisin and subtilosin caused a total depletion of the ΔpH (Fig. 3a, b) through formation of transmembrane pores.
Fig. 3.
Subtilosin immediately depletes the transmembrane pH gradient (ΔpH) in G. vaginalis cells. Cells were energized with 2.2 mM glucose at start of fluorescence readings. Two μmol/L of valinomycin (Val) was used to transform the ΔΨ of the PMF into ΔpH (a, b). Subtilosin (Sub, a) caused an immediate decrease in the fluorescent signal, indicating a depletion of the ΔpH. Nisin (Nis, b) triggered a slower, gradual decrease in fluorescence. Both negative controls, (control (−)) subtilosin diluent (a) and nisin diluent (b), had no effect on the ΔpH. Two μmol/L of nigericin (Nig) was used to dissipate any remaining ΔpH (a, b). For both subtilosin and nisin, there was no subsequent drop in fluorescence, signifying that both bacteriocins totally depleted the ΔpH portion of the PMF
Discussion
In this study, we clarified the molecular mechanism of action of the bacteriocin subtilosin against the vaginal pathogen G. vaginalis. To the best of our knowledge, this is the first report detailing subtilosin’s mode of action against a specific target microorganism.
Our results clearly show that subtilosin acts by fully depleting the transmembrane pH gradient (ΔpH) and causing an immediate efflux of intracellular ATP, but has no effect on the transmembrane electric potential (ΔΨ). The fact that subtilosin does not affect both portions of the PMF is not surprising, as several other bacteriocins are known to selectively dissipate only one PMF component. For example, enterocin P is able to dramatically decrease intracellular ATP concentrations and membrane potential (ΔΨ) in Enterococcus faecium T136 without affecting the ΔpH [17]. In contrast, pediocin PD-1 creates pores in target cell membranes that allow for leakage of K+ ions but not ATP and other essential metabolites [4]. Taken together, the current results strongly suggest that the changes in the PMF brought about by subtilosin are due to the formation of transient pores in the cytoplasmic membrane of G. vaginalis. To our knowledge, this is the first report of the mode of action of subtilosin conducted on live cells. The in vivo results from this study support those of Thennarasu et al. [38], who demonstrated via an in vitro, cell-free system that subtilosin binds and inserts itself into the lipid bilayer of their target cell membrane. Their work showed that only the hydrophobic region of the cyclic peptide is submerged in the bilayer, while the anionic portion of the molecule remains free above the membrane. Further, they found that membrane permeabilization occurred at subtilosin concentrations well above the MIC level for the tested strain of E. coli, a phenomenon that has also been reported for the bacteriocins nisin and mersacidin [19, 20]. At these high concentrations, aggregation occurs that creates multimeric units of subtilosin, thereby greatly increasing destabilization of the cell membrane. Thus, this destabilization event likely leads to subsequent formation of transient pores, which then cause the ΔpH dissipation and ATP efflux seen in the current investigation.
Our previous research [35, 36] established the antimicrobial activity of subtilosin against a variety of pathogens involved in human health, as well as its safety for human tissues. However, the possibility of its inclusion in personal care products aimed at the treatment and/or prophylaxis of BV required that its specific mode of action against the target pathogen, G. vaginalis, be fully characterized. The data gathered in this study will allow for an intelligent design of subtilosin-based formulations for the effective control of BV-associated microorganisms. The growing problem of bacterial resistance to common antimicrobials is often due to prolonged exposure that allows the microorganisms to develop resistance. This can be avoided or delayed through the use of multiple compounds with differing modes of action that synergize to more effectively control the growth of the pathogen. The target cells will have less time to adapt to the various stresses, and it is far less likely that resistant organisms will appear. Now that subtilosin’s mode of action has been clarified, future research will focus on evaluating potential synergies with other natural antimicrobials that possess differing mechanisms of action in a multiple-hurdle approach [12] against G. vaginalis. Furthermore, model studies will be performed using membrane vesicles and liposomes derived from G. vaginalis’ cell wall in order to fully ascertain whether a cell wall receptor molecule plays a role in subtilosin binding and eventual cell death. These assays will provide a final key piece of information in the intelligent design and formulation of a novel, safe product containing subtilosin for the treatment of BV.
Acknowledgments
This research was sponsored by the NIH NIAID Grant 1R01AI084137 “Multiplex Nanocarrier-Based Hydrogels for Prevention of Vaginal HIV Transmission, Highly Innovative Tactics to Interrupt Transmission of HIV (HIT-IT)” and the Rutgers University Life Science Commercialization Fund “Antimicrobial peptide subtilosin for control of bacterial vaginosis and feminine health care” (2008–2009).
References
- 1.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 2.Babasaki K, Takao T, Shimonishi Y, Kurahashi K. Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. J Biochem. 1985;98:585–603. doi: 10.1093/oxfordjournals.jbchem.a135315. [DOI] [PubMed] [Google Scholar]
- 3.Bannatyne RM, Smith AM. Recurrent bacterial vaginosis and metronidazole resistance in Gardnerella vaginalis. Sex Transm Infect. 1998;74:455–456. [PubMed] [Google Scholar]
- 4.Bauer R, Chikindas ML, Dicks LMT. Purification, partial amino acid sequence and mode of action of pediocin PD-1, a bacteriocin produced by Pediococcus damnosus NCFB 1832. Int J Food Microbiol. 2005;101:17–27. doi: 10.1016/j.ijfoodmicro.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Chikindas ML, García-Garcerá MJ, Driessen AJ, Ledeboer AM, Nissen-Meyer J, Nes IF, Abee T, Konings WN, Venema G. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol. 1993;59:3577–3584. doi: 10.1128/aem.59.11.3577-3584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen DP, Hutkins RW. Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici. Appl Environ Microbiol. 1992;58:3312–3315. doi: 10.1128/aem.58.10.3312-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;10:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 8.Eckert LO, Moore DE, Patton DL, Agnew KJ, Eschenbach DA. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in vitro fertilization. Infect Dis Obstet Gynecol. 2003;11:11–17. doi: 10.1155/S1064744903000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falagas ME, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007;13:657–664. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs RS. Asymptomatic bacterial vaginosis: is it time to treat? Am J Obstet Gynecol. 2007;196:495–496. doi: 10.1016/j.ajog.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005;32:523–529. doi: 10.1016/j.clp.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould GW, Leistner L. Update on hurdle technology approaches to food preservation. In: Davidson PM, Sofos JN, Branen AL, editors. Antimicrobials in food. 3. CRC Press; Boca Raton: 2005. pp. 621–630. [Google Scholar]
- 13.Guihard G, Bénédetti H, Besnard M, Letellier L. Phosphate efflux through the channels formed by colicins and phage T5 in Escherichia coli cells is responsible for the fall in cytoplasmic ATP. J Biol Chem. 1993;268:17775–17780. [PubMed] [Google Scholar]
- 14.Haggerty CL, Hillier SL, Bass DC, Ness RB PID Evaluation and Clinical Health Study Investigators. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis. 2004;39:990–995. doi: 10.1086/423963. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi FB, Ghassemi M, Roebuck KA, Spear GT. Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J Infect Dis. 1999;179:924–930. doi: 10.1086/314674. [DOI] [PubMed] [Google Scholar]
- 16.Hashemi FB, Ghassemi M, Faro S, Aroutcheva A, Spear GT. Induction of human immunodeficiency virus type 1 expression by anaerobes associated with bacterial vaginosis. J Infect Dis. 2000;181:1574–1580. doi: 10.1086/315455. [DOI] [PubMed] [Google Scholar]
- 17.Herranz C, Chen Y, Chung HJ, Cintas LM, Hernandez PE, Montville TJ, Chikindas ML. Enterocin P selectively dissipates the membrane potential of Enterococcus faecium T136. Appl Environ Microbiol. 2001;67:1689–1692. doi: 10.1128/AEM.67.4.1689-1692.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, 2nd, Rao AV, McNellis D, Regan JA, Carey JC, Klebanoff MA. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 19.Hsu S-T, Breukink E, de Kruiff B, Kaptein R, Bonvin AMJJ, van Nuland NAJ. Mapping the targeted membrane pore formation mechanism by solution NMR: the Nisin Z and lipid II interaction in SDS micelles. Biochemistry. 2002;41:7670–7676. doi: 10.1021/bi025679t. [DOI] [PubMed] [Google Scholar]
- 20.Hsu S-T, Breukink E, Bierbaum G, Sahl HG, de Kruiff B, Kaptein R, van Nuland NAJ, Bonvin AMJJ. NMR study of mersacidin and lipid II interaction in dodecylphosphocholine micelles. Conformational changes are a key to antimicrobial activity. J Biol Chem. 2003;278:13110–13117. doi: 10.1074/jbc.M211144200. [DOI] [PubMed] [Google Scholar]
- 21.Liebetrau A, Rodloff AC, Behra-Miellet J, Dubreuil L. In vitro activities of a new des-fluoro(6) quinolone, garenoxacin, against clinical anaerobic bacteria. Antimicrob Agents Chemother. 2003;47:3667–3671. doi: 10.1128/AAC.47.11.3667-3671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, Song J, Kimber N, Shott S, Tangora J, Aroutcheva A, Mazees MB, Wells A, Cohen A, Faro S. The role of bacterial vaginosis in infection after major gynecologic surgery. Infect Dis Obstet Gynecol. 1999;7:169–174. doi: 10.1002/(SICI)1098-0997(1999)7:3<169::AID-IDOG10>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubbe MM, Botha PL, Chalkley LJ. Comparative activity of eighteen antimicrobial agents against anaerobic bacteria isolated in South Africa. Eur J Clin Microbiol Infect Dis. 1999;18:46–54. doi: 10.1007/s100960050225. [DOI] [PubMed] [Google Scholar]
- 24.Marx R, Stein T, Entian KD, Glaser SJ. Structure of the Bacillus subtilis peptide antibiotic subtilosin A determined by 1H NMR and matrix assisted laser desorption/ionization time-of-flight mass spectrometry. J Protein Chem. 2001;20:501–506. doi: 10.1023/a:1012562631268. [DOI] [PubMed] [Google Scholar]
- 25.Molenaar D, Abee T, Konings WN. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991;1115:75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DB, Bellamy S, Nachamkin I, Ness RB, Macones GA, Allen-Taylor L. First trimester bacterial vaginosis, individual microorganism levels, and risk of second trimester pregnancy loss among urban women. Fertil Steril. 2007;88:1396–1403. doi: 10.1016/j.fertnstert.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton ER, Piper J, Peairs W. Bacterial vaginosis and intraamniotic infection. Am J Obstet Gynecol. 1997;176:672–677. doi: 10.1016/s0002-9378(97)70568-4. [DOI] [PubMed] [Google Scholar]
- 28.Oakeshott P, Kerry S, Hay S, Hay P. Bacterial vaginosis and preterm birth: a prospective community-based cohort study. Br J Gen Pract. 2004;54:119–122. [PMC free article] [PubMed] [Google Scholar]
- 29.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 30.Shelburne CE, An FY, Dholpe V, Ramamoorthy A, Lopatin DE, Lantz MS. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J Antimicrob Chemother. 2007;59:297–300. doi: 10.1093/jac/dkl495. [DOI] [PubMed] [Google Scholar]
- 31.Silkin L, Hamza S, Kaufman S, Cobb SL, Vederas JC. Spermicidal bacteriocins: lacticin 3147 and subtilosin A. Bioorg Med Chem Lett. 2008;18:3103–3106. doi: 10.1016/j.bmcl.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Sims PJ, Waggoner AS, Wang CH, Hoffman JF. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan S, Fredericks DN. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis. 2008;2008:750479. doi: 10.1155/2008/750479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein T, Düsterhus S, Stroh A, Entian KD. Subtilosin production by two Bacillus subtilis subspecies and variance of the sbo-alb cluster. Appl Environ Microbiol. 2004;70:2349–2353. doi: 10.1128/AEM.70.4.2349-2353.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutyak KE, Wirawan RE, Aroutcheva AA, Chikindas ML. Isolation of the Bacillus subtilis antimicrobial peptide from the dairy product-derived Bacillus amyloliquefaciens. J Appl Microbiol. 2007;104:1067–1074. doi: 10.1111/j.1365-2672.2007.03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutyak KE, Anderson RA, Dover SE, Feathergill KA, Aroutcheva AA, Faro S, Chikindas ML. Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect Dis Obstet Gynecol. 2008;2008:540758. doi: 10.1155/2008/540758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taha TE, Gray RH, Kumwenda NI, Hoover DR, Mtimavalye LA, Liomba GN, Chiphangwi JD, Dallabetta GA, Miotti PG. HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:52–59. doi: 10.1097/00042560-199901010-00008. [DOI] [PubMed] [Google Scholar]
- 38.Thennarasu S, Lee D-K, Poon A, Kawulka KE, Vederas JC, Ramamoorthy A. Membrane permeabilization, orientation, and antimicrobial mechanism of subtilosin A. Chem Phys Lipids. 2005;137:38–51. doi: 10.1016/j.chemphyslip.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Turvoskiy Y, Ludescher RD, Aroutcheva AA, Faro S, Chikindas ML. Lactocin 160, a bacteriocin produced by vaginal Lactobacillus rhamnosus, targets cytoplasmic membranes of the vaginal pathogen, Gardnerella vaginalis. Probiotics Antimicrob Proteins. 2009;1:67–74. doi: 10.1007/s12602-008-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weir E. Bacterial vaginosis: more questions than answers. Can Med Assoc J. 2004;171:448. doi: 10.1503/cmaj.1041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkowski K, Bruno MEC, Montville TJ. Correlation of bioenergetic parameters with cell death in Listeria monocytogenes cells exposed to nisin. Appl Envrion Microbiol. 1994;60:4186–4188. doi: 10.1128/aem.60.11.4186-4188.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]