Abstract
Maize, with its excellent forward genetics and male sterility screens, was used to identify >50 meiotic mutants representing at least 35 genes that affect key prophase processes such as pairing, synapsis, and homologous recombination. Most of these mutants were found by Inna Golubovskaya during the course of her remarkable career as a cytogeneticist. In addition to undertaking general cytological surveys to classify mutant phenotypes, Golubovskaya focused her efforts on characterizing several key regulatory mutants: ameiotic1 (am1), required to establish the meiotic cell cycle in maize; absence of first division (afd1), required for proper prophase chromosome morphology and for meiotic sister-chromatid cohesion leading to a reductive chromosome segregation at the first meiotic division; and plural abnormalities of meiosis (pam1), required for the clustering of telomeres on the nuclear envelope needed for pairing and synapsis. Her dramatic childhood in Leningrad during its siege in World War II, her fortuitous education in genetics at Leningrad State University, her continued research at the forward-looking Institute of Cytology and Genetics of the USSR Academy of Science Siberian branch, her plight at the fall of the Soviet Union, and her work in America helped engender a unique and valuable plant geneticist. Inna Golubovskaya related this personal history to the authors in conversation.
MEIOSIS is the specialized cell division required in all eukaryotes with a sexual life cycle to produce gametes with a haploid content of chromosomes. During meiosis one round of DNA replication is associated with two rounds of chromosome segregation. The general progression of meiosis is conserved evolutionarily, and hence meiotic prophase and chromosome segregation are similar in plants, animals, and fungi. Following S phase, at leptotene, chromosomes condense and the two sister chromatids are held together along their length by the sister-chromatid cohesin complexes that help to form the axial element that runs the length of the leptotene chromosome. The double-strand breaks that initiate homologous recombination usually occur at this stage. At the leptotene–zygotene transition there is a transient remodeling of chromosome architecture that can include the attachment and clustering of telomeres on the nuclear envelope. These events facilitate the pairing of homologous chromosomes. Coincident with pairing, the homologs zip up—i.e., synapse—as a tripartite synaptonemal complex (SC) forms along the length of the two chromosomes between their axial elements. At pachytene, the homologs are completely synapsed and homologous recombination is completed, leading to crossing over between homologs and chiasmata formation. During diplotene and diakinesis, the SCs fall apart as chromosomes condense further. The homologs are held together by the chiasmata until they segregate away from each other at the first meiotic division (MI). During the subsequent second meiotic division (MII), sister chromatids separate from each other to produce the haploid gametes.
The unique morphology and behavior of meiotic prophase chromosomes have fascinated developmental and cell biologists for >100 years, and the genetics of meiosis, i.e., the “genetics of genetics,” have inspired many to search for mutants that affect this process. The first meiotic mutants that were recognized were the asynaptic mutants found in maize by Beadle (1930) and the c3G mutant in Drosophila melanogaster, in which crossing over is arrested (Gowen and Gowen 1922; Gowen 1933). The initial genetic dissection of Drosophila meiosis was particularly successful (Sandler et al. 1968). But it was especially during the 1960s that a systematic analysis of meiotic mutants took place, as induced by X rays and/or chemical mutagens in a diversity of organisms, including Drosophila, fungi, and various plants (reviewed in Golubovskaya 1979; John 1990). As presented in Golubovskaya’s 1979 review, the rationale for this approach is straightforward:
Meiotic mutants provide meaningful clues to the regulation of meiotic cells; they also help determine the role of cytological entities, their relationships (those between the SC and chiasmata) and the significance of cytological events of meiosis. Furthermore, they reveal similarities and differences in the mechanisms of meiotic recombination, DNA repair and mutability in eukaryotes. And, finally, they permit one to retrace the pathways along which meiosis was arrested in apomictic plants and parthogenetic animal species (p. 248).
“The perfection and beauty of meiosis”
Maize has excellent forward genetics. Primarily using male sterility screens, maize geneticists have obtained >50 meiotic mutants representing ∼35 genes. In Table 1, these mutants are classified and listed in order of the timing of the events that they impact during meiosis. Most of these were found and characterized by Inna Golubovskaya (Figure 1) and collaborators during the course of her remarkable career as a cytogeneticist, both in the former Soviet Union and also in America. In addition to general cytological surveys to classify mutant phenotypes, based, for example, on initiation of recombination or extent of synapsis (Golubovskaya 1989, 2011; Pawlowski et al. 2003), Golubovskaya has focused her cytological studies on several important mutants: ameiotic1 (am1), required to establish the meiotic cell cycle in maize; absence of first division (afd1), required to establish prophase chromosome morphology and sister-chromatid cohesion; and plural abnormalities of meiosis (pam1), required for the clustering of telomeres on the nuclear envelope (the bouquet) (references are listed in Table 1). All three mutants affect multiple early meiotic prophase events, and their analysis has led to a more profound understanding of their regulation.
TABLE 1.
Maize meiotic mutants
| Gene symbol and chromosome arm location | Reference or origin | Allele symbol after genetic analysis |
| Differentiation of meiocytes | ||
| multiple archesporial cells 1 (mac1), 10S | Abramova et al. (2002); Sheridan et al. (1996, 1999) | mac1 (former name lar487) |
| Switch to meiotic cell cycle | ||
| ameiotic 1 (am1), (six alleles) 5S | Rhoades (1956); Golubovskaya et al. (1992, 1993, 1997); Palmer (1971); Pawlowski et al. (2009) | am1-1, am1-2, am1-485, am1-489, am1-6, am1-praI |
| Sister-chromatid cohesion | ||
| absence of the first division (afd1), (five alleles), 6.08 | Golubovskaya and Mashnenkov (1975); Golubovskaya et al. (2006); | afd1-1, afd1-2, afd1-3, afd1-4, afd1-5 |
| Zm shugoshin 1 (sgo1), 7.02 | Golubovskaya et al. (2003); Hamant et al. (2005) | sgo1 |
| Chromosome condensation | ||
| Meiotic025 (Mei025), 5L | Beadle (1937); Golubovskaya (1979); | Mei025 |
| sticky1 (st1) | Golubovskaya et al. (2003) | st1 |
| elongate1 (el1), 8L | Rhoades and Dempsey (1966) | el1 |
| Meiotic bouquet | ||
| plural abnormalities of meiosis (pam1), 1L | Golubovskaya et al. (2002); Golubovskaya (1977) | pam1 |
| Homologous synapsis | ||
| asynaptic 1 (as1), 1S | Beadle (1930) | as1 |
| desynaptic1 (dy1) (SP) | Nelson and Clary (1952) | dy1 |
| desynaptic 1 (dsy1) (two alleles) | Golubovskaya et al. (1997); Golubovskaya and Mashnenkov (1976) | dsy1-1, dsy1-9101 |
| desynaptic2 (dsy2), 5.03–05 | Golubovskaya (1989); Franklin et al. (2003) | dsy2 |
| desynaptic 9303 (dsy*9303) | Golubovskaya et al. (2003) | dsya9303 |
| desynaptic 9305 (dsy*9305) | dsya9305 | |
| desynaptic (dsy9904a) | dsy9904a | |
| desynaptic (dsy9904) | dsy9904b | |
| desynaptic (dsy9905a) | dsy9905a | |
| desynaptic (dsy9905b) | dsy9905b | |
| desynaptic (dsy9906a) | dsy9906a | |
| desynaptic (dsy9906) | dsy9906b | |
| mtm99-14a | Golubovskaya et al. (2003) | mtm99-14 |
| mtm99-25 | Golubovskaya et al. (2003) | mtm99-25 |
| mtm99-30 | mtm99-30 | |
| Homology search | ||
| desynaptic 498, renamed poor homology synapsis (phs1) 9.03 | Golubovskaya et al. (2003); Pawlowski et al. (2004) | phs1 |
| mutator male sterile (mms25), renamed desynaptic CS | Staiger and Cande (1990); Golubovskaya et al. (2003) | dsyCS |
| segregation II | Golubovskaya et al. (2003) | segII |
| Recombination | ||
| ZmRAD51A, 3.04 | Franklin et al. (1999) | Renamed Zm Rad51A1 |
| ZmRad51B, 7.04 | Li et al. (2007) | ZmRad51A2 |
| Monopolar centromere attachment | ||
| mtm00-10 | Inna Golubovskaya, 2006b | mtm00-10 |
| Meiotic cytoskeleton/spindle | ||
| divergent 1 (dv1) | Clark (1940) | dv1 |
| divergent EMS new | Inna Golubovskaya, 2008b | Same phenotype as dv1 |
| male sterile 43 (ms43) | Golubovskaya (1989) | ms43 |
| male sterile 28 (ms28) | Golubovskaya (1989) | ms28 |
| variable 1 (va1), 7L | Beadle (1932) | va1 |
| Meiosis exit | ||
| polymitotic1, 2 alleles, 6S | Beadle (1929, 1931) | po1, po1-ms6 |
| male sterile 6 | Beadle (1929, 1931) | New allele of po1 |
| po1-ms4 | Beadle (1932); Liu et al. (1993) | New allele of po1-ms4 |
mtm represents maize targeted mutagenesis.
Mutants found recently have not been published yet.
Figure 1.—

Inna Golubovskya, 2010, Berkeley, California.
Dr. Golubovskaya “fell in love with meiosis” as a young Ph.D. at the Institute of Cytology and Genetics of the Siberian branch of the USSR Academy of Science, in Akademgorodok, a rural suburb of Novosibrisk. This was the first research center in the Soviet Union where post-Lysenko plant and animal genetic research was permitted. Indeed, replacing Lysenkoism was a primary mission of the institute founded by geneticist Nicholei Dubininin during the Khrushchev administration (Kupershtokh 2009). Enthusiastic professors from Moscow, Leningrad, and similar established research centers congregated in rural Akademgorodok, distant from political control. Here they established a democratic new science. Dr. Golubovskaya credits this intellectually free period in her career with the development of her personal vision. She pursued cytogenetic research on the wheat–Agropyron amphidiploid complex and specifically quantified chromosome instability and infertility among various genotypes. She earned her first Ph.D. degree in 1970. Professor V. V. Khvostova was her Ph.D. supervisor, and Inna published her research on wheat cytogenetics as a chapter in a book (Golubovskaya 1971) that became the primary text for the agricultural genetics students of the USSR at that time.
“I was amazed by the perfection and beauty of meiosis for the whole of my life.” During her Ph.D. studies, Dr. Golubovskaya became aware of the power of a genetic strategy for studying meiosis. For example, geneticists working with Drosophila, maize, and a few other eukaryotes had characterized several meiotic mutants, and the details of how meiosis was altered made it clear to Inna that meiosis could be dissected into its parts genetically (Golubovskaya 1975). Some defects were at the beginning of the regulatory cascade, like Rhoades’ maize mutant ameiotic1 (Rhoades 1956). Others disrupted homologous synapsis specifically, like Beadle’s asynaptic1 (Beadle 1930). At the Siberian Institute, Inna began her ultimate genetics project—the saturation mutagenesis of maize meiosis—and she isolated her first 13 new mutants. Some mutant phenotypes looked similar to those described by Beadle in the 1930s. So, in 1976, she asked the Maize Genetics Cooperation Stock Center to mail her the reference alleles for the six extant maize meiotic genes. Immediately, Inna began allelism tests, mapping and making all possible combinations in comparable genetic backgrounds. One of her mutants appeared to be allelic to polymitotic (Beadle 1929), and another, the praI gene that blocked meiosis at early prophase 1 stage, was allelic to ameiotic1 (Golubovskaya et al. 1997) in which cells undergo mitosis rather than meiosis. The absence of first division1 (afd1) mutant was a new and unique type of mutant. In afd1, sister chromatids segregated equationally (mitosis-like) at the first meiotic division instead of the normal reductional segregation of homologous chromosomes in wild-type meiosis (Golubovskaya et al. 2006). Golubovskaya’s maize work was first published in 1979, and in several subsequent reviews she classified meiotic processes and analyzed the phenotypes of her mutants within this framework (Golubovskaya 1979, 1988; Golubovskaya and Khristolyubova 1985). Inna defended a second Ph.D. degree in 1983, this one in genetics. Inna rapidly earned the reputation in the international plant genetics community as “the best maize geneticist in the USSR.” Her work focused attention on meiosis in flowering plants and has helped to revitalize maize cytogenetics worldwide.
“The road to life”
Early in World War II, 3-year-old Inna Golubovskaya was left for safekeeping with her grandparents near the Soviet city of Leningrad. In the fall of 1941, cut off from supplies by the German army, her grandparents starved to death. Approximately a million people starved to death during this siege [a feeling for this situation has been captured in fiction (Blackwell 2003)]. Inna was placed in an orphanage house. In the winter of 1943, well into the Siege of Leningrad (September 1941–January 1944), a very much alone 4-year-old Inna and others in her orphanage were led out of the wasted city across frozen Lake Ladozhsky, the “road to life.” This road was routinely bombed, and many died. But Inna survived and returned to Leningrad in July 1945 when the war ended. During the war her father and two uncles died defending Leningrad. Inna’s mother was deafened by bombs but managed to escape the city with her infant son, Inna’s brother, although Inna was left knowing nothing about their whereabouts until late 1945 when Inna was reunited with her mother and brother. Although her entire extended family was homeless, it was definitely a family. “It was a poor but joyful childhood.” Largely thanks to an exceptional mother, Inna loved her studies and worked hard. Having begun to show her talents, Inna enrolled in Leningrad State University, and her “road to genetics” began.
To set Inna’s scientific generation in perspective, while Inna was growing up in the late 1940s and 1950s in war-torn Russia, the maize geneticists who trained with R. A. Emerson at Cornell's Department of Plant Breeding (Rhoades 1984)—George Beadle, Charles Burnham, Barbara McClintock, and Marcus Rhoades—were well into their research. That research, when combined with that in Drosophila, firmly placed genes on chromosomes, defined various forms of genetic recombination, and examined meiosis. When Inna chose genetics as her major in 1959, the field of maize cytogenetics in the United States was starting to be pushed aside by research with a more molecular and more microbial bent. George Beadle’s 1930s collection of about five reference mutant alleles of meiotic genes in maize had not been grown in 30 years, and they were rarely studied. Inna preserved the old maize mutants and characterized many more over the course of her career.
Leningrad State University was unique in the Soviet Union; it was a world-class university and a suitable place to be trained in genetics. The wrong-headed genetic dogma of Trofim Lysenko—a politicized brand of Lamarckianism sanctioned by Stalin—was the official genetics of the Soviet Union and poisoned almost all biology curricula until ca. 1963. However, the biology faculty at Leningrad State University had a long history of disrespecting Lysenko, perhaps a legacy from their beloved plant geneticist and germplasm collector colleague N. I. Vavilov, who died a martyr in prison in 1943 for blatantly opposing Lysenko (Crow 1993). Inna was in the first genetics class following Lysenko’s downfall. M. E. Lobashov, chair of the Genetics Department, and V. S. Fedorof, her major professor for her master’s degree, were at the top of a long list of faculty whom Dr. Golubovskaya held in high regard. In 1963, Inna left her beloved Leningrad to take the position of Geneticist at the Institute of Cytology and Genetics, Siberian Branch of the USSR Academy of Science, near Novosibrisk. She felt properly trained in classical genetics and ready “for my own way.”
Professor Golubovskaya lands a great job but then Perestroika begins
After moving from Akademgorodok in Novosibirsk to Leningrad in 1986, Inna was given an appointment as group leader (professor) in the Genetics Department of the N. I. Vavilov Institute in St. Petersburg (formerly Leningrad). By 1987, Gorbachev’s economic reforms, known as perestroika, were making for change. Inna found perestroika an exciting time for freedom in her country, but a terrible time for science. Science in Russia survived only by support from Soros grants (funded 50:50 by the United States and Russia) and the Russian Fund for Fundamental Investigations grants.
American colleagues turned out to be useful at this bend in the road. One of us (Mike Freeling) had the responsibility, as an assistant professor at the University of California at Berkeley (UC-Berkeley), to be the liaison between the Genetics Society of America and the Israeli delegation to the 14th International Congress of Genetics, held in Moscow in 1978. The meeting was particularly controversial. Listening to Inna speak about her work, work she so clearly loved, was difficult because she was both speaking reasonable English and arguing in Russian with her minder on the stage. Inna told Mike Freeling later that it was against the rules to speak anything but Russian, but she broke the rules so she could communicate directly with foreign geneticists. Among a few other American geneticists, senior maize geneticist Ed Coe showed particular interest in Inna’s work and would play a seminal role 10 years hence. Freeling asked Inna on behalf of his colleague, Zac Cande, also a professor at UC-Berkeley, whether she would collaborate with Cande on better visualizing meiotic defects. Zac was a cell biologist interested in cell division but had little background at that time in maize genetics. No one could miss Inna's authentic delight.
In 1979 Ed Coe put Inna's name on the mailing list for The Maize Genetics Cooperation Newsletters, and she received these and various genetics books from him. These were valuable resources that were difficult to obtain even in the post-Lysenko era. In 1990 Inna received an invitation to participate in a United States–USSR workshop of maize geneticists, initiated by W. F. Sheridan (University of North Dakota) and Ed Coe (U.S. Department of Agriculture, University of Missouri), and funded by the National Science Foundation. The idea was to gather Russian and U.S. maize geneticists around one round table to discuss scientific problems. While waiting for this workshop, Dr. Sheridan invited Inna to participate in the 33rd Annual Maize Meeting in 1991, and the United States–Russia workshop took place the next year. This was Inna's first time in the United States, and she met many of the leading maize geneticists and cytogeneticists (Figure 2). At this time, several important collaborations with American scientists were initiated.
Figure 2.—

“She was my goodness,” said Inna of Barbara McClintock (left) at Cold Spring Harbor, New York, in 1991. Inna learned plant cytogenetics at Leningrad State University from Burnham's Discussions in Cytogenetics (Burnham 1962) and a collection of McClintock's papers.
Inna finds new mutants with Bill Sheridan
Inna spent part of the next 7 years in Bill Sheridan's lab and field in Grand Forks, North Dakota, and at the Hawaiian Research Center. Here, Inna isolated and characterized 14 Mu-tagged meiotic mutants, most with defects in pairing and synapsis (Golubovskaya et al. 2003). In particular, the discovery of two new alleles of the ameiotic1 gene (am485 and am489) (Golubovskaya et al. 1993) and the alleles of phs1 and mac1 were critical for their subsequent cloning. The phs1 mutant is deficient in homologous synapsis (Pawlowski et al. 2004; Ronceret et al. 2009). The mac1 gene controls cell fate during anther and ovule development. In developing mac1 anthers, the tapetal cell layer is missing, and there are more meiocytes than in wild type; and in mac1 ovules multiple archesporial cells differentiate into many megaspore mother cells, instead of the normal one cell (Sheridan et al. 1996, 1999). Inna's time in the Sheridan lab gave her the resources needed to maintain and propagate her original collection of mutants induced with chemical mutagenesis, and graduate students Don Auger (now professor at South Dakoda State University) and Guy Farish (Associate Academic Dean, Baldwin-Wallace College) helped Inna adjust to the American style of life and science.
These were dramatic times in Russia. A week after Inna arrived in Grand Forks, on August 19, 1991, GKChP generals conspired to take the leader of the Soviet Union, Mikhail S. Gorbachev, prisoner during his vacation in the Crimea. Inna's family in St. Petersburg (her two daughters Vita and Yuliya and husband Michael Golubovsky) spent nights at Palace Square in support of Gorbachev. Communications to St. Petersburg were blocked during this crisis, and Inna could monitor her homeland and family only by watching TV.
Cytogenetics in the Cande lab
To further her cytological studies, in 1999 Inna moved to UC-Berkeley and joined Zac Cande's lab. Ten years before Inna's arrival at Berkeley, W. Zacheus Cande had been convinced by a former graduate student, Chris Staiger (now a professor at Purdue University), to look at the cytoskeleton in wild-type and mutant maize meiocytes. Even at this time, Inna provided Chris with mutants and guidance from Russia as to what mutants may be most promising. Inna has always been very generous with her mutants and her time. John Sedat, one of the pioneers in developing deconvolution light microscopy, helped Cande to study the architecture of maize pachytene chromosomes, and a joint postdoc, Kelly Dawe (a Mike Freeling student, now a professor at the University of Georgia), used Sedat's computerized 3D fluorescence light microscope system to describe the behavior of chromosomes during meiotic prophase in wild-type cells. As part of that project, other members of the Cande lab worked out how to use FISH and immunostaining of meiotically relevant proteins to study plant chromosome structure in 3D.
Inna had an immediate and major impact on the Cande lab as she adopted these technologies to study her mutants. Her analysis of plural abnormalities of meiosis1 (pam1), a unique mutation with multiple defects in meiotic prophase, was representative of the value of this collaborative effort. Inna had found and described pam1 mutant behavior previously in Russia, but in the Cande lab she showed that its primary defect is to retard bouquet formation, the clustering of telomeres on the nuclear envelope during early meiotic prophase (Golubovskaya 1977; Golubovskaya et al. 2002). No other mutant like it had been found in any organism at that time. Her analysis of pam1 helped to revitalize the idea that bouquet formation played a central role in regulation of pairing and synapsis (Harper et al. 2004). Analysis of this and similar mutants spurred many in the meiosis field working with such diverse organisms as yeast, nematodes, and maize to develop methods of looking at prophase chromosome movements in living meiocytes (reviewed in Sheehan and Pawlowski 2009).
Using the great genetics resources provided by Inna and her insight into the mutant phenotypes, the Cande lab cloned many of the maize meiotic genes that she had found in her forward genetic screens: phs1, cloned with Wojteck Pawlowski (now a professor at Cornell University); afd1 (her favorite), cloned with David Braun (a Mike Freeling postdoc, now a professor at the University of Missouri); the Rhoades’ ameiotic1 gene, cloned by Wojteck Pawlowski and Rachel Wang using Inna's mutator alleles; sgo1 by Oliver Hamant (now a group leader at InRA, Lyon); and recently, mac1 by Rachel Wang (now a Research Fellow at the Academia Sinica, Taiwan) (Pawlowski et al. 2004, 2009; Hamant et al. 2005; Golubovskaya et al. 2006).
The absence of first division1 (afd1) gene was first identified as a mutant that fails to maintain the centromere cohesion required for the reductional division in meiosis (Golubovskaya and Mashnenkov 1975). adf1 mutants bypass the early stages of meiotic chromosome formation blocking the installation of RAD51 foci and are epistatic to all other meiotic mutants tested except am1 (Golubovskaya et al. 1993). When cloned, afd1 was found to be an α-kleisin homolog that is part of the cohesin complex. In her analysis of weak alleles of afd1, Inna demonstrated that afd1 regulates not only meiotic sister-chromatid cohesion, but also the maintenance of the lateral elements of the synaptonemal complex in maize (Golubovskaya et al. 2006).
In ameiotic1 (am1-1), meiotic divisions are replaced by mitosis (Rhoades 1956). In most am1 alleles, male meiocytes undergo mitosis, while female meiocytes either undergo mitosis or arrest at premitotic interphase. One exception is the am1-praI allele, in which both male and female meiocytes enter meiosis and then arrest at the leptotene/zygotene transition (Golubovskaya et al. 1992, 1993, 1997). Molecular lesions in the five ameiotic1 alleles define two key domains: The first domain is an N-terminal domain defined by am1-1 and am1-2 that is required for the switch from mitosis to meiosis. The second domain, defined by am1-praI, is required for progression from leptotene into zygotene. Am1 is a novel gene found in monocots that shares partial similarity to SWITCH1 in Arabidopsis (Pawlowski et al. 2009), a gene that has functions that overlap am1 in that it regulates meiotic prophase chromosome behavior.
Inna continues to walk through fields and screen for new mutants. The phenotype of her new favorite, mtm00-10, is illustrated in Figure 3, and efforts to clone it are in progress. As demonstrated using centromere and chromosome-specific markers, this mutant is defective in pairing of homologs, and the univalent chromosomes at metaphase I show bipolar sister kinetochore orientation rather than the expected unipolar orientation of sister kineotochores in bivalents. The mechanism of kinetochore orientation during the two meiosis divisions is not well understood, and the genes responsible for controlling this behavior have not been identified in plants (Watanabe 2006).
Figure 3.—
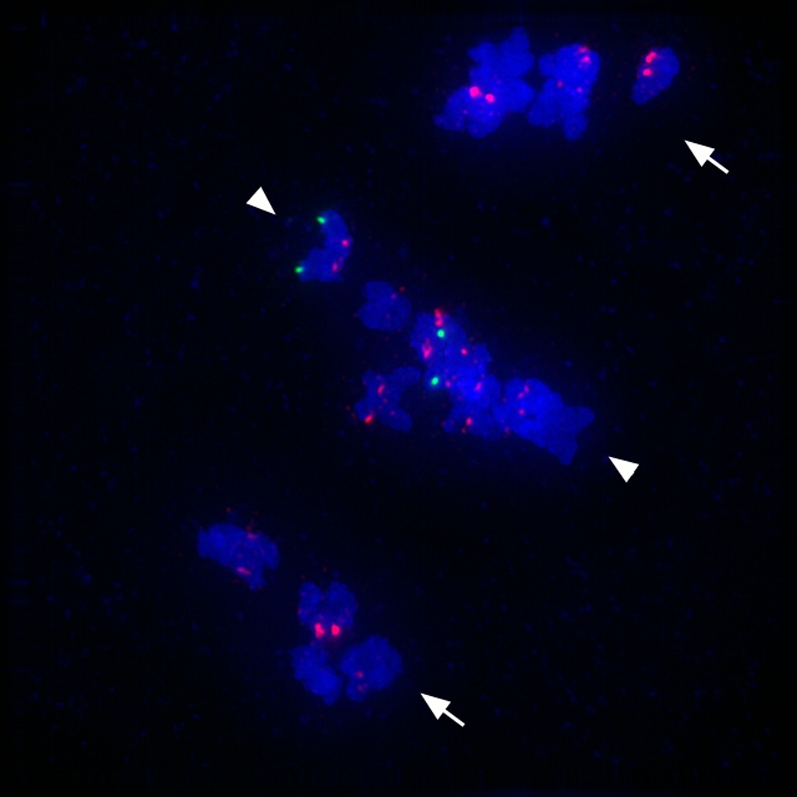
A mtm00-10 mutant with defects in pairing and centromere orientation at metaphase I of meiosis. Shown is an image of anaphase 1 in maize mtm00-10 mutant meiocyte, stained for Cent C (a red centromere probe) and for 5S rDNA (a green probe), and DAPI-stained chromosomes (blue). The Cent C repeat FISH probe marks all centromeres; however, the 5S rDNA FISH probe marks only the chromosome 2 long arm, where the 5S ribosomal DNA locus is located. The mtm00-10 gene affects chromosome segregation at anaphase 1. Homologous chromosomes that pair properly during prophase 1 segregate regularly to opposite poles at anaphase 1 (arrows). However, some homologous chromosomes remain unpaired, and they line up at the middle of cell. The sister centromeres are oriented toward opposite spindle poles; however, sister-chromatid cohesion is not released at centromeric regions, and the chromosomes do not move poleward (arrowheads). In this nucleus, the homologs of chromosome 2 (green signals) did not pair and remain in the middle of the cell.
In 2010, the 72-year-old Inna propagated her collection of multiple alleles of ∼40 maize meiotic genes (Table 1). Inna discovered ∼30 of these genes, and all have been illuminated by her analyses. The grow-out at the Oxford Tract cornfield—in the heart of Berkeley, California—marks Inna's 50 years as a geneticist and, so she says, her last summer with her plants. Now her main goal is to prepare her collection of maize meiotic mutants for transfer to the maize community via the Maize Genetics Cooperation Stock Center.
Family matters
Inna married Mikhail D. Golubovsky in 1961 when they were both sophomores at Leningrad State University. “Neither catastrophes nor distance could break our happy union.” Professor Golubovsky, a Drosophila geneticist and a full member of the Russian Academy of Sciences, found his own ways to pursue excellent science, sometimes located near Inna and sometimes not. Pursuit of a scientific dream and family unity are not incompatible, but they are not always compatible either. That Inna and Mikhail found a way is to their immeasurable credit.
Teaching and awards
During her staying in Novosibirsk Inna participated in the Extension Summer Program to educate high school biology teachers in Mendelian genetics. She taught cytogenetics classes for students of Novosibirsk State University and supervised five masters graduate students and one Ph.D. student. In addition, two Ph.D. students graduated under her supervision at the N. I. Vavilov Research Institute in St. Petersburg. She was elected as a full member of the Russian Academy of Natural Science in 2002.
Perspective
In America, most of us have not had to choose between our science and our safety. This is exactly what Inna Golubovskaya had to do in the early years of her scientific career. Her contributions to the field of meiosis are immense, and her passion for science is authentic. We are truly thankful to have known and worked with Inna Golubovskaya.
References
- Abramova L. I., Avalkina N. A., Golubeva E. A., Pyzhenkova Z. S., Golubovskaya I. N., 2002. Embryological effect of the mac1 mutation in Zea Mays (Poacaae). Bot. J. (Russ) 87: 28–32 [Google Scholar]
- Beadle G., 1929. A gene for supernumerary mitosis during spore development in Zea mays. Science 50: 406–407 [DOI] [PubMed] [Google Scholar]
- Beadle G. W., 1930. Genetic and cytological studies of a Mendelian asynaptic in Zea mays. Cornell Agric. Exp. Sta. Mem. 129: 1–23 [Google Scholar]
- Beadle G. W., 1931. A gene in maize for supernumerary cell divisions following meiosis. Cornell Univ. Agr. Exp. Sta. Mem. 135: 1–12 [Google Scholar]
- Beadle G. W., 1932. Genes in maize for pollen sterility. Genetics 17: 413–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., 1937. Chromosome aberration and gene mutation in sticky chromosome plants of Zea mays. Cytologia Fujii Jubilee Volume: 43–56 [Google Scholar]
- Blackwell E., 2003. Hunger. Little Brown, London [Google Scholar]
- Burnham C., 1962. Discussions in Cytogenetics. Burgess Publishing Company, Minneapolis [Google Scholar]
- Clark F. J., 1940. Cytogenetic studies of divergent meiotic spindle formation in Zea Mays. Am. J. Bot. 27: 547–559 [Google Scholar]
- Crow J. F., 1993. N. I. Vavilov, martyr to genetic truth. Genetics 134: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A. E., McElver J., Sunjevaric I., Rothstein R., Bowen B., et al. , 1999. Three-dimensional microscopy of the Rad51 recombination protein during meiotic prophase. Plant Cell 11: 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A. E., Golubovskaya I. N., Bass H. W., Cande W. Z., 2003. Improper chromosome synapsis is associated with elongated RAD51 structures in the maize desynaptic2 mutant. Chromosoma 112: 17–25 [DOI] [PubMed] [Google Scholar]
- Golubovskaya I. N., 1971. Cytogenetics of distant wheat hybrids and perspectives on their using in breeding, pp. 234–286 Cytogenetics of Wheat and Wheat Hybrids, edited by Khvostova V. V. Nauka, Moscow (in Russian) [Google Scholar]
- Golubovskaya I. N., 1975. Genetic control of chromosomes behavior in meiosis, pp. 312–343 Cytology and Genetic of Meiosis, edited by Khvostova V. V., Bogdanov Y. F. Nauka, Moscow (in Russian) [Google Scholar]
- Golubovskaya I. N., 1979. Genetic control of meiosis. Int. Rev. Cytol. 58: 247–290 [DOI] [PubMed] [Google Scholar]
- Golubovskaya I. N., 1988. Double maize meiotic mutants and genetic control of meiosis. Genetika (Russ.) 24: 1649–1657 [Google Scholar]
- Golubovskaya I. N., 1989. Meiosis in maize: mei genes and conception of genetic control of meiosis. Adv. Genet. 26: 149–192 [Google Scholar]
- Golubovskaya I. N., Khristolyubova N. B., 1985. Maize meiosis and maize genes, pp. 723–738 Plant Genetics, edited by Freeling M. Alan R. Liss, New York [Google Scholar]
- Golubovskaya I. N., Mashnenkov A. S., 1975. Genetic control of meiosis. I. Meiotic mutation in corn (Zea mays L.) afd, causing the elimination of the first meiotic division. Genetika (Russ.) 11: 810–816 [Google Scholar]
- Golubovskaya I. N., Mashnenkov A. S., 1976. Genetic control of meiosis: II. A desynaptic mutant in maize induced by N-nitroso-N-methylurea. Genetika (Russ.) 12: 7–14 [Google Scholar]
- Golubovskaya I. N., Mashnenkov A. S., 1977. Multiple disturbances of meiosis in corn are caused by a single recessive mutation pamA-A344. Genetika (Russ.) 13: 1278–1287 [Google Scholar]
- Golubovskaya I. N., Grebennikova Z. K., Avalkina N. A., 1992. New allele of ameiotic1 gene in maize and problem of gene control of meiosis initiation in high plants. Genetika (Russ.) 27: 137–146 [Google Scholar]
- Golubovskaya I., Grebennikova Z. K., Avalkina N. A., Sheridan W. F., 1993. The role of the ameiotic1 gene in the initiation of meiosis and in subsequent meiotic events in maize. Genetics 135: 1151–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya I., Avalkina N., Sheridan W. F., 1997. New insights into the role of the maize ameiotic1 locus. Genetics 147: 1339–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya I. N., Harper L. C., Pawlowski W. P., Schichnes D., Cande W. Z., 2002. The pam1 gene is required for meiotic bouquet formation and efficient homologous synapsis in maize (Zea mays L.). Genetics 162: 1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya I. N., Sheridan W. F., Harper L. C., Cande W. Z., 2003. Novel meiotic mutants of maize identified from Mu transposon and EMS mutant screens. Maize Genet. Coop. Newsl. 77: 10–13 [Google Scholar]
- Golubovskaya I. N., Hamant O., Timofejeva L., Wang C. J., Braun D., et al. , 2006. Alleles of afd1 dissect REC8 functions during meiotic prophase I. J. Cell Sci. 119: 3306–3315 [DOI] [PubMed] [Google Scholar]
- Golubovskaya I. N., Wang C.-J. R., Timofejeva L., Cande W. Z., 2011. Maize meiotic mutants with improper or nonhomologous synapsis due to problems in pairing or synaptonemal complex formation. J. Exp. Bot. 62: 1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen J. W., 1933. Meiosis as a genetic character in Drosophila melanogaster. J. Exp. Zool. 65: 83–106 [Google Scholar]
- Gowen M. S., Gowen J. W., 1922. Complete linkage in Drosophila melanogaster. Am. Nat. 61: 286–288 [Google Scholar]
- Hamant O., Golubovskaya I., Meeley R., Fiume E., Timofejeva L., et al. , 2005. A REC8-dependent plant Shugoshin is required for maintenance of centromeric cohesion during meiosis and has no mitotic functions. Curr. Biol. 15: 948–954 [DOI] [PubMed] [Google Scholar]
- Harper L., Golubovskaya I., Cande W. Z., 2004. A bouquet of chromosomes. J. Cell Sci. 117: 4025–4032 [DOI] [PubMed] [Google Scholar]
- John B., 1990. Meiosis. Cambridge University Press, New York [Google Scholar]
- Kupershtokh N. A., 2009. The Institute of Cytology and Genetics of the RAS Siberian Branch. Herald of the Russian Academy of Sciences 79: 307–315 [Google Scholar]
- Li J., Harper L. C., Golubovskaya I., Wang C. R., Weber D., et al. , 2007. Functional analysis of maize RAD51 in meiosis and double-strand break repair. Genetics 176: 1469–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Golubovskaya I., Cande W. Z., 1993. Abnormal cytoskeletal and chromosome distribution in po, ms4 and ms6, mutant alleles of polymitotic that disrupt the cell cycle progression from meiosis to mitosis in maize. J. Cell Sci. 106: 1169–1178 [DOI] [PubMed] [Google Scholar]
- Nelson O. E., Clary G. B., 1952. Genetic control of semisterility in maize. J. Hered. 43: 205–210 [Google Scholar]
- Palmer R. G., 1971. Cytological studies of ameiotic and normal maize with reference to premeiotic pairing. Chromosoma 35: 233–246 [Google Scholar]
- Pawlowski W. P., Golubovskaya I. N., Cande W. Z., 2003. Altered nuclear distribution of recombination protein RAD51 in maize mutants suggests the involvement of RAD51 in meiotic homology recognition. Plant Cell 15: 1807–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski W. P., Golubovskaya I. N., Timofejeva L., Meeley R. B., Sheridan W. F., et al. , 2004. Coordination of meiotic recombination, pairing, and synapsis by PHS1. Science 303: 89–92 [DOI] [PubMed] [Google Scholar]
- Pawlowski W. P., Wang C. J., Golubovskaya I. N., Szymaniak J. M., Shi L., et al. , 2009. Maize AMEIOTIC1 is essential for multiple early meiotic processes and likely required for the initiation of meiosis. Proc. Natl. Acad. Sci. USA 106: 3603–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M. M., 1956. Genic control of chromosomal behavior. Maize Genet. Coop. Newsl. 30: 38–48 [Google Scholar]
- Rhoades M. M., 1984. The early years of maize genetics. Annu. Rev. Genet. 18: 1–29 [DOI] [PubMed] [Google Scholar]
- Rhoades M. M., Dempsey E., 1966. Induction of chromosome doubling at meiosis by the elongate gene in maize. Genetics 54: 505–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronceret A., Doutriaux M. P., Golubovskaya I. N., Pawlowski W. P., 2009. PHS1 regulates meiotic recombination and homologous chromosome pairing by controlling the transport of RAD50 to the nucleus. Proc. Natl. Acad. Sci. USA 106: 20121–20126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler L., Lindsley D. L., Nicoletti B., Trippa G., 1968. Mutants affecting meiosis in natural populations of Drosophila melanogaster. Genetics 60: 525–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan M. J., Pawlowski W. P., 2009. Live imaging of rapid chromosome movements in meiotic prophase I in maize. Proc. Natl. Acad. Sci. USA 106: 20989–20994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan W. F., I. Shamrov N. A. Avalkina, I., Batygina T. B., Golubovskaya I. N., 1996. The mac1 gene: controlling the commitment to the meiotic pathway in maize. Genetics 142: 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan W. F., Golubeva E. A., Abrhamova L. I., Golubovskaya I. N., 1999. The mac1 mutation alters the developmental fate of the hypodermal cells and their cellular progeny in the maize anther. Genetics 153: 933–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger C. J., Cande W. Z., 1990. Microtubule distribution in dv, a maize meiotic mutant defective in the prophase to metaphase transition. Dev. Biol. 138: 231–242 [DOI] [PubMed] [Google Scholar]
- Watanabe Y., 2006. A one-sided view of kinetochore attachment in meiosis. Cell 126: 8–10 [DOI] [PubMed] [Google Scholar]


