Abstract
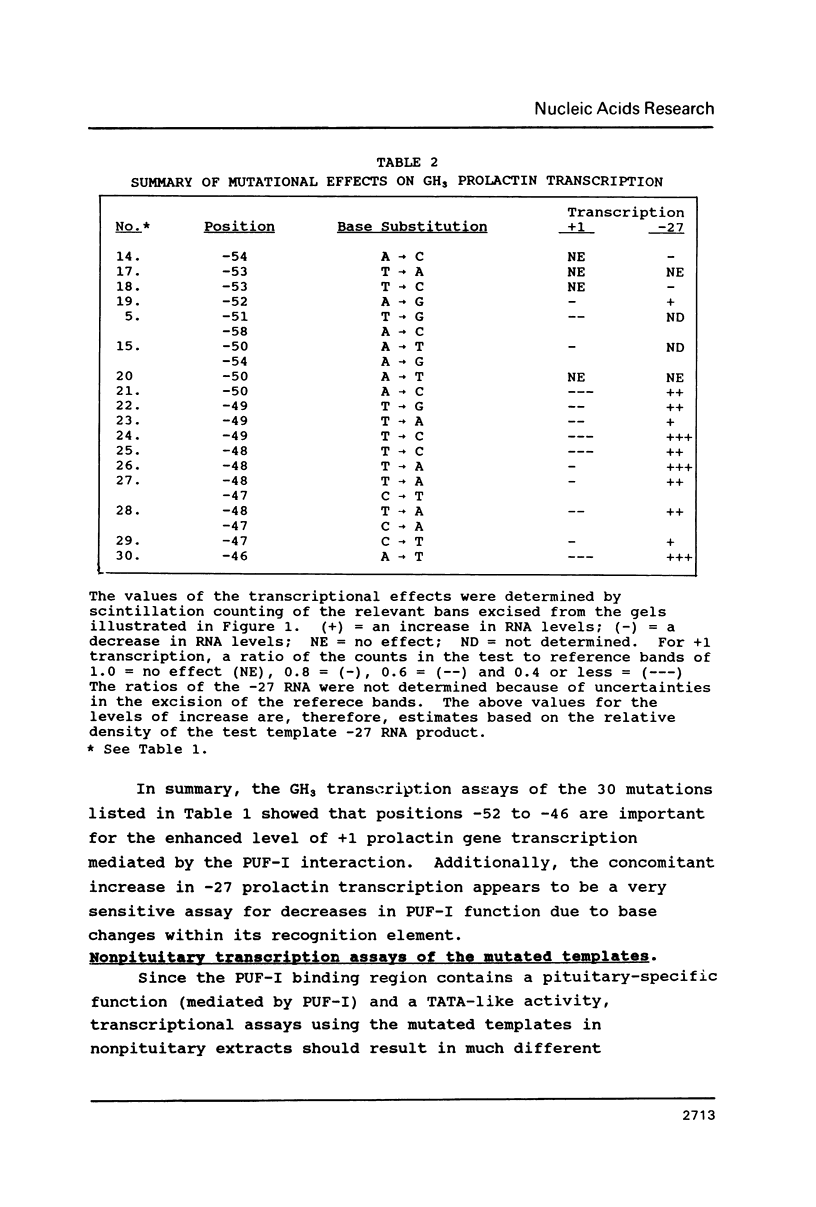
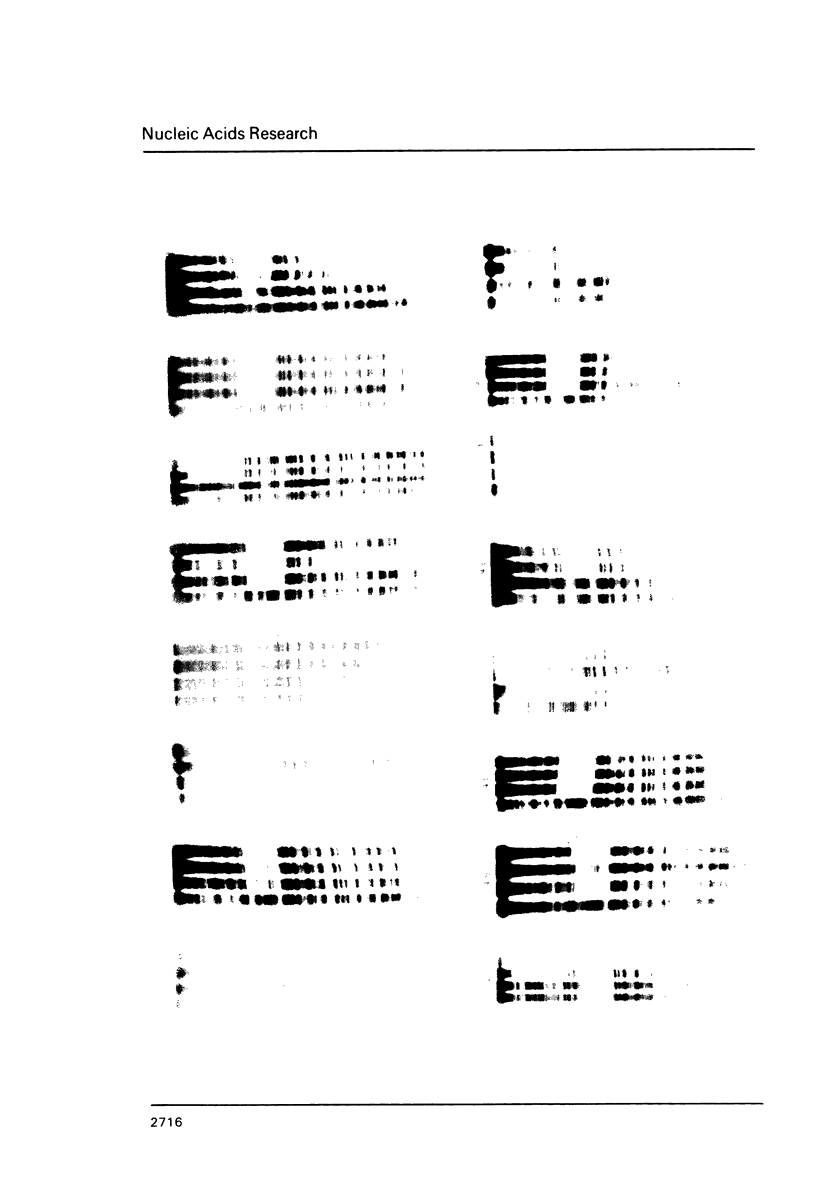
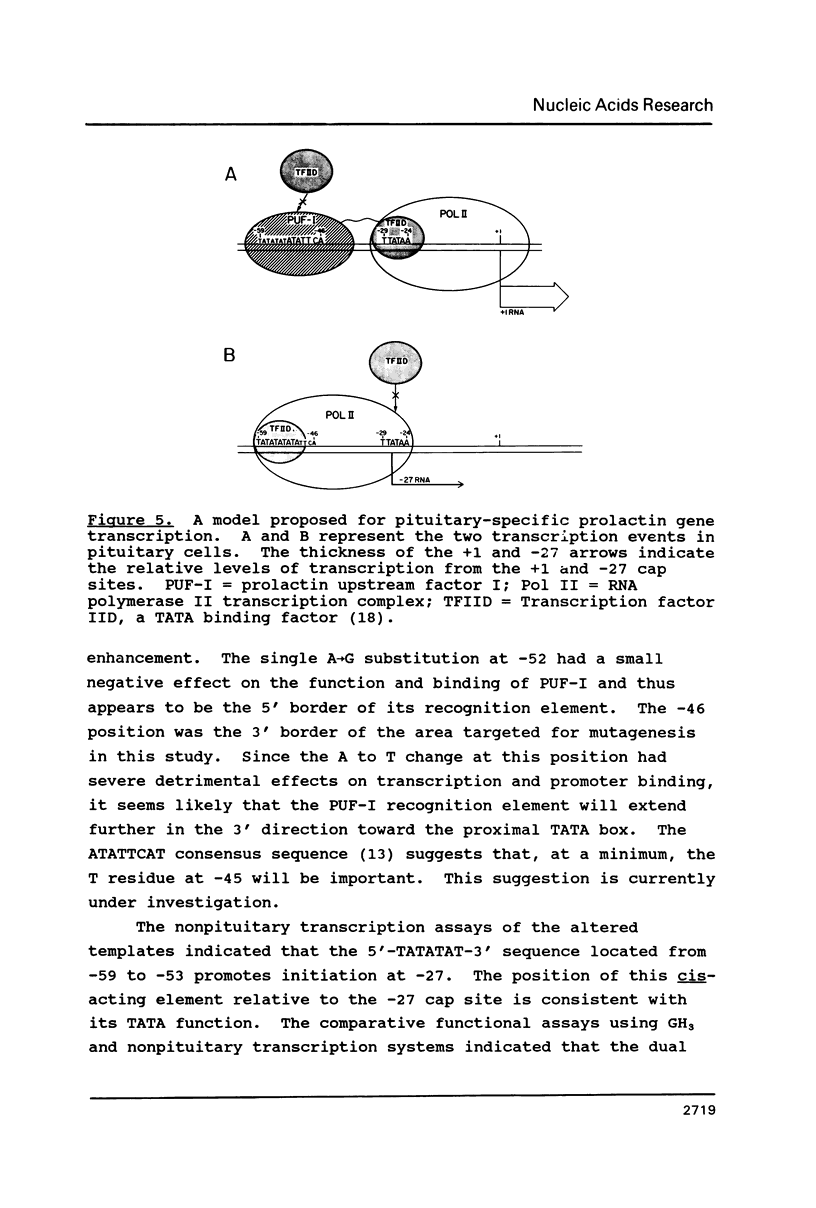
The cell-type-specific transcription of the prolactin gene in vitro is mediated through the interaction of prolactin upstream factor I (PUF-I) with a 28 basepair region of the gene promoter (-63 to -36) which contains an 18 bp A+T-rich imperfect palindrome (-63 to -46). Base substitutions were introduced into 16 of the 18 palindromic residues by targeted saturation mutagenesis. The GH3 binding and in vitro transcription assays of the mutated promoters showed that base substitutions within the 5'-ATATTCA-3' sequence located at -52 to -46 were detrimental to PUF-I binding and its cell-type-specific transcriptional enhancement activity. Transcription assays of the mutated promoters performed with several nonpituitary-derived extracts demonstrated that a distal TATA box located from -59 to -53 promotes initiation at -27. Thus, the cell-type-specific cis-acting element required by PUF-I for DNA recognition is immediately adjacent to a general TATA sequence. Base substitutions that decreased +1 transcription and PUF-I binding concomitantly increased -27 initiation of RNA in vitro. We suggest that PUF-I binding in pituitary cells potentiates +1 transcription and represses alternative TATA box activity for initiation events occurring at -27. This is the first known report of a eukaryotic DNA binding protein that has both an activator and repressor activity for a single transcription unit.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki K., Maeda H., Wang J., Kitamura D., Watanabe T. Purification of a nuclear trans-acting factor involved in the regulated transcription of a human immunoglobulin heavy chain gene. Cell. 1988 Jun 3;53(5):723–730. doi: 10.1016/0092-8674(88)90090-6. [DOI] [PubMed] [Google Scholar]
- Barron E. A., Cao Z., Schneider B. G., Kraig E., Carrillo A. J., Sharp Z. D. Dual functions of a cis-acting element within the rat prolactin gene promoter. Mol Cell Biol. 1989 Feb;9(2):817–819. doi: 10.1128/mcb.9.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Cao Z. D., Barron E. A., Carillo A. J., Sharp Z. D. Reconstitution of cell-type-specific transcription of the rat prolactin gene in vitro. Mol Cell Biol. 1987 Oct;7(10):3402–3408. doi: 10.1128/mcb.7.10.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Heguy A., Roeder R. G. Identification and purification of a human lymphoid-specific octamer-binding protein (OTF-2) that activates transcription of an immunoglobulin promoter in vitro. Cell. 1987 Dec 4;51(5):783–793. doi: 10.1016/0092-8674(87)90101-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederrecht G., Shuey D. J., Kibbe W. A., Parker C. S. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell. 1987 Feb 13;48(3):507–515. doi: 10.1016/0092-8674(87)90201-7. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Roeder R. G. Binding of transcription factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell. 1987 Nov 20;51(4):613–622. doi: 10.1016/0092-8674(87)90130-9. [DOI] [PubMed] [Google Scholar]