Abstract
Numerous transposable element insertions in Drosophila melanogaster cause hypomorphic mutations. We report that transcription initiation within a region found in many P-element constructs provides an explanation for why some gene function is retained. We detected evidence of this transcription in four different types of P constructs, regardless of whether the insertion was in a coding exon, intron, 5′ untranslated region, or upstream of the gene span.
Results and Discussion
Genetically engineered P elements have been used as tools for experimental manipulation of the Drosophila genome (Bellen et al. 2004; Thibault et al. 2004). These elements tend to insert near transcription start sites, frequently resulting in hypomorphic mutations (Spradling et al. 1995). This result is sometimes surprising, as insertions within early exons might be expected to produce null mutations rather than hypomorphs. Here, we report that transcription initiation from within a P element may be responsible for some mutations being hypomorphic rather than null.
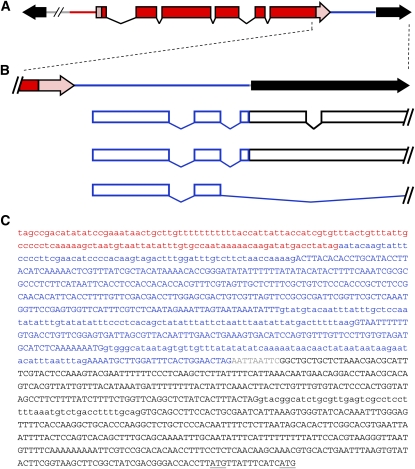
We first observed this phenomenon in a P insertion allele of nbs. The nbsEY15506 allele contains a 10.9-kb P{EPgy2} element inserted into the coding sequence in the second exon. Although the insertion separates two conserved domains, it creates a hypomorphic, separation-of-function allele, rather than a null allele. The nbsSM9 derivative has an internal deletion of ∼7 kb of this P element, but retains the hypomorphic nature of nbsEY15506. We previously reported that nbsSM9 has a longer transcript than wild-type nbs on a Northern blot and that the hypomorphic character of nbsSM9 was due, at least in part, to transcription that initiated from within the P element (Figure 1) (Mukherjee et al. 2009). Using 5′ RACE, we found that transcription initiated in the region downstream of the 3′ end of the white (w) gene carried on the P element—a region that is located 3′ to the endogenous w locus, but is not part of the annotated w gene span. In addition, we detected that three introns, with canonical splice donor and acceptor sequences, had been spliced out (Figure 1, B and C). Two of these introns are located in the region downstream of w, but the third is within the P-element 5′ end, which is transcribed in the direction opposite to P transposase transcription. The terminal 3 bp of the P end form a start codon that is in-frame with the nbs-coding sequence. Results from our genetic assays suggested that nbsSM9 does produce an N-terminally truncated protein that lacks the FHA domain, resulting in loss of some NBS functions but retention of others.
Figure 1.—
A cryptic transcript initiates from within P elements. (A) All four types of P elements analyzed shared a copy of w (shown in red, with UTRs in pink) oriented toward the 5′ P end (black). The hash marks indicate additional features of the various P elements. Blue indicates the region downstream of the endogenous w locus, which was present on all the P elements that we examined. (B) Three splicing patterns that we observed are shown relative to their position on the P element. The first was observed in adult nbsSM9 and SlbpEP3182 flies, the second in SlbpEP3182 larvae, and the third in adult GlcAT-SKG01446 flies and adult flies carrying P{wHy}DG30201. These transcripts were detected by the 5′ RACE System for Rapid Amplification of cDNA Ends, Version 2.0 (Invitrogen; catalog #18374-058). Our PCR did not facilitate the detection of the very 5′ end of the transcript in every case, but the position of the upstream primer is consistent with transcription initiation that occurs in the region downstream of w. (C) The sequence of the relevant part of the P element, with splicing as detected in nbsSM9 and SlbpEP3182 adults. The sequence of the cryptic transcript is represented in capital letters; lowercase letters represent sequences present in the DNA that are spliced out in the transcript. Two potential start codons in the 5′ P end are underlined. Red letters: the 5′ UTR of w; blue letters: the region downstream of w; gray letters: vector sequence; black letters: the 5′ P end.
The region from which this intra-P transcription appears to initiate corresponds with the genomic location X:2,683,995...2,684,631, directly upstream of the gene CG32795 (D. melanogaster genome release 5.34; Tweedie et al. 2009). The proximity to CG32795 led us to investigate the possibility of promoter elements in this region. There are no EST cDNAs in GenBank for this region and no annotated exons; nevertheless, there is evidence for transcriptional activity. Importantly, transcripts from the region between w and the first annotated exon of CG32795 have been detected in white pre-pupae and adult male flies (Celniker et al. 2009). On the basis of the position of RNA-seq reads in the modENCODE genome browser, these transcripts appear to contain exons in the same relative position as those within the P-element transcripts. A notable difference is that the downstream intron of the endogenous transcript is longer and appears to splice to the second exon in the 5′ untranslated region (UTR) of transcript CG32795-RA. The levels of the unannotated transcript appear to be substantially lower than those of the flanking genes, but are sufficient to explain the origin of the transcript produced from P{EPgy2}.
We tested other P elements to see if this transcription was an isolated phenomenon. The nbsSM9 allele is unusual in that the P element is inserted into coding sequences. To determine whether insertions in other regions can also produce hypomorphic alleles due to transcription from a P element, we analyzed an insertion into the 5′ UTR of the Slbp gene (Figure 2). This insertion also creates a hypomorphic allele (Sullivan et al. 2001). The structure of the P{EP} element in this allele differs from that of the P{EPgy2}element that we analyzed in nbs, but in both cases the element is inserted so that the w gene is transcribed in the same direction as the gene into which the P element is inserted. Sullivan et al. (2001) carried out a P excision screen and generated hypomorphic and null alleles, both caused by internal deletions within the P element, without deletion of the Slbp sequence. Deletions that removed the region downstream of w, such as the null allele Slbp15, do not produce Slbp transcript. In contrast, the hypomorphic allele Slbp10, which lost much of the P element but retained the region downstream of w, produces both Slbp transcript and SLBP protein, as determined by in situ hybridization to mRNA and Western blot, respectively (Sullivan et al. 2001).
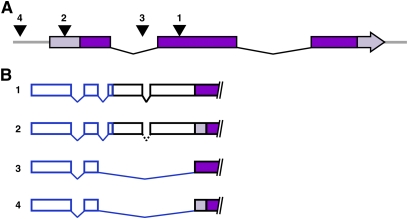
Figure 2.—
Transcription can initiate from P elements in various locations relative to the gene. (A) We examined P elements in various positions relative to nearby genes. A generic schematic of those insertions is displayed: an insertion into an exon (1), 5′ UTR (2), an intron (3), and an intergenic region 5′ of the gene span (4). In all cases, w was oriented in the same direction as the endogenous gene. Lighter-colored regions indicate UTRs. (B) The structures of the four types of transcripts detected are represented, with numbers corresponding to their position in A. Three introns are spliced out in adult RNA samples. The exonic insertion (1) transcribes directly into the exon; in nbsSM9, the final three bases of the P end form an in-frame start codon. The insertion in the 5′ UTR (2) transcribes in a similar manner. Here, the intron in the 5′ P end is represented with dotted lines because this region was spliced out in SlbpEP3182 adult flies, but not in larvae. Both the intronic (3) and the intergenic (4) insertions splice past the P end into the first splice acceptor sequence in the next available exon, regardless of whether it is a UTR or a coding region.
We performed PCR on cDNA from Slbp10/TM6B adults and second instar larvae and found that there is also transcription from the region downstream of w in this P{EP} element. In adults, the sequence and splice sites are identical to those that we detected in nbsSM9 (Figure 1, B and C; Figure 2B). Intriguingly, splicing of the intron in the P end was not detected in cDNA isolated from larvae, raising the possibility that splicing of the intra-P transcript may be regulated by tissue and/or developmental timing.
P elements also frequently insert into introns (Spradling et al. 1995). To determine whether transcription from the region downstream of w can contribute to the mutagenic properties of intronic insertions, we analyzed an insertion of a P{SUPor-P} element into the first intron of GlcAT-S (Figure 2). By performing PCR on cDNA obtained from GlcAT-SKG01446 adults, we found that transcripts were produced in the region downstream of the P-element w gene, but with a slightly different splicing pattern than in the two previous examples. The first intron was spliced out as described above, but the second intron was found to splice to the next downstream exon of GlcAT-S (Figure 1B and Figure 2B). This may be due to the fact that the P element was in an intron, or it could be related to the su(Hw) binding region between the end of the w span and the 5′ P end of P{SUPor-P}.
We also tested a P element inserted into an intergenic region to determine if it could promote transcription of a nearby gene. We examined P{wHy}DG30201, which is inserted upstream of CG9775 (Figure 2A). PCR on cDNA from P{wHy}DG30201 adult flies revealed a situation similar to that of GlcAT-SKG01446 and the endogenous w locus. Transcription initiated in the region downstream of w, and the first intron was spliced out. The second intron spliced into the first splice acceptor sequence of the first exon of the 5′ UTR of CG9775, 23 bp downstream of the annotated transcription start site. This exon can be found in both transcript CG9775-RA and transcript CG9775-RD (Figure 2B). Thus, this P{wHy} insertion is responsible for additional transcription of a downstream gene.
These findings provide insight into the nature of hypomorphic alleles that arise from insertion of certain engineered transposable elements. For example, there is no guarantee that transcription from within the transposable element will match the expression pattern of the gene’s native promoter. If this is the case, altered expression may result in different severities, potentially ranging from wild-type function in some tissues to complete absence of function in other tissues. It is interesting to note that there are two potential ORF-initiating start codons at the end of the 5′ P end: the final three bases, and another that begins 13 bp upstream (Figure 1C). As the resulting ORFs are in different reading frames, one would expect as many as 2/3 of correctly oriented w-containing P elements in exons to produce at least a partial protein product.
Features of these cryptic transcripts that affect translation efficiency may play a role in the reduced function as well. While Slbp10 mutants produce SLBP protein, they do so at reduced levels (Sullivan et al. 2001). This may be due to characteristics of the cryptic transcript. Among the exons on the P-element–Slbp transcript, the exon in which translation begins—a fusion of the 5′ P end and the remaining sequence of the 5′ UTR and start of the coding region of Slbp—is the only exon that does not contain a short open reading frame. This suggests that translation can successfully take place only when ribosomes bind to that key exon, as binding to the upstream exons would lead to premature termination of translation and potentially to nonsense-mediated decay (NMD) (Peltz et al. 1993; Gatfield et al. 2003). This feature may contribute to the hypomorphic character of some mutants with intra-P transcription: even if transcripts were being produced at the normal rate, transcript degradation may ensure that not all transcripts would survive to be translated, leading to a reduced level of protein. The cryptic transcript of nbsSM9 does not share this characteristic. Rather, we found that levels of the nbsSM9 transcript appeared to be similar to that of wild type (Mukherjee et al. 2009). This suggests that NMD may not be involved in reducing the levels of nbs transcript. This is curious, as the P elements in Slbp10 and nbsSM9 have identical sequence in the region of the cryptic transcript. This suggests that the stability of these transcripts is affected by additional factors that are not immediately apparent.
The position and orientation of a P element insertion may have additional ramifications for mutagenesis. Insertions in introns may invoke alternative splicing of the native transcript, possibly resulting in impaired functionality of the transcript or protein. Additionally, it may be that insertions that take place in a gene span, but which transcribe the region downstream of w in the opposite direction as the gene does, may produce antisense RNA. These transcripts could initiate RNA interference of the native transcript, providing another means of producing a hypomorph.
The w gene is a common marker in engineered transposable elements in Drosophila. We have demonstrated that the region downstream of w can transcribe into the gene into which the P element is inserted or is near. The examples presented here encompass four different P-element constructs in four different gene regions (coding sequence, 5′ untranslated region, intron, and upstream). Thus, this is likely to be a common effect, and transcription initiation from within these elements is likely to contribute to the hypomorphic mutations generated by many of these insertion alleles.
Acknowledgments
We thank Deirdre Tatomer for providing Slbp mutant flies; the laboratory of Lillie Searles for assistance with RNA analysis; and Bob Duronio, William Marzluff, and members of the Sekelsky and Duronio labs for helpful discussions of the manuscript. This work was supported by a grant from the National Institutes of Health (GM061252) to J.S.
Literature Cited
- Bellen H. J., Levis R. W., Liao G., He Y., Carlson J. W., et al. , 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167: 761–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A., Gerstein M. B., Gunsalus K. C., Henikoff S., et al. , 2009. Unlocking the secrets of the genome. Nature 459: 927–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield D., Unterholzner L., Ciccarelli F. D., Bork P., Izaurralde E., 2003. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways. EMBO J. 22: 3960–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., LaFave M. C., Sekelsky J., 2009. DNA damage responses in Drosophila nbs mutants with reduced or altered NBS function. DNA Repair (Amst.) 8: 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz S. W., Brown A. H., Jacobson A., 1993. Messenger-RNA destabilization triggered by premature translational termination depends on at least 3 cis-acting sequence elements and one trans-acting factor. Genes Dev. 7: 1737–1754 [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Stern D. M., Kiss I., Roote J., Laverty T., et al. , 1995. Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc. Natl. Acad. Sci. USA 92: 10824–10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E., Santiago C., Parker E. D., Dominski Z., Yang X., et al. , 2001. Drosophila stem loop binding protein coordinates accumulation of mature histone mRNA with cell cycle progression. Genes Dev. 15: 173–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault S. T., Singer M. A., Miyazaki W. Y., Milash B., Dompe N. A., et al. , 2004. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36: 283–287 [DOI] [PubMed] [Google Scholar]
- Tweedie S., Ashburner M., Falls K., Leyland P., McQuilton P., et al. , 2009. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 37: D555–D559 [DOI] [PMC free article] [PubMed] [Google Scholar]