Summary
Neural progenitor cells (NPCs) can repair damaged myelin in neurodegenerative diseases. In this issue of Immunity, Cao et al., 2011, report that NPCs also produce the cytokine LIF, which suppresses Th17 cell-driven inflammation and autoimmunity by upregulating the protein Socs3.
Stem cell-based therapies hold the key to next generation treatments for many debilitating neurodegenerative diseases. Pluripotent embryonic stem (ES) cells and neural-tissue derived progenitor cells possess both self-renewal capacity and the ability to generate neurons or oligodendrocytes, which makes them an attractive target for cell-based therapies in neurodegenerative diseases (Pluchino, et al., 2003). Transplantation of NPCs in the central nervous system provides a means by which demyelinated nerve fibers can be repaired in neuroinflammatory diseases like multiple sclerosis (MS). Using an experimental animal model of human MS, it was shown that NPCs are indeed capable of differentiating into oligodendrocytes and repairing damaged myelin (Pluchino, et al., 2003). However, in addition to their ability to repair damaged myelin, NPCs may also have anti-inflammatory properties and therefore suppress ongoing inflammation by inhibiting proinflammatory effector T cells.
Although the etiology of multiple sclerosis is not well understood, it is clear that myelin sheath of the white matter of the CNS is the target of immune attack, resulting in inflammation and subsequent demyelination, which leads to axonal resection and neuronal degeneration. Self-reactive CD4+ T helper (Th) and CD8+ T cells that produce proinflammatory cytokines play a critical role in inducing the disease. Naïve CD4+ T cells differentiate into different effector T cells subsets upon activation. These effector T cells have been classified into Th1, Th2 and Th17 cells, based on their cytokines and effector functions. Emerging evidence indicates that proinflammatory Th1 cells, which produce Interferon-γ (IFN-γ), Interleukin (IL-) 2 and lymphotoxin β, and Th17 cells, which produce IL-17A, IL-17F and IL-22, play a key role in inducing the disease (Korn et al., 2009; Kuchroo et al., 2002). The critical role of Th17 cells has been further demonstrated in a number of autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), an animal of model of human MS (Korn et al., 2009). Genetic deficiencies of Th17 associated molecules such as RoRγt, STAT3, IL-23p19 and IL-23R lead to almost complete resistance to development of EAE (Korn et al., 2009). Based on these observations, IL-17-producing Th17 cells have been proposed to play a critical role in initiating tissue inflammation in EAE and many other autoimmune diseases.
In addition to differentiating into oligodendrocytes in the areas of demyelination, NPC treatment has also been shown to control tissue inflammation in EAE, suggesting that NPCs might suppress the effector functions of self-reactive T cells (Einstein et al., 2003). However, it was not clear until now as to how NPCs dampen T cell response and regulate tissue inflammation in EAE. In this issue of Immunity (Cao et al., 2011), Zhang and colleagues report the underlying mechanism by which NPCs control tissue inflammation during EAE (Figure 1). This paper illustrates that NPCs produce a number of anti-inflammatory cytokines including LIF, which normally plays a role in neurogenesis. However, this paper (Cao et al., 2011) reports that LIF ameliorates EAE by selectively and specifically suppressing the development of Th17 cells. LIF belongs to the IL-6 family of cytokines and signals via a LIF receptor together with gp130, a common receptor chain that is also used by IL-6 and other IL-6 family cytokines. We and others have previously shown that IL-6 together with IL-1 and Transforming growth factor beta (TGF-β) induce Th17 differentiation (Bettelli et al., 2006; Veldhoen et al., 2006) and, in fact IL-6 acts as a pivot that dictates the balance of whether a naïve T cell would become a effector Th17 cells or regulatory FoxP3+ T cell (Bettelli et al., 2006).
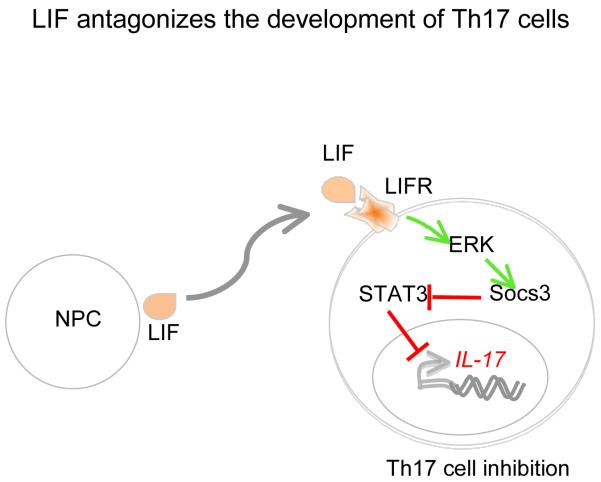
Figure 1.
NPC-derived leukemia inhibitory factor (LIF) induced ERK-1 and 2 dependent activation of Socs3 expression. Overexpression of Socs3 by LIF attenuates the activation of STAT3, which in turn suppress IL-17 production.
The idea that NPCs must suppress T cell response in the peripheral immune compartment came from the observation that whereas a single intravenous injection of NPCs ameliorated EAE, immunized mice treated with NPCs did not show any infiltration of NPCs into their CNS (Cao et al., 2011). In fact, the vast majority of NPCs were found to be present in the secondary lymphoid organs (lymph nodes and spleen). This implies that NPCs may not simply inhibit EAE by differentiating into oligodendrocytes and thus repairing the damaged myelin sheath, but must also suppress effector T cells in the peripheral immune compartments. This was further supported by the observation that administering irradiated NPCs, which had lost their ability to differentiate into oligodendrocytes could still suppress EAE. This raised the issue of whether NPCs-derived soluble factors could suppress EAE by inhibiting effector T cells responses (Cao et al., 2011).
To identify the soluble factor(s) produced by NPCs that inhibit proinflammatory effector T cells, Cao et al screened a known panel of cytokines and neurotropins produced by NPCs. Among other factors produced by NPCs, Cao et al found that fully differentiated NPCs predominantly produce LIF, which inhibited differentiation of Th17 cells—and neutralization of LIF by a LIF antibody restored IL-17 production (Cao et al., 2011). Similarly, pretreatment of the NPCs supernatant with LIF antibody abrogated the protective effect of NPC-derived supernatant in EAE, with the reappearance of Th17 cells in the periphery and CNS of the affected mice. Consistent with this observation, in vivo administration of LIF inhibited the development of EAE with a commensurate decrease in Th17 cells (Cao et al., 2011). Cao et al showed that LIF-receptor is actively expressed on differentiated Th17 cells, which makes them responsive to LIF dependent inhibition. From these data, it is clear that the two family members, IL-6 and LIF, have opposite effects on Th17 development (Bettelli et al., 2006; Cao et al., 2011).
During Th17 cells differentiation, IL-6 induces phosphorylation of STAT3 (pSTAT3) that binds to promoter elements of the gene encoding RoRγt, a transcription factor that induces development of Th17 cells (Durant et al., 2011). The critical role of STAT3 in Th17 cell development has also been supported by a number of experimental observations. For example, conditional deficiency of STAT3 in CD4+ T cells abrogated the development of Th17 cells, and these mice were highly resistant to EAE (Liu et al., 2008). Because LIF binding to LIFR regulates proximal signaling events during Th17 development, Cao and colleagues found that LIF selectively inhibited STAT3 activation without affecting STAT1 and STAT6 activation, the transcription factors that are essential for the development of Th1 and Th2 cells, respectively. LIF treatment induced Socs3 expression both in vitro and in vivo, which is known to inhibit generation and activation of pSTAT3. Inhibition of Socs3 expression by introduction of Socs3-specific siRNA, abrogated the inhibitory effects of LIF on Th17 development. The authors further showed that LIF-induced Socs3 expression was dependent on ERK-1 and 2 activation as a selective inhibitor of MEK-ERK pathway inhibited LIF-induced Socs3 expression. Taken together, these observations provide the evidence that LIF-induced inhibition of Th17 cells is dependent on ERK1 and 2-induced expression of Socs3, which is a critical negative regulator of Stat3 activation (Figure 1). Cao et al further translated their findings to human Th17 cells. Similarly to mouse NPCs, human NPCs also produce large amount of LIF in their culture supernatant, which inhibited the development of human Th17 cells in Socs3-dependent manner (Cao et al., 2011). Furthermore, LIF treatment inhibited the production of IL-17 in CD4+ T cells from MS patients.
These observations add a new dimension to stem cell based therapy for inflammatory neurodegenerative diseases. The NPCs may not only suppress CNS autoimmune demyelinating disease by differentiating into oligodendrocytes and repairing injured myelin sheath but also by inhibiting proinflammatory Th17 cells. In addition, these observations highlight LIF as an interesting target for treating many autoimmune diseases including multiple sclerosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Cao W, Yang Y, Wang Z, Liu A, Fang L, Wu F, Hong J, Shi Y, Leung S, Dong C, Zhang JZ. Immunity. 2011 doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O’Shea JJ. Immunity. 2011;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Mol Cell Neurosci. 2003;24:1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. Annu Rev Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- Liu X, Lee YS, Yu CR, Egwuagu CE. J Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Carro UD, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino1 G. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]