Abstract
In this study, we show that the haplo-enhancer effect of JIL-1 has the ability to counterbalance the haplo-suppressor effect of both Su(var)3-9 and Su(var)2-5 on position-effect variegation, providing evidence that a finely tuned balance between the levels of JIL-1 and the major heterochromatin components contributes to the regulation of gene expression.
THE essential JIL-1 histone H3S10 kinase (Jin et al. 1999; Wang et al. 2001) is a major regulator of chromatin structure (Deng et al. 2005, 2008) that functions to maintain euchromatic domains while counteracting heterochromatization and gene silencing (Ebert et al. 2004; Lerach et al. 2006; Zhang et al. 2006; Bao et al. 2007). In the absence of the JIL-1 kinase, the major heterochromatin markers H3K9me2, HP1a [Su(var)2-5], and Su(var)3-7 spread to ectopic locations on the chromosome arms (Zhang et al. 2006; Deng et al. 2007, 2010). These observations suggested a model for a dynamic balance between euchromatin and heterochromatin (Ebert et al. 2004; Zhang et al. 2006; Deng et al. 2010), where, as can be monitored in position-effect variegation (PEV) arrangements, the boundary between these two states is determined by antagonistic functions of a euchromatic regulator (JIL-1) and the major determinants of heterochromatin assembly, e.g., Su(var)3-9, HP1a, and Su(var)3-7 (for review see Weiler and Wakimoto 1995; Girton and Johansen 2008). In support of this model, Deng et al. (2010) recently showed that Su(var)3-7 and JIL-1 loss-of-function mutations have an antagonistic and counterbalancing effect on gene expression using PEV assays; however, potential dynamic interactions between JIL-1 and the other two heterochromatin genes, Su(var)3-9 and Su(var)2-5, were not addressed in this study. Interestingly, in other genetic interaction assays monitoring the lethality as well as the chromosome morphology defects associated with the null JIL-1 phenotype, only a reduction in the dose of the Su(var)3-9 gene (Zhang et al. 2006; Deng et al. 2007) rescued both phenotypes. In contrast, in the same assays a reduction of Su(var)3-7 rescued the lethality, but not the chromosome defects (Deng et al. 2010), and no genetic interactions were detectable between JIL-1 and Su(var)2-5 (Deng et al. 2007). Thus, these findings indicate that while Su(var)3-9 activity may be a major factor in the lethality and chromatin-structure perturbations associated with loss of the JIL-1 histone H3S10 kinase, these effects are likely to be uncoupled from HP1a and, to a lesser degree, from Su(var)3-7. This raises the question of whether JIL-1 dynamically interacts with the two other heterochromatin genes, Su(var)2-5 and Su(var)3-9, in regulating gene expression, as it does with Su(var)3-7.
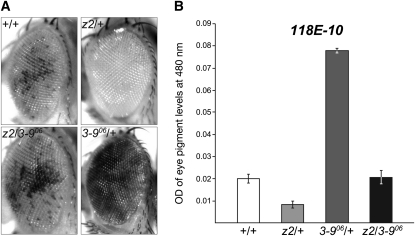
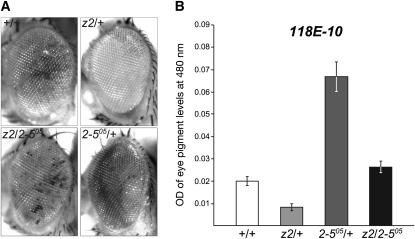
To answer this question, we explored the effect of various combinations of loss-of-function alleles of JIL-1 and Su(var)3-9 or Su(var)2-5 on PEV caused by the P-element insertion line 118E-10 (Wallrath and Elgin 1995; Wallrath et al. 1996). Insertion of this P element (P[hsp26-pt, hsp70-w]) into euchromatic sites results in a uniform red-eye phenotype whereas insertion into a known heterochromatin region of the fourth chromosome results in a variegating eye phenotype (Cryderman et al. 1998; Bao et al. 2007) (Figures 1 and 2). It has been demonstrated that loss-of-function JIL-1 alleles can act as haplo-enhancers of PEV, resulting in increased silencing of gene expression (Deng et al. 2010), whereas loci for structural components of heterochromatin such as Su(var)3-9, Su(var)2-5, and Su(var)3-7 act as strong haplo-suppressors (Eissenberg et al. 1990; Reuter et al. 1990; Tschiersch et al. 1994). In the experiments, the transgenic reporter line 118E-10 was crossed into JIL-1z2/+, Su(var)3-906/+, and Su(var)2-505/+ mutant backgrounds as well as into JIL-1z2/Su(var)3-906 and JIL-1z2/Su(var)2-505 double-mutant backgrounds. The JIL-1z2 allele is a true null allele (Wang et al. 2001; Zhang et al. 2003), the loss-of-function Su(var)3-906 allele is due to a DNA insertion (Schotta et al. 2002), and the Su(var)2-505 loss-of-function allele is associated with a frameshift resulting in a nonsense peptide containing only the first 10 amino acids of HP1a (Eissenberg et al. 1992). Thus, to test whether the heterozygous JIL-1z2 allele could counterbalance the suppression of the Su(var)3-906 or Su(var)2-505 loss-of-function alleles of the PEV of 118E-10, we compared the eye pigment levels of the various genotypes (Figures 1 and 2 and Table 1). Pigment assays were performed essentially as in Kavi and Birchler (2009) using three sets of 10 pooled fly heads from each genotype. Although both male and female flies were scored, due to sex differences only results from male flies are shown. However, the trend observed in female flies was identical to that in male flies (supporting information, Figure S1). As illustrated in Figures 1 and 2, the heterozygous JIL-1z2/+ genotype enhances PEV as indicated by the increased proportion of white ommatidia and a 59% decrease in the optical density (OD) of the eye pigment levels (0.0083 ± 0.0015; n = 3) as compared to +/+ flies (0.0203 ± 0.0021; n = 3). This reduction was statistically significant (Table 1). In contrast, the heterozygous Su(var)3-906/+ and Su(var)2-505/+ genotypes suppress PEV as indicated by an increase of the proportion of red ommatidia and a statistically significant (Table 1) 384% (0.078 ± 0.001; n = 3) and 330% (0.067 ± 0.004; n = 3) increase, respectively, in the OD of the eye pigment levels. However, in the JIL-1z2/Su(var)3-906 and JIL-1z2/Su(var)2-505 double-mutant backgrounds, variegation of the proportion of red ommatidia was intermediate, and the eye pigment levels (0.0207 ± 0.0031 and 0.0263 ± 0.0035, respectively; n = 3) were statistically indistinguishable from genotypes with +/+ levels of JIL-1, Su(var)3-9, and Su(var)2-5 proteins (Figures 1 and 2 and Table 1).
Figure 1.—
Counterbalancing effect of JIL-1 and Su(var)3-9 loss-of-function alleles on the PEV of the P-element insertion line 118E-10. (A) Examples of the degree of PEV in the eyes of wild-type JIL-1 and Su(var)3-9 (+/+), JIL-1z2/+ (z2/+), Su(var)3-906/+ (3-906/+), and JIL-1z2/Su(var)3-906 (z2/3-906) flies in a 118E-10/+ background. All images are from male flies. (B) Histograms of the amount of eye pigment in +/+, JIL-1z2/+ (z2/+), Su(var)3-906/+ (3-906/+), and JIL-1z2/Su(var)3-906 (z2/3-906) male flies heterozygous for 118E-10. Fly stocks were maintained according to standard protocols (Roberts 1998). Oregon-R was used for wild-type preparations. The JIL-1z2 allele is described in Wang et al. (2001) and in Zhang et al. (2003). The Su(var)3-906 and Su(var)2-505 alleles are described in Schotta et al. (2002) and in Eissenberg et al. (1992). The P-element P[hsp26-pt, hsp70-w] insertion line 118E-10 was the generous gift of L. Wallrath. The hsp70 promoter is leaky and promotes sufficient expression to generate a variegated eye phenotype under non-heat-shock conditions (Wallrath and Elgin 1995; Bao et al. 2007). PEV assays were performed as previously described in Lerach et al. (2006) and in Bao et al. (2007). In short, various combinations of JIL-1, Su(var)3-9, or Su(var)2-5 alleles were introduced into the 118E-10 or wm4 PEV arrangements by standard crossing. To quantify the variegated phenotype, adult flies were collected from the respective crosses at eclosion, aged 6 days at 25°, frozen in liquid nitrogen, and stored at −80°C until assayed. The pigment assays were performed essentially as in Kavi and Birchler (2009) using three sets of 10 fly heads of each genotype collected from males and females. For each sample, the heads from the 10 flies were homogenized in 200 μl of methanol with 0.1% hydrochloric acid and centrifuged, and the OD of the supernatant was spectrophotometrically measured at a wavelength of 480 nm.
Figure 2.—
Counterbalancing effect of JIL-1 and Su(var)2-5 loss-of-function alleles on the PEV of the P-element insertion line 118E-10. (A) Examples of the degree of PEV in the eyes of wild-type JIL-1 and Su(var)2-5 (+/+), JIL-1z2/+ (z2/+), Su(var)2-505/+ (2-505/+), and JIL-1z2/Su(var) 2-505 (z2/2-505) flies in a 118E-10/+ background. All images are from male flies. (B) Histograms of the levels of eye pigment in +/+, JIL-1z2/+ (z2/+), Su(var2-5)05/+ (2-505/+), and JIL-1z2/Su(var)2-505 (z2/2-505) male flies heterozygous for 118E-10.
Table 1.
Statistical comparison of eye pigment assays
| Genotypea | JIL-1z2/+ | 3-906/+ | JIL-1z2/3-906 |
| 118E-10/+ | |||
| +/+ | P < 0.005 | P < 0.0001 | P > 0.8 |
| JIL-1z2/+ | — | P < 0.0001 | P < 0.005 |
| 3-906/+ | — | — | P < 0.0001 |
| Genotypea | JIL-1z2/+ | 2-505/+ | JIL-1z2/2-505 |
| 118E-10/+ | |||
| +/+ | P < 0.005 | P < 0.0001 | P > 0.06 |
| JIL-1z2/+ | — | P < 0.0001 | P < 0.005 |
| 2-505/+ | — | — | P < 0.0005 |
| Genotype | JIL-1z2/+ | 3-906/+ | JIL-1z2/3-906 |
| wm4/Y | |||
| +/+ | P < 0.005 | P < 0.0001 | P < 0.0001 |
| JIL-1z2/+ | — | P < 0.0001 | P < 0.0001 |
| 3-906/+ | — | — | P < 0.001 |
| Genotype | JIL-1z2/+ | 2-505/+ | JIL-1z2/2-505 |
| wm4/Y | |||
| +/+ | P < 0.005 | P < 0.0001 | P < 0.0001 |
| JIL-1z2/+ | — | P < 0.0001 | P < 0.0001 |
| 2-505/+ | — | — | P < 0.001 |
For each genotype, the average pigment level from three sets of measurements from 10 pooled fly heads were compared using a two-tailed Student’s t-test.
Only male flies were scored.
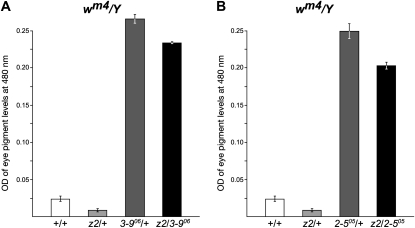
To test whether a heterozygous JIL-1 null allele also could counterbalance the suppression of the Su(var)3-906 or Su(var)2-505 alleles of the PEV of wm4, we performed experiments similar to those described above for 118E-10. As illustrated in Figure 3 for male flies, the heterozygous JIL-1z2/+ genotype enhances PEV as indicated by a 67% decrease in the OD of the eye pigment levels (0.008 ± 0.002; n = 3) as compared to +/+ flies (0.024 ± 0.003, n = 3). This reduction was statistically significant (Table 1). In contrast, the heterozygous Su(var)3-906/+ and Su(var)2-505/+ genotypes suppress PEV as indicated by a statistically significant (Table 1) 1115% (0.2677 ± 0.0061; n = 3) and 1023% (0.2454 ± 0.0103; n = 3) increase, respectively, in the OD of the eye pigment levels. However, in the JIL-1z2/Su(var)3-906 and JIL-1z2/Su(var)2-505 double-mutant backgrounds, the eye pigment levels (0.2337 ± 0.0011 and 0.2043 ± 0.0037, respectively; n = 3) were significantly reduced (Table 1) by 13% and 17% as compared to heterozygous Su(var)3-906/+ and Su(var)2-505/+ genotypes, indicating that a heterozygous JIL-1 null allele has the abilty to counterbalance the suppression of the Su(var)3-906 or Su(var)2-505 alleles of the PEV of wm4. However, it should be noted that it has been demonstrated that JIL-1 can act both as an enhancer and as a suppressor of wm4 PEV, depending on the precise levels of JIL-1 (Lerach et al. 2006; Deng et al. 2010). Thus, the genetic interactions between JIL-1 and the Su(var)3-9 and Su(var)2-5 alleles in regulating the PEV of wm4 are likely to be more complex than in the case of 118E-10 where reduced levels of JIL-1 always act as an enhancer (Bao et al. 2007). In females where the enhancer effect of the heterozygous JIL-1z2 allele is less pronounced than in males, a statistically significant counterbalancing effect was detected only in flies of the JIL-1z2/Su(var)2-505 genotype (Figure S2).
Figure 3.—
Counterbalancing effect of JIL-1 with Su(var)3-9 and Su(var)2-5 loss-of-function alleles on the PEV of wm4. (A) Histograms of the levels of eye pigment in +/+, JIL-1z2/+ (z2/+), Su(var)3-906/+ (3-906/+), and JIL-1z2/Su(var)3-906 (z2/3-906) wm4 male flies. (B) Histograms of the levels of eye pigment in +/+, JIL-1z2/+ (z2/+), Su(var)2-505/+ (2-505/+), and JIL-1z2/Su(var)2-505 (z2/2-505) wm4 male flies.
These results demonstrate that the haplo-enhancer effect of JIL-1 has the ability to counterbalance the haplo-suppressor effect of both Su(var)3-9 and Su(var)2-5 on the PEV of two different alleles. In previous experiments, a genetic interaction between JIL-1 and Su(var)2-5 was not detected (Deng et al. 2007). However, the assays used to probe for interactions were viability and rescue of polytene chromosome morphology. As indicated by the experiments presented here, these parameters are likely to be independent of and separate from the mechanisms contributing to epigenetic regulation of PEV and gene silencing. Consequently, the present experiments, taken together with those of Deng et al. (2010) using a JIL-1 null allele, provide strong evidence that a finely tuned balance between the levels of JIL-1 and all of the major heterochromatin components Su(var)3-9, HP1a, and Su(var)3-7 contributes to the regulation of PEV and gene expression.
Acknowledgments
We thank members of the laboratory for discussion, advice, and critical reading of the manuscript. We also acknowledge V. Lephart for maintenance of fly stocks and Kevin Bienik for technical assistance. We especially thank L. Wallrath for providing fly stocks. This work was supported by National Institutes of Health grant GM062916 (to K.M.J. and J.J.).
Literature Cited
- Bao X., Deng H., Johansen J., Girton J., Johansen K. M., 2007. Loss-of-function alleles of the JIL-1 histone H3S10 kinase enhance position-effect variegation at pericentric sites in Drosophila heterochromatin. Genetics 176: 1355–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryderman D. E., Cuaycong M. H., Elgin S. C. R., Wallrath L. L., 1998. Characterization of sequences associated with position-effect variegation at pericentric sites in Drosophila heterochromatin. Chromosoma 107: 277–285 [DOI] [PubMed] [Google Scholar]
- Deng H., Zhang W., Bao X., Martin J. N., Girton J., et al. , 2005. The JIL-1 kinase regulates the structure of Drosophila polytene chromosomes. Chromosoma 114: 173–182 [DOI] [PubMed] [Google Scholar]
- Deng H., Bao X., Zhang W., Girton J., Johansen J., et al. , 2007. Reduced levels of Su(var)3–9 but not Su(var)2–5 (HP1) counteract the effects on chromatin structure and viability in loss-of-function mutants of the JIL-1 histone H3S10 kinase. Genetics 177: 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Bao X., Cai W., Blacketer M. J., Belmont A. S., et al. , 2008. Ectopic histone H3S10 phosphorylation causes chromatin structure remodeling in Drosophila. Development 135: 699–705 [DOI] [PubMed] [Google Scholar]
- Deng H., Cai W., Wang C., Lerach S., Delattre M., et al. , 2010. JIL-1 and Su(var)3–7 interact genetically and counteract each other’s effect on position-effect variegation in Drosophila. Genetics 185: 1183–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A., Schotta G., Lein S., Kubicek S., Krauss V., et al. , 2004. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18: 2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., James T., Foster-Hartnett D., Hartnett D., Ngan V., et al. , 1990. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 87: 9923–9927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Morris G. D., Reuter G., Hartnett T., 1992. The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girton J., Johansen K. M., 2008. Chromatin structure and regulation of gene expression: the lessons of PEV in Drosophila. Adv. Genet. 61: 1–43 [DOI] [PubMed] [Google Scholar]
- Jin Y., Wang Y., Walker D. L., Dong H., Conley C., et al. , 1999. JIL-1: a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol. Cell 4: 129–135 [DOI] [PubMed] [Google Scholar]
- Kavi H. H., Birchler J. A., 2009. Interaction of RNA polymerase II and the smal RNA machinery affects heterochromatic silencing in Drosophila. Epigenetics Chromatin 2: 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerach S., Zhang W., Bao X., Deng H., Girton J., et al. , 2006. Loss-of-function alleles of the JIL-1 kinase are strong suppressors of position effect variegation of the wm4 allele in Drosophila. Genetics 173: 2403–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Giarre M., Farah J., Gausz J., Spierer A., et al. , 1990. Dependence of position-effect variegation in Drosophila on dose of a gene encoding an unusual zinc-finger protein. Nature 344: 219–223 [DOI] [PubMed] [Google Scholar]
- Roberts D. B., 1998. Drosophila: A Practical Approach. IRL Press, Oxford [Google Scholar]
- Schotta G., Ebert A., Krauss V., Fischer A., Hoffmann J., et al. , 2002. Central role of Drosophila SU(VAR)3–9 in histone H3–K9 methylation and heterochromatic gene silencing. EMBO J. 21: 1121–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschiersch B., Hofmann A., Krauss V., Dorn R., Korge G., et al. , 1994. The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3–9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 13: 3822–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath L. L., Elgin S. C. R., 1995. Position effect variegation in Drosophila is associated with altered chromatin structure. Genes Dev. 9: 1263–1277 [DOI] [PubMed] [Google Scholar]
- Wallrath L. L., Guntur V. P., Rosman L. E., Elgin S. C. R., 1996. DNA representation of variegating heterochromatic P-element inserts in diploid and polytene tissues of Drosophila melanogaster. Chromosoma 104: 519–527 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang W., Jin Y., Johansen J., Johansen K. M., 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105: 433–443 [DOI] [PubMed] [Google Scholar]
- Weiler K. S., Wakimoto B. T., 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29: 577–605 [DOI] [PubMed] [Google Scholar]
- Zhang W., Jin Y., Ji Y., Girton J., Johansen J., et al. , 2003. Genetic and phenotypic analysis of alleles of the Drosophila chromosomal JIL-1 kinase reveals a functional requirement at multiple developmental stages. Genetics 165: 1341–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Deng H., Bao X., Lerach S., Girton J., et al. , 2006. The JIL-1 histone H3S10 kinase regulates dimethyl H3K9 modifications and heterochromatic spreading in Drosophila. Development 133: 229–235 [DOI] [PubMed] [Google Scholar]