Abstract
OBJECTIVE
To examine whether factors related to the patient or her treatment influence asthma severity during pregnancy.
METHODS
Symptom and medication data were collected by in-person and telephone interviews. Women were recruited before 24 weeks of gestation through private obstetricians and hospital clinics. Eight hundred seventy-two women had physician-diagnosed asthma, 686 were active asthmatics, and 641 with complete data were analyzed. The Global Initiative for Asthma measured severity. Cumulative logistic regression models for repeated measures assessed changes in asthma severity during each month of pregnancy.
RESULTS
Two factors had significant and profound effects on the course of asthma: prepregnancy severity and use of medication according to Global Initiative for Asthma guidelines. Although several factors were analyzed (race, age, atopic status, body mass index, parity, fetal sex, and smoking), none were significant risk factors for changes in asthma severity, measured in a clinically important way as a one-step change in Global Initiative for Asthma category. Women with milder asthma received most benefit from appropriate treatment, 62% decreased risk for worsening asthma among those with intermittent asthma (0.38, 95% confidence interval 0.23–0.64) and 52% decreased risk among those with mild persistent asthma (odds ratio 0.48, 95% confidence interval 0.28–0.84). Month or trimester of gestation was not consistently associated with changes in asthma severity.
CONCLUSION
Asthma severity during pregnancy is similar to severity in the year before pregnancy, provided patients continue to use their prescribed medication. If women discontinue medication, even mild asthma is likely to become significantly more severe.
LEVEL OF EVIDENCE
II
Asthma is the most common chronic disease affecting women of childbearing age. Between 8.4% and 8.8% of pregnant women in the United States have asthma,1 and the increasing incidence of asthma(9.1%) among children in the United States2 suggests that the prevalence of asthma during pregnancy is likely to increase. Asthma is also one of the most common health conditions affecting pregnant women,3 and it is important to consider the effect of pregnancy on asthma. Early investigators4,5 suggested a rule of thirds: in one third of women, asthma improves during pregnancy; in one third, asthma becomes worse; and in one third it remains the same. However, assessment of improvement has often been subjective,4 and exacerbations have been measured by hospitalizations and emergency department visits.6 No studies have used the more common clinical endpoints of symptoms and medication use to assess exacerbation during pregnancy.6 In addition, seasonal effects were uncontrolled, and women were often recruited from specialty asthma clinics and may not represent the distribution of asthma severity seen in more typical obstetric practice.
Recent reviews have attempted to define subgroups of women who may experience more severe asthma during pregnancy. Schatz et al7 reported that asthma exacerbations during pregnancy were more likely among women classified with more severe asthma at enrollment (mean gestational age 19 weeks). Other investigators have suggested that African-American women8 and women carrying a female fetus9,10 are at greater risk of more severe asthma during pregnancy.
This study examines the severity of asthma during the course of pregnancy among a large sample of women recruited through community obstetricians and prenatal clinics. It investigates patient characteristics that may be associated with changes in asthma severity and the effects of medication. Asthma symptoms and medication use were collected at multiple points during pregnancy, and exacerbation or improvement is assessed using the Global Initiative for Asthma guidelines.11
MATERIALS AND METHODS
Between April 1997 and June 2000, pregnant women were recruited from 56 obstetric practices and six clinics associated with hospitals in Connecticut and Southern Massachusetts. They participated in a prospective study designed to examine the effects of asthma, asthma symptoms, and asthma therapy on pregnancy outcomes. The study was approved by the Yale University Human Investigations Committee, and all women gave informed consent for participation. Details of the enrollment procedure have been previously published.12 Briefly, 2,379 women were enrolled before 24 weeks of gestation (mean 15±3 weeks). Exclusion criteria were inability to speak either English or Spanish and insulin-dependent diabetes. At enrollment, women were interviewed in their homes by trained research assistants; follow-up interviews were administered by phone at 20, 28, and 36 weeks (±5 days) of gestation and in the hospital postpartum. The baseline interview collected detailed information about symptoms and medication use during pregnancy and in the year before pregnancy, as well as maternal characteristics including age, height, weight, race, pregnancy history, and cigarette smoking. Women who had a liveborn neonate (2,205), reported a physician diagnosis of asthma (872), and reported experiencing asthma symptoms (wheeze, persistent cough, chest tightness, shortness of breath) or using asthma medications in the year before pregnancy (686) were selected for the current analysis. Women who did not complete a postpartum interview were excluded due to incomplete data; 641 women with complete data throughout pregnancy are included in this analysis.
The 2002 Global Initiative for Asthma identifies four categories of asthma severity that we have adapted to match our methods of data collection (Table 1). For a more detailed description refer to the Global Initiative for Asthma guidelines11 and their application in this project.12 Global Initiative for Asthma categories were used to measure the severity of both prepregnancy and gestational asthma. Categories were determined by using respondent reported asthma symptom frequency and medication use. Prepregnancy Global Initiative for Asthma categories were calculated by using the most severe or “worst” month(s) in the year before pregnancy as reported by the respondent at her initial interview. If the respondent did not identify particular months as worse than others, severity was calculated for a “typical” month.
Table 1.
Adapted Global Initiative for Asthma Severity Classification Guidelines
| GINA Score | Severity Class | Symptom Frequency* | Medication Use |
|---|---|---|---|
| 1 | Intermittent | Symptoms 1–7 days per month or nocturnal symptoms fewer than 2 nights per month | Reliever medication† used as necessary No daily controller medication‡ needed |
| 2 | Mild Persistent | Symptoms more than 7 days but less than every day per month or nocturnal symptoms 2–7 nights per month | Reliever medication used as necessary and/or use of one controller medication per month |
| 3 | Moderate Persistent | Symptoms daily and nocturnal symptoms fewer than 14 nights per month | Reliever medication used as necessary and/or use of two controller medications per month |
| 4 | Severe Persistent | Symptoms daily and nocturnal symptoms more than 14 nights per month | Reliever medication used as necessary and/or use of three or more controller medications per month |
GINA, Adapted Global Initiative for Asthma.
Data from Bracken MB, Triche EW, Belanger K, Saftlas A, Beckett WS, Leaderer BP. Asthma Symptoms, Severity, and Drug Therapy: A Prospective Study of Effects on 2205 Pregnancies. Obstet Gynecol 2003;102:739–52.
Symptom days are counted as any day where at least one of the following four symptoms occur: wheeze, persistent cough, chest tightness, shortness of breath.
Reliever medication refers to short-acting β2-agonists.
Controller medications include systemic steroids, inhaled steroids, long-acting β2-agonists, anticholinergics, leukotriene inhibitors, chromones, and xanthine derivatives (eg, theophylline).
Telephone interviews were conducted at 20, 28, and 36 weeks gestation, and postpartum interviews were conducted in the hospital shortly after delivery. Respiratory symptom and medication data were collected at each of these intervals and used to calculate Global Initiative for Asthma categories for each gestational month (ie, 10 months of 28 days each if pregnancy reached a full 40 weeks gestation, beginning from date of last menstrual period). Data were collected for all four asthma symptoms (wheeze, persistent cough, shortness of breath, chest tightness), but shortness of breath, without other symptoms, was excluded when counting symptom days during the months of pregnancy as this is itself not an uncommon pregnancy symptom.
Prepregnancy body mass index (BMI) was calculated based on height and prepregnancy weight as reported by the respondent. Women with BMI less than 26 kg/m2 were considered underweight or normal. Women with BMI 26 kg/m2 or greater were considered overweight or obese.
Race, age, atopy, and parity were all reported by the respondent at the initial interview. Smoking history was based on data collected at all interviews. Nonsmokers include those who never smoked as well as those who quit smoking before pregnancy.
Cumulative logistic regression models for repeated measures13 were constructed based on a transition model, in which the monthly severity score for each respondent depends on her score in the prior month of pregnancy.14 This approach considered the severity of asthma in one month of gestation as a factor related to severity in the following month, which could be a continuation of the current exacerbations, worsening, or improvement. A model that only included main effects for a factor, provided estimates of odds ratios for risk of a one-level change in Global Initiative for Asthma category for women who exhibited a one-unit change for the characteristic.
To evaluate the effect of appropriate treatment on asthma severity during pregnancy variables were included in the final stratified model to indicate if the respondent was appropriately medicated (according to Global Initiative for Asthma guidelines) for their symptom severity, in each month of gestation. Any gestational month when a respondent did not experience asthma symptoms or use asthma medications was excluded. The effect of appropriate medication use varied by monthly asthma severity and so failed to meet the proportional odds assumption across all levels of the response variable. A test of this assumption was highly significant (P<.001). Thus, an ordinal logistic nonproportional odds regression model for repeated measures was constructed, which allowed the effect of medication use to vary along with the level of the response variable, monthly severity15; SAS code (SAS Institute, Cary, NC) for this approach is provided at (http://tigger.uic.edu/-hedeker/long.html).
Global Initiative for Asthma scores for gestational months 2 through 9 were included in the models. Gestational month 1 was not included because there was no score for the month prior. Gestational month 10 was excluded because significantly fewer respondents (n=476) were eligible to contribute a score due to many women delivering during this month and not providing enough data for calculation of a Global Initiative for Asthma score.
Based on prior studies7 we anticipated that asthma severity in the year before pregnancy would affect asthma course during pregnancy and all analysis is stratified by prepregnancy Global Initiative for Asthma categories. Compared with women with intermittent (n=369) or mild persistent (n=129) asthma, there were few women with moderate (n=74) or severe persistent (n=70) asthma, and they were combined to create one stratum for analysis.
RESULTS
The distribution of study population characteristics by prepregnancy asthma severity is shown in Table 2. A majority of women (57.5%) had intermittent asthma, 129 women (20.1%) had mild persistent asthma, and 144 (22.5%) women had moderate or severe persistent asthma.
Table 2.
Selected Characteristics of Study Population by Prepregnancy Asthma Status
| All | Prepregnancy Asthma Severity* |
|||
|---|---|---|---|---|
| Intermittent (n=368) | Mild Persistent (n=129) | Moderate/Severe (n=144) | ||
| Race | ||||
| White | 407 (63.5) | 217 (59.0) | 86 (66.7) | 104 (72.2) |
| Black | 66 (10.3) | 37 (10.1) | 17 (13.2) | 12 (8.3) |
| Hispanic | 147 (22.9) | 99 (26.9) | 25 (19.4) | 23 (16.0) |
| Age (y) | ||||
| Younger than 20 | 99 (15.4) | 70 (19.0) | 18 (14.0) | 11 (7.6) |
| 20–34 | 457 (71.3) | 255 (69.3) | 91 (70.5) | 111 (77.1) |
| 35 or older | 85 (13.3) | 43 (11.7) | 20 (15.5) | 22 (15.3) |
| Atopy | ||||
| No | 158 (24.6) | 115 (31.3) | 24 (18.6) | 19 (13.2) |
| Yes | 483 (75.4) | 253 (68.8) | 105 (81.4) | 125 (86.8) |
| Prepregnancy BMI (kg/m2) | ||||
| Less than 26 | 383 (59.8) | 235 (63.9) | 65 (50.4) | 83 (57.6) |
| 26 or higher | 255 (39.8) | 132 (35.9) | 62 (48.1) | 61 (42.4) |
| Parity | ||||
| 0 | 300 (46.8) | 172 (46.7) | 61 (47.3) | 67 (46.5) |
| 1 or more | 341 (53.2) | 196 (53.3) | 68 (52.7) | 77 (53.5) |
| Fetal sex | ||||
| Male | 330 (51.5) | 191 (51.9) | 60 (46.5) | 79 (54.9) |
| Female | 308 (48.0) | 176 (47.8) | 68 (52.7) | 64 (44.4) |
| Smoking history | ||||
| Nonsmoker | 509 (79.4) | 301 (81.8) | 95 (73.6) | 113 (78.5) |
| 1st trimester only | 75 (11.7) | 37 (10.1) | 18 (14.0) | 20 (13.9) |
| 1st and 3rd trimesters | 57 (8.9) | 30 (8.2) | 16 (12.4) | 11 (7.6) |
| No. of months of asthma symptoms (during pregnancy) | ||||
| None | 50 (7.8) | 43 (11.7) | 6 (4.7) | 1 (0.7) |
| 1–3 | 146 (22.8) | 107 (29.1) | 24 (18.6) | 15 (10.4) |
| 4–6 | 201 (31.4) | 134 (36.4) | 42 (32.6) | 25 (17.4) |
| 7 or more | 244 (38.1) | 84 (22.8) | 57 (44.2) | 103 (71.5) |
| No. months of controller medication use (during pregnancy) | ||||
| None | 207 (32.3) | 172 (46.7) | 25 (19.4) | 10 (6.9) |
| 1–3 | 144 (22.5) | 99 (26.9) | 23 (17.8) | 22 (15.3) |
| 4–6 | 97 (15.1) | 55 (14.9) | 26 (20.2) | 16 (11.1) |
| 7 or more | 193 (30.1) | 42 (11.4) | 55 (42.6) | 96 (66.7) |
| No. of emergency department visits during pregnancy | ||||
| None | 542 (84.6) | 325 (88.3) | 110 (85.3) | 107 (74.3) |
| One | 58 (9.0) | 26 (7.1) | 12 (9.3) | 20 (13.9) |
| Two or more | 41 (6.4) | 17 (4.6) | 7 (5.4) | 17 (11.8) |
| No. of hospital visits during pregnancy | ||||
| None | 627 (97.8) | 365 (99.2) | 126 (97.7) | 136 (94.4) |
| One or more | 14 (2.2) | 3 (0.8) | 3 (2.3) | 8 (5.6) |
BMI, body mass index.
Data are n (%). N values might not sum to total because of missing data.
Prepregnancy asthma severity measured by maximum Adapted Global Initiative for Asthma score in the year before pregnancy.
More white women were in the moderate/severe asthma group, while African-American women were more likely to have mild persistent asthma and Hispanics intermittent asthma. Increased age at pregnancy is associated with more severe asthma, as well as prepregnancy BMI greater than 26. Women with mild persistent asthma were somewhat more likely to smoke during their pregnancy (26.4%) when compared with those with intermittent (18.2%) and moderate/severe asthma (21.5%).
All of the variables indicating increased asthma severity during pregnancy (number of months of symptoms, number of months of controller medication use, emergency department visits and hospitalization) were associated with prepregnancy asthma severity (Table 2).
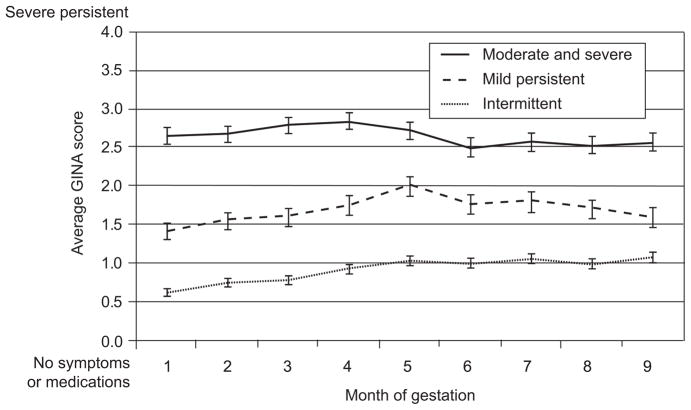
Figure 1 plots the mean (standard error) Global Initiative for Asthma score for each month of pregnancy, stratified by prepregnancy asthma severity. Mean Global Initiative for Asthma scores for transient asthma ranges from 0.6 to 1.1. For mild persistent asthma, the range is 1.5 to 2.0 and for moderate/severe asthma 2.5 to 2.9. Mean Global Initiative for Asthma scores appear to increase through month 5 of pregnancy (month 4 in the moderate/severe group); however, the mean Global Initiative for Asthma scores for each group never intersect. There is little evidence of a trend across pregnancy.
Fig. 1.
Mean (standard error [SE]) monthly Global Initiative for Asthma (GINA) score by prepregnancy asthma severity.
Belanger. Asthma and Medication Use During Pregnancy. Obstet Gynecol 2010.
Initial models confirmed a significant interaction between asthma severity in the year before pregnancy and gestational month (χ2 24.5; P=.04) as well as asthma severity in the prior month of pregnancy (χ2 21.65; P=.006). When compared with women with intermittent asthma in the year before pregnancy, women with mild persistent and moderate/severe asthma before pregnancy were more likely to experience severe asthma during pregnancy with odds ratio (OR) 1.51 (95% confidence interval [CI] 0.96, 2.37) and 2.79 (95% CI 1.65, 4.74), respectively.
Table 3 presents the results of the repeated measures model, stratified by prepregnancy asthma severity and controlled for season, Global Initiative for Asthma score in the prior gestational month, and all factors in the model. Among women with intermittent or moderate/severe asthma before pregnancy, none of the patient characteristics were significantly associated with asthma severity during pregnancy. Among women with mild persistent asthma before pregnancy, prepregnancy BMI greater than 26 kg/m2 was associated with a 31% increased likelihood for asthma to increase one level of Global Initiative for Asthma score (OR 1.31, CI 1.04–1.65) during pregnancy.
Table 3.
Adjusted* Associations Between Selected Respondent Characteristics and Increased Adapted Global Initiative for Asthma Score During Pregnancy by Prepregnancy Asthma Severity
| Factor | Prepregnancy Asthma Severity
|
||
|---|---|---|---|
| Intermittent [OR† (95% CI)] | Mild Persistent [OR† (95% CI)] | Moderate/Severe [OR† (95% CI)] | |
| Race | |||
| White | Reference | Reference | Reference |
| Black | 1.03 (0.81–1.32) | 0.69 (0.46–1.05) | 1.45 (0.86–2.43) |
| Hispanic | 0.96 (0.79–1.17) | 0.88 (0.63–1.22) | 0.85 (0.59–1.22) |
| Age (y) | |||
| 20–34 | Reference | Reference | Reference |
| Younger than 20 or 35 or older | 1.05 (0.83–1.34) | 0.96 (0.61–1.51) | 1.17 (0.65–2.11) |
| Atopy | |||
| No | Reference | Reference | Reference |
| Yes | 1.09 (0.91–1.32) | 0.83 (0.57–1.19) | 1.17 (0.74–1.86) |
| Prepregnancy BMI | |||
| Less than 26 kg/m2 | Reference | Reference | Reference |
| 26 kg/m2 or higher | 1.03 (0.88–1.21) | 1.31 (1.04–1.65) | 0.89 (0.70–1.13) |
| Parity | |||
| 0 | Reference | Reference | Reference |
| 1 or more | 1.03 (0.87–1.21) | 1.06 (0.83–1.35) | 1.08 (0.86–1.36) |
| Fetal sex | |||
| Male | Reference | Reference | Reference |
| Female | 0.91 (0.78–1.06) | 0.93 (0.72–1.19) | 0.81 (0.64–1.03) |
| Smoking history | |||
| Nonsmoker | Reference | Reference | Reference |
| 1st trimester only | 1.14 (0.88–1.47) | 0.93 (0.69–1.25) | 1.04 (0.73–1.48) |
| 1st and 3rd trimesters | 0.95 (0.71–1.26) | 1.29 (0.84–1.97) | 1.17 (0.68–2.02) |
| Gestational month | |||
| 2 | Reference | Reference | Reference |
| 3 | 0.98 (0.72–1.32) | 0.85 (0.57–1.27) | 1.33 (0.87–2.02) |
| 4 | 1.33 (0.99–1.78) | 0.99 (0.63–1.57) | 1.13 (0.73–1.74) |
| 5 | 1.29 (0.96–1.74) | 1.46 (0.93–2.29) | 0.85 (0.55–1.32) |
| 6 | 1.06 (0.78–1.44) | 0.57 (0.36–0.90) | 0.88 (0.56–1.39) |
| 7 | 1.31 (0.99–1.74) | 0.82 (0.53–1.27) | 1.10 (0.74–1.65) |
| 8 | 0.90 (0.65–1.24) | 0.76 (0.45–1.27) | 0.86 (0.51–1.43) |
| 9 | 1.13 (0.87–1.48) | 0.65 (0.45–0.94) | 1.05 (0.71–1.57) |
OR, odds ratio; CI, confidence interval; BMI, body mass index.
Transitional ordinal regression model of repeated measures adjusted for race, age, atopy, prepregnancy BMI, parity, fetal sex, smoking history, gestational month and season, and Adapted Global Initiative for Asthma score in prior gestational month.
Odds of Adapted Global Initiative for Asthma score being one level higher than if the respondent exhibited the referent characteristic.
There was little evidence of a change in asthma severity over the course of pregnancy (Table 3). Only women with mild persistent asthma before pregnancy demonstrated any significant change. These women experienced a decrease in asthma severity during gestational months 6 (OR 0.57, 95% CI 0.36–0.90) and 9 (OR 0.65, 95% CI 0.45–0.94) compared with gestational month 2.
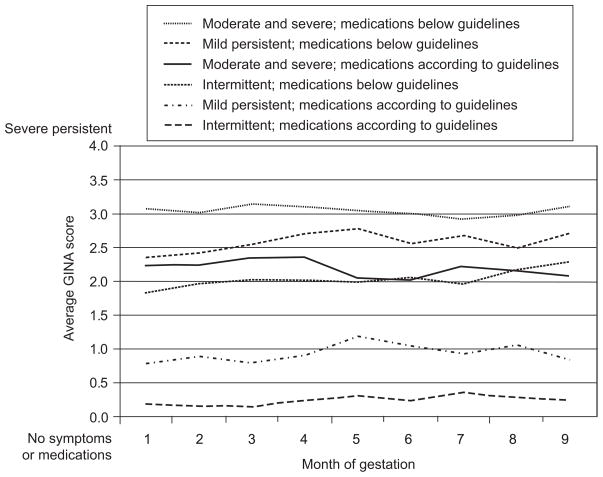
The effect of using asthma medication during pregnancy as recommended by the Global Initiative for Asthma guidelines is shown in Figure 2. For all three categories of asthma severity before pregnancy, mothers whose medication fell below the recommended guideline experienced more severe asthma during pregnancy than women using their recommended medication.
Fig. 2.
Mean monthly Global Initiative for Asthma (GINA) score by prepregnancy asthma severity.
Belanger. Asthma and Medication Use During Pregnancy. Obstet Gynecol 2010.
A final model examined the minimum effectiveness of asthma medication use as prescribed by the Global Initiative for Asthma guidelines. This model includes monthly observations only for the subset of women who experienced asthma symptoms or used asthma medication in that month and controls for BMI, gestational month, season, and asthma severity in the prior month. The protective effect of medication was strongest among women with intermittent asthma before pregnancy. Using medications as recommended by Global Initiative for Asthma guidelines, these women were 62% less likely to experience an increase in asthma severity (OR 0.38, 95% CI 0.23–0.64). The effect of using recommended medication was also significant in the mild persistent group in which women were 52% less likely to experience an increase in asthma severity if they were medicated according to Global Initiative for Asthma guidelines (OR 0.48, 95% CI 0.28–0.84). Among women with moderate or severe asthma before pregnancy, the effect of appropriate medication also reduced asthma severity but to a lesser degree and did not achieve statistical significance (OR 0.71, 95% CI 0.46–1.09). The inherent structure of Global Initiative for Asthma categories prevents the measurement of worsening asthma in a woman who is already classified as severe (category 4), and the effect of medication use may be under estimated in this group.
DISCUSSION
Two factors were demonstrated to have significant and profound effects on the course of asthma: 1) prepregnancy asthma severity; and 2) use of asthma medication according to Global Initiative for Asthma guidelines. Although a number of other factors were analyzed (race, age, atopic status, BMI, parity, fetal sex, and smoking), none were significant risk factors for changes in asthma severity, measured in a clinically important way as a one-step change in Global Initiative for Asthma category. Women with mild asthma received the most benefit from appropriate treatment, a 60% decrease in risk for worsening asthma among those with intermittent asthma and a 50% decreased risk among women with mild persistent asthma. There was little indication that month or trimester of gestation were themselves associated with changes in asthma severity.
Strengths of this study include women being recruited from prenatal care providers and may represent the range of asthma severity usually seen in obstetric practice (57.4% intermittent and 20.1% mild persistent). Data were collected prospectively and the study was able to examine effects of medication use, controlling for severity of asthma. Asthma severity was measured using Global Initiative for Asthma categories, an accepted classification that allowed measurement of severity for each month of gestation. A limitation of the Global Initiative for Asthma scale (and all similar scales) is that when women are classified as severe (category 4) a worsening of their condition cannot be demonstrated; also, lung function was not directly measured. Symptom and medication data were collected by interview, and errors in recall may have occurred. Follow-up interviews were conducted at 8-week intervals to minimize this error.
Schatz et al7 reported the importance of predicting asthma morbidity during pregnancy by asthma severity classification using NAEPP guidelines,15 a precursor of the Global Initiative for Asthma. Women were classified based on asthma status at enrollment, usually the second trimester of pregnancy. This classification was associated with risk for hospitalization, unscheduled physician visits and oral corticosteroid use during pregnancy. Similar results were found in our study where asthma was classified based on severity in the year before pregnancy. Hospitalization, emergency department visits, and higher Global Initiative for Asthma scores during pregnancy were all associated with asthma severity in the year before pregnancy.
Murphy et al16 studied the incidence of asthma exacerbations during pregnancy among women classified as having mild, moderate or severe asthma using Australian asthma management guidelines (similar to the National Asthma Education and Prevention Program). Severe exacerbations (defined as hospitalization, emergency department visit, unscheduled doctor visit, or oral corticosteroid use) occurred in 8% of those with mild asthma, 47% of those with moderate asthma, and 65% of those with severe asthma during pregnancy. They reported 29% of severe exacerbations were associated with nonadherence to inhaled corticosteroid medication.
Women may discontinue asthma medication when realizing they are pregnant, fearing negative effects on the fetus. Enriquez et al17 used the Tennessee Medicaid database to examine the likelihood that asthmatic women discontinue medication. The study used pharmacy claims data, which included the number of prescriptions filled and the number of days supplied. Each asthmatic woman was classified as a user or nonuser of medication for each week of pregnancy. Between week 5 and week 13 of pregnancy, there was a decrease of 13.2% (95% CI 9.0–17.2) in the use of short-acting β agonists and a decline of 22.9% (95% CI 13.9–31.0) in the use of inhaled corticosteroids.
Physicians advising women about medication use during pregnancy must consider the effect of medication on the fetus balanced by any effect of worsening maternal asthma on the fetus. Previously, we reported an increased risk of fetal growth restriction associated with higher average Global Initiative for Asthma scores (test for trend 1.24, 95% CI 1.05–1.47). No increased fetal risks were associated with bronchodilator or inhaled steroid use. Increased risks of preterm delivery were associated with average dose of oral steroid (OR 1.11, 95% CI 1.03–1.18) and average dose of theophylline (OR 1.05, 95% CI 1.01–1.09).12 Similar results were reported by Schatz et al18 in a prospective study conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Network. No association was found between exposure to β agonists, inhaled steroids, or theophylline and gestational hypertension, preterm delivery, low birth weight, or weight for gestational age. Controlling for asthma severity, use of oral steroids was associated with preterm delivery less than 37 weeks of gestation (OR 1.54, 95% CI 1.02–2.33) and low birth weight (OR 1.80, 95% CI 1.13–2.88). Olesen et al19 investigated associations between asthma medication use and perinatal outcomes in the Birth Registry of North Jutland, Denmark. No associations were found between receipt of prescriptions for β agonists or inhaled steroids and gestational age, birth weight, or birth length. Women receiving prescriptions for theophylline or inhaled steroids were more likely to have neonates with low birth weight or who were small for gestational age. In addition, women who discontinued inhaled steroid therapy had term birth weights that were lower than women who did not purchase any prescription drugs during pregnancy (−219.6 g, 95% CI −417.6 to −21.7).
Several studies have specifically investigated the risks of congenital malformations with asthma medication and asthma exacerbations. Kallen and Otterblad Olausson20 observed a 9% increased risk of any congenital malformation but no consistent pattern of specific defect, and many comparisons were made. The authors recognized that residual confounding could have produced this result. Tata et al21 reported a small increase in risk of any malformation among women diagnosed with asthma (OR 1.10, 95% CI 1.01–1.20) compared with nonasthmatic women. No association was found for women receiving asthma treatment (OR 1.06, 95% CI 0.94–1.20) or for any specific medication. Blais and Forget22 investigated the association of asthma exacerbations with congenital malformations. Among women who had an asthma exacerbation during the first trimester, there was an increased risk of any malformation (OR 1.48, 95% CI 1.04–2.09). Among women who did not fill a prescription for oral steroids, women who experienced an exacerbation in the first trimester had an increased risk of a major malformation (OR 1.95, 95% CI 1.03–3.72). This suggests that it was the exacerbation of asthma, not the treatment, that may be causally related to the malformation. A case–control study23 specifically investigating risks of asthma medication in relation to gastroschisis reported an increased risk of bronchodilator use (OR 2.06, 95% CI 1.19–3.59). No adverse effect was found for antiinflammatory medication or bronchodilators used with antiinflammatory drugs. This study did not assess asthma severity, so it cannot distinguish the effect of asthma from the effect of treatment. Lin et al23 reported an increased risk of cardiac defects but concluded that uncontrolled asthma, severe asthma, or bronchodilators may increase risk. In summary, the risk, if any, for congenital malformation among the children of asthmatic women is inconclusive. The few reported risks are small, there is no consistency for specific defect, and the bias that can follow from highly selected study populations, from differences in recall or ascertainment of a malformation, or from multiple comparisons and indication (whether the drug or asthma is associated) frustrate any conclusion from this limited body of literature.
We found no support for the notion of thirds in change of asthma severity in pregnancy; rather asthma during pregnancy is likely to be similar in severity to asthma in the year before pregnancy, provided patients continue to use prescribed medication. If women discontinue medication, even mild asthma is likely to become significantly more severe. Recent research indicates that the fetus may experience significant risk from exacerbations of asthma in the mother. The American College of Obstetricians and Gynecologists (the College)24 recommends continuation of medication for the health of both mother and fetus. The current paper provides empirical support for the College guidelines: exacerbations during pregnancy are best prevented when the mother uses asthma medication appropriate to her level of symptoms.
Acknowledgments
Supported by grants AI41040 & DA05484 from the National Institutes of Health.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Kwon HL, Triche EW, Belanger K, Bracken MB. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin North Am. 2006;26:29–62. doi: 10.1016/j.iac.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Bloom B, Cohen RA. Summary health statistics for U.S. children: National Health Interview Survey, 2007. Vital Health Stat. 2009;10:239. [PubMed] [Google Scholar]
- 3.Namazy JA, Schatz M. Update in the treatment of asthma during pregnancy. Clin Rev Allergy Immunol. 2004;26:139–48. doi: 10.1385/CRIAI:26:3:139. [DOI] [PubMed] [Google Scholar]
- 4.Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81:509–17. [PubMed] [Google Scholar]
- 5.Juniper EF, Newhouse MT. Effect of pregnancy on asthma: a critical appraisal of the literature. In: Schatz M, Zeiger RS, editors. Asthma and allergy in pregnancy. New York (NY): Dekker; 1993. pp. 223–49. [Google Scholar]
- 6.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61:169–76. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schatz M, Dombrowski MP, Wise R, Thom EA, Landon M, Mabie W, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112:283–8. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- 8.Carroll KN, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, Hartert TV. Racial differences in asthma morbidity during pregnancy. Obstet Gynecol. 2005;106:66–72. doi: 10.1097/01.AOG.0000164471.87157.4c. [DOI] [PubMed] [Google Scholar]
- 9.Kwon HL, Belanger K, Holford TR, Bracken MB. Effect of fetal sex on airway lability in pregnant women with asthma. Am J Epidemiol. 2006;163:217–21. doi: 10.1093/aje/kwj032. [DOI] [PubMed] [Google Scholar]
- 10.Bakhireva LN, Schatz M, Jones KL, Tucker CM, Slymen DJ, Klonoff-Cohen HS, et al. Fetal sex and maternal asthma control in pregnancy. J Asthma. 2008;45:403–7. doi: 10.1080/02770900801971826. [DOI] [PubMed] [Google Scholar]
- 11.Global Initiative for Asthma. NHLBI/WHO Workshop Report: Global Strategy for Asthma Management and Prevention, National Institutes of Health, National Heart, Lung, and Blood Institute. NIH Publication #02-3659. 2002 Available at: http://www.ginasthma.com/guidelineitem.asp?11=2&12=1&intld=82. Retrieved January 19, 2010.
- 12.Bracken MB, Triche EW, Belanger K, Saftlas A, Beckett WS, Leaderer BP. Asthma symptoms, severity, and drug therapy: a prospective study of effects on 2205 pregnancies. Obstet Gynecol. 2003;102:739–52. doi: 10.1016/s0029-7844(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 13.Agresti A. Categorical data analysis. New York (NY): Wiley; 1990. [Google Scholar]
- 14.Diggle P, Liang KY, Heagerty P, Zeger S. Analysis of longitudinal data. Oxford (UK): Oxford University Press; 2001. [Google Scholar]
- 15.National Asthma Education Program Report of the Working Group on Asthma and Pregnancy, Management of asthma during pregnancy. NIH Publication #93–3279A. Bethesda (MD): National Heart, Lung, and Blood Institute; 1993. [Google Scholar]
- 16.Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: mechanisms and treatment implications. Eur Respir J. 2005;25:731–50. doi: 10.1183/09031936.05.00085704. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez R, Wu P, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol. 2006;195:149–53. doi: 10.1016/j.ajog.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 18.Schatz M, Dombrowski MP, Wise R, Momirova V, Landon M, Mabie W, et al. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol. 2004;113:1040–5. doi: 10.1016/j.jaci.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Olesen C, Thrane N, Nielsen GL, Sorensen HT, Olsen J. A population-based prescription study of asthma drugs during pregnancy: changing the intensity of asthma therapy and perinatal outcomes. Respiration. 2001;68:256–61. doi: 10.1159/000050507. [DOI] [PubMed] [Google Scholar]
- 20.Kallen B, Otterblad Olausson P. Use of anti-asthmatic drugs during pregnancy. 2. Infant characteristics excluding congenital malformations. Eur J Clin Pharmacol. 2007;63:375–81. doi: 10.1007/s00228-006-0258-0. [DOI] [PubMed] [Google Scholar]
- 21.Tata LJ, Lewis SA, McKeever TM, Smith CJ, Doyle P, Smeeth L, et al. Effect of maternal asthma, exacerbations and asthma medication use on congenital malformations in offspring: a UK population-based study. Thorax. 2008;63:981–7. doi: 10.1136/thx.2008.098244. [DOI] [PubMed] [Google Scholar]
- 22.Blais L, Forget A. Asthma exacerbations during the first trimester of pregnancy and the risk of congenital malformations among asthmatic women. J Allergy Clin Immunol. 2008;121:1379–84. 84, e1. doi: 10.1016/j.jaci.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 23.Lin S, Munsie JP, Herdt-Losavio ML, Bell E, Druschel C, Romitti PA, et al. Maternal asthma medication use and the risk of gastroschisis. Am J Epidemiol. 2008;168:73–9. doi: 10.1093/aje/kwn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asthma in pregnancy. ACOG Practice Bulletin No. 90. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;111:457–64. doi: 10.1097/AOG.0b013e3181665ff4. [DOI] [PubMed] [Google Scholar]