Abstract
Genetic studies have implicated the evolutionary novel, anthropoid primate-specific gene locus G72/G30 in psychiatric diseases. This gene encodes the protein LG72 that has been discussed to function as a putative activator of the peroxisomal enzyme -amino-acid-oxidase (DAO) and as a mitochondrial protein. We recently generated ‘humanized' bacterial artificial chromosome transgenic mice (G72Tg) expressing G72 transcripts in cells throughout the brain. These mice exhibit several behavioral phenotypes related to psychiatric diseases. Here we show that G72Tg mice have a reduced activity of mitochondrial complex I, with a concomitantly increased production of reactive oxygen species. Affected neurons display deficits in short-term plasticity and an impaired capability to sustain synaptic activity. These deficits lead to an impairment in spatial memory, which can be rescued by pharmacological treatment with the glutathione precursor N-acetyl cysteine. Our results implicate LG72-induced mitochondrial and synaptic defects as a possible pathomechanism of psychiatric disorders.
Keywords: G72Tg mice exhibit altered mitochondrial activity and increased production of ROS, G72Tg mice show synaptic deficits and impaired spatial learning, NAC treatment completely restores spatial learning ability in G72Tg mice
INTRODUCTION
G72 (also named -amino-acid oxidase activator, DAOA) is among the best replicated vulnerability genes for schizophrenia (Addington et al, 2004; Chumakov et al, 2002; Li and He, 2007; Schumacher et al, 2004) and bipolar affective disorder (BPAD) (Addington et al, 2004; Prata et al, 2008; Schumacher et al, 2004; for a review see Detera-Wadleigh and McMahon, 2006). G72 was also recently found to be associated with major depression (Jansen et al, 2009), which together supports the notion that these disorders have a partially overlapping etiology. The G72 gene locus emerged late during evolution of higher primates (Chumakov et al, 2002). It encodes two genes, G72 and G30, that are transcribed contrariwise from overlapping DNA strands (reviewed in Abou Jamra et al, 2006). LG72, the longest open reading frame, represents an exceptional case of a primate-specific gene with a rapidly changing protein structure, probably related to a rapid evolution of underlying brain function (Kvajo et al, 2008). Although the N terminus of the protein is well conserved among anthropoid primates, variations in the C terminus result in proteins of different sizes. Human beings have the largest protein with 153 amino acids, followed by the gibbon LG72 with 121 amino acids. The LG72 protein in chimpanzees and gorillas consists of 66 amino acids (Chumakov et al, 2002).
Yeast two-hybrid and biochemical studies have demonstrated that LG72 binds to -amino-acid-oxidase (DAO), the main degrading enzyme of -serine. -serine, produced by astrocytes, is an allosteric co-activator of NMDA-type glutamate receptors. The effects of LG72 on peroxisomal DAO functioning is controversial, because both enhancement (Chumakov et al, 2002) and repression (Sacchi et al, 2008) of DAO activity have been reported. An alternative role for the LG72 protein was suggested by the demonstration that it contains an N-terminal mitochondrial translocation sequence and is transported into the mitochondria. Experiments performed in neuronal cultures also showed that it affects mitochondrial morphology (Kvajo et al, 2008).
We recently generated BAC (bacterial artificial chromosome) transgenic mice carrying the entire human G72/G30 gene locus (G72Tg; Otte et al, 2009). These ‘humanized' mice expressed all known G72 splice variants, as well as G30 transcripts. Behavioral analyses of these mice showed several phenotypes, such as a PPI deficit that is reversible by haloperidol treatment, increased compulsive behaviors, impaired locomotor coordination, increased sensitivity to phencyclidine, and impaired odorant discrimination. These behavioral phenotypes are regarded as rodent correlates to symptoms of schizophrenia (Bottas et al, 2005; Braff and Geyer, 1990; Murray, 2002; Nguyen et al, 2010). However, many clinical features of schizophrenia can also be observed in other psychiatric disorders like bipolar disorder (Taylor et al, 1993; Tsuang et al, 1980; Valles et al, 2000) and genetic risk factors contributing to psychiatric disorders, including G72/G30 that are typically associated with more than one disorder.
In this study, we focus on the analysis of hippocampal learning in G72Tg mice, because cognitive deficits including spatial working memory, short-term spatial memory, and long-term episodic memory are observed in schizophrenia. (Joyce et al, 2002). We demonstrate a striking spatial learning deficit in G72Tg mice and concomitant synaptic deficits, which are most likely due to LG72-related mitochondrial dysfunctions resulting in elevated brain reactive oxygen species (ROS). Interestingly, an oral treatment with N-acetyl cysteine (NAC), a precursor of glutathione (GSH), increases the antioxidant capacity and rescues the spatial learning deficit.
MATERIALS AND METHODS
Transgenic Mice
Transgenic mice (G72Tg) were generated as described (Otte et al, 2009). G72Tg mice were crossed to CD1 wild-type (Wt) mice and maintained as a hemizygote line. Transgenic animals were genotyped by dot-blot analysis. Briefly, 15 μl of denatured (5 min 95 °C) genomic mouse-tail DNA was spotted onto nitrocellulose membranes (Schleicher and Schull, Dassel, Germany) and allowed to dry. Dot blots were hybridized with an intronic G72 PCR probe that was synthesized with the primers BACSouthF (5′-AGTTAGCTGTGCCTGACCTCG-3′) and BACSouthR (5′-TGAAAGCTCTCATCGGTCCTG-3′).
Expression Analysis
Total RNA and cDNA preparation as well as Taqman and differential protein expression analyses were performed as described in the Supplementary Methods.
Measurement of DAO Activity
Tissue from specified organs of Wt and G72Tg mice were prepared and processed as published previously (D'Aniello et al, 1993) utilizing the Pyruvate Assay Kit (BioVision, Mountain View, CA) to detect α-keto acid production. Briefly, mouse cerebelli were homogenized on ice with a Polytron homogenizer in Pyruvate Assay Buffer (0.25 g tissue per ml) containing protease inhibitors (Complete, EDTA-free; Roche Diagnostics, Indianapolis, IN). The homogenate was clarified by centrifugation at 30 000 g for 30 min at 4 °C. A measure of 100 μl of the supernatant was mixed with 100 μl of 0.1 M -alanine (in 0.1 M Tris-HCl, pH 8.2) or with 100 μl of 0.1 M Tris-HCl, pH 8.2, and then incubated at 37 °C for 30 min. Pyruvate formed by the oxidation of -alanine by DAO was measured using the Pyruvate Assay Kit from BioVison. A measure of 50 μl of the reaction product was added to 50 μl of Pyruvate Detection Mix and incubated at room temperature for 30 min. A standard curve covering a range of 10–0.1 nmol per well was used as control. Absorbance was measured at 570 nm and the pyruvate concentration was calculated relative to the standard curve. Pyruvate production was normalized to total protein as determined using the Coomassie Plus Protein Assay Kit (Pierce Chemical, Rockford, IL).
Measurement of -Serine by HPLC
Amino-acid enantiomers were separated by HPLC using a C18 reverse-phase column (250 mm) (Knauer, Berlin, Germany) with fluorimetric detection after derivatization with N-isobutyryl--cysteine and o-phthaldialdehyde, as described (Grant et al, 2006) (see Supplementary Methods).
Determination of Mitochondrial Enzyme Activities
Aconitase activity was measured according to Gardner et al (1994) with small modifications. Activity was determined at 30 °C using a dual-wavelength spectrophotometer (Aminco DW 2000; SLM Instruments) following the absorbance change at 340–380 nm ered−ox=5.5/mM/cm). The reaction mixture contained 50 mM Tris-Cl (pH 7.4), 5 mM sodium citrate, 0.6 mM MnCl2, 0.2 mM NADP+, 0.1 mg/ml isocitrate dehydrogenase, 0.2% laurylmaltoside and 40–70 μg/ml of protein. The mitochondrial homogenate was diluted 10 times in a solution containing 50 mM Tris-Cl and 0.6 mM MnCl2 (pH 7.4) just before the activity measurement. Then, the sample was sonicated for 15 s with the ultrasonic processor GEX-600. The activity of citrate synthase was determined by standard methods as described elsewhere (Wiedemann et al, 2000). The activity of rotenone-sensitive NADH : CoQ1-reductase was measured at 340–380 nm (ered−ox=5.5/mM/cm) as described (Kudin et al, 2002). Briefly, the reaction was performed in a reaction buffer containing 50 mM KCl, 10 mM Tris-HCl and 1 mM EDTA (pH 7.4) in the presence of 150 μM NADH, 100 μM coenzyme Q1 and 2 mM KCN with a dual-wavelength spectrophotometer (Aminco DW 2000; SLM Instruments). The protein content of brain tissue homogenates was determined using a protein assay kit based on Peterson's modification of the micro-Lowry method according to the instructions of the manufacturer (Sigma, Munich, Germany).
Determination of Reduced GSH Content
The mitochondrial suspension was dissolved in MTP medium at a final concentration of ∼0.8 mg/ml. After the addition of monochlorobimane (100 μM) and GSH S-transferase (1 U/ml), the reaction mixture was sonicated for 15 s with the ultrasonic processor GEX-600. After 30 min development of reaction, the mitochondrial suspension was centrifuged at 16 000 g for 7 min. Thereafter, the fluorescence of each supernatant was measured (lex=380 nm, lem=470 nm) using a fluorescence reader (Spectra MAX Gemini; Molecular Devices). As a standard we used GSH dissolved in MTP medium in a range 0–100 μM (Kamencic et al, 2000). All data are expressed as mean±SEM.
Measurement of Malondialdehyde
Cerebellar malondialdehyde was measured by enzymatic detection, according to the method described by Esterbauer and Cheeseman (1990).
Slice Preparation and Electrophysiology
For a detailed method description see Supplementary Methods.
Cell Culture and Transfection
Detailed information about the performed experiments is given in the Supplementary Methods.
Pharmacological NAC Treatment
After weaning, 3-week-old Wt and G72Tg male mice received a 1 g/l NAC solution (pH 7.0) as the only drinking source. This dose was related to Chen et al, (2007), Lehmann et al, (1994), and Lin and Yin (2008), where it was effective in treating several disease models in mice when given via drinking water. The control mice drank water. After 5 weeks, mice were behaviorally tested and then killed for biochemical studies. For the generation of a GSH time curve, mice were treated as described above, but 3, 5.5, and 8 weeks old mice were killed and brain GSH content was measured.
Morris Water Maze
One week before starting the experiments, male mice (9–10 weeks old) were transferred to standard single-mouse cages and maintained at a 12 : 12-h inverted cycle (light on 1900–0700 hours) Animals were tested during the dark period. The home cages were brought into the test room at least 30 min before starting the experiment. The Morris water maze (MWM) test was conducted using heterozygous transgenic animals, which were compared with their Wt littermates. A circular pool (diameter 120 cm and height 30 cm) made of black PVC was used in a dimly illuminated room. The pool was filled with water (24 °C) opacified with blue staining to a depth of 15 cm. Four orthogonal starting positions were situated around the perimeter of the pool, dividing its surface into four quadrants. A platform in the form of a transparent Plexiglas cylinder (15 cm tall and 8 cm diameter) covered with a white aluminum perforated plate (14 cm diameter) was placed in the center of one quadrant, approximately 1.5 cm under the water level and served as an escape platform. The pool was located in a room containing numerous extra-maze visual cues. A camera was fixed at the ceiling above the water maze. Each trial was recorded with the help of a Noldus 2.1 video tracker camera system.
Water Maze Procedure
Each mouse was tested for four consecutive sessions daily over 7 days. The hidden platform remained at a fixed spatial location for the entire acquisition period and each mouse was assigned a different escape sector. For each trial session, the mice were released, facing the wall of the maze. During the first 2 days, animals were placed to the same starting point (N) for each session. From days 3 to 7, animals were placed to one of the four positions (N, E, S, W; Supplementary Figure S6A/B), respectively, for each session. A trial ended when the mouse reached the hidden platform and managed to remain there for 5 s. If a mouse did not manage to escape to the platform within 70 s, it was guided to the platform and the trial was recorded as an escape failure with an arbitrary latency of 70 s. The mouse was left in a dark, dry container for a 15 s inter-trial interval. After the 7-day acquisition phase, each mouse was subjected to a reversal test for 3 consecutive days. To assess their spatial memory ability, the platform was removed from the maze. The animals were tested for retention of spatial memory 24 h after the final trial. In this probe trial, lasting for 70 s, each mouse was placed into the water as described for the training trials. The time (s) to reach the target quadrant and the time spent in the target quadrant was recorded.
Data Analysis and Statistics
Brain GSH content of NAC-treated mice was analyzed using one-way analysis of variance (ANOVA), followed by a Dunn's multiple comparison test. The GSH time curve of NAC-treated mice was evaluated statistically by using two-way ANOVA and Bonferroni post hoc test.
The GSH time curve of untreated mice was analyzed by using one-way ANOVA and Bonferroni post hoc test. Electrophysiological and all other biochemical studies were analyzed using Mann–Whitney U-test. MWM escape latency and swim speed were analyzed by three-way ANOVA using genotype, treatment and time as main factors. In case of significance, a Fisher LSD post hoc test was performed. The analysis of the probe trial was carried out by Kruskal–Wallis test followed by a Dunn's multiple comparison test as post hoc test. Statistical analyses were conducted using the software STATISTICA 7 (Statsoft, Tulsa, OK) and Prism 4 (GraphPad Software, San Diego, CA). All data are expressed as mean±SEM. P-values <0.05 were considered to represent significant effects.
RESULTS
Mitochondrial Dysfunction and Altered Redox State in G72Tg Mice
To gain insight into pathogenic processes induced by G72 expression, we performed a systematic analysis of differentially expressed proteins using two-dimensional gel electrophoresis of crude cerebellar protein extracts from G72Tg and Wt mice. Gel spots that differed significantly between the two genotypes were identified by mass spectrometry. Interestingly, we found an increased expression of the GSH-S-transferase (GSTs) M1 in both transgenic mouse (G72Tg1 and G72Tg2) lines and also an increased GST P1 expression in the G72Tg2 line (Table 1). GSTs belong to a multi-gene family of detoxifying enzymes. They are involved in the metabolism of a wide range of endogenous and xenobiotic compounds by catalyzing the conjugation of these compounds with GSH (Board, 2007). Furthermore, we also found an upregulation of phosphoglycerate mutase 1, an enzyme involved in glycolysis. Upregulation of this enzyme enhances the glycolytic flux and may occur under conditions of insufficient mitochondrial energy supply (Kondoh et al, 2007).
Table 1. Differentially Expressed Proteins in the Cerebellum of G72Tg Mice.
| Name | Accession ID | Ratio G72Tg : Wt |
|---|---|---|
| G72Tg1 | ||
| Rho GDP dissociation inhibitor | GDIR_MOUSE | 2.04 : 1 |
| Tubulin α1 | Q3TGF0_MOUSE | 0.5 : 1 |
| Tubulin α1 | Q3TGF0_MOUSE | 0.34 : 1 |
| Cofilin-1 (Cofilin, non-muscle isoform) | COF1_MOUSE | 1.69 : 1 |
| Glutathione S-transferase P1 | GSTP1_MOUSE | 2.29 : 1 |
| Glutathione S-transferase M1 | GSTM1_MOUSE | 1.54 : 1 |
| G72Tg2 | ||
| Tubulin β2 | Q99JZ6_MOUSE | 2.52 : 1 |
| Glutamine synthetase | Q91VC6_MOUSE | 2.84 : 1 |
| Phosphoglycerate mutase 1 | PGAM1_MOUSE | 2.41 : 1 |
| Cofilin-1 (Cofilin, non-muscle isoform) | COF1_MOUSE | 1.45 : 1 |
| Glutathione S-transferase M1 | GSTM1_MOUSE | 1.59 : 1 |
Relative expression levels were evaluated independently by densitometry from three Wt and three G72Tg gels.
Given the mitochondrial localization of heterologously expressed LG72 in human (Kvajo et al, 2008) and mouse cells (Supplementary Figure S1A), these findings suggested the possibility that LG72 affected mitochondrial function and lead to an increased production of ROS. We therefore investigated mitochondrial density and expression of mitochondrial marker enzymes in G72Tg mice. Cytochrome oxidase and succinate dehydrogenase stainings of cerebellar sections (Supplementary Figure S2B (A and B)), and citrate synthase activity in cerebellar homogenates (Supplementary Figure S2B (C and D)) were indistinguishable from Wt littermates. The mitochondrial density was thus not altered in transgenic mice. However, we observed a striking difference between Wt and G72Tg animals in the citrate synthase-normalized activity of complex I in cerebellar homogenates (p=0.014; Figure 1c), which showed strong LG72 expression (Otte et al, 2009). In contrast, complex I activity in cortex homogenates, where G72 was expressed at much lower levels (Otte et al, 2009), was not different from Wt. We next studied aconitase activity, which catalyzes the stereo-specific isomerization of citrate to isocitrate. This enzyme is known to be very sensitive to oxygen radicals owing to an Fe–S cluster at the catalytic center and can thus serve as an ROS indicator (Flint et al, 1993). Indeed, citrate synthase-normalized aconitase activity was also significantly lower. This effect was again specific for the cerebellar homogenates (p=0.002; Figure 1d). To determine if an increased generation of ROS in the mitochondria of G72Tg mice resulted in an increased accumulation of oxidative damage, we next investigated oxidized lipids and reduced GSH levels. As shown in Figure 1e, oxidized lipids in the cerebellum were indeed increased (p=0.002) in G72Tg mice. GSH levels, a measure of global antioxidant activity, were lower in G72Tg cerebellar homogenates (p=0.014; Figure 1f). These significant difference in GSH levels were first observed at 8 weeks of age (F5, 29=2.976, p<0.0314; Supplementary Figure S3). Taken together, these results demonstrate a decreased mitochondrial complex I activity in the presence of LG72, presumably causing a concomitant increase in ROS production.
Figure 1.
(a) Quantification of mitochondrial citrate synthase activity in cerebellar (Cb) and cortical (Co) lysates revealed no differences between the genotypes. (b) The ratios of complex I activity/citrate synthase activity mice and (c) aconitase activity/citrate synthase activity were decreased in cerebellar lysates of G72Tg animals. (d) Quantification of reduced glutathione (GSH) content per μg protein in cortex and cerebellum of male wild-type (Wt) and G72Tg mice. (e) Concentration of thiobarbituric acid-reactive substances (TBARS), which are an indicator of oxidized biomolecules in the cerebellum of G72Tg and Wt male animals. (f) -serine content (%-serine) in Wt and G72Tg mice. Co=cerebral cortex; Cb=cerebellum; Hc=hippocampus; Fb=forebrain. (g) DAO activity in Wt and G72Tg mice. **p<0.01 and *p<0.05 for Mann–Whitney U-test. All results are shown as mean±SEM., n=6, scale bar a–c represent 10 μm.
Unchanged DAO Activity and -Serine Levels
We next determined if the activity of DAO was changed in G72Tg mice. Thus, we measured -serine in the total serine pool (%-serine) in the forebrain, the hippocampus, and the cerebral cortex. As shown in Figure 1g, we found no difference between G72Tg and Wt mice. In addition, we measured DAO activity in the cerebellum of Wt and G72Tg mice and found no alteration in DAO activity (Figure 1h).
Synaptic Deficits
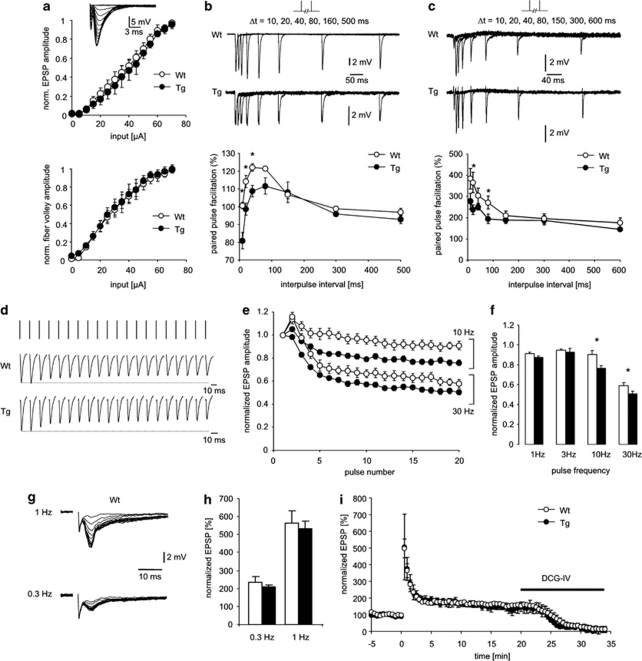
Because mitochondria are remarkably concentrated at synaptic sites in neurons (Palay, 1956) and have been suggested to have a role in both the development and function of synapses (Li et al, 2004; Stowers et al, 2002; Verstreken et al, 2005), we examined whether central synaptic transmission is altered in G72Tg mice. For this purpose, we studied the perforant path-dentate granule (PP-DG) cell and the mossy fiber-CA3 (MF-CA3) synapses in the hippocampus. G72 mRNA is strongly expressed in the entorhinal cortex and dentate granule neurons (Supplementary Figure S4; Otte et al, 2009), and therefore present at the pre- and postsynaptic neurons of the PP-DG synapse. In CA3 pyramidal cells, G72 expression was low and thus preferentially located presynaptically at the MF-CA3 synapse.
At the PP-DG synapse, input–output curves revealed no differences in basal synaptic transmission. Both the size of the presynaptic fiber volley (reflecting presynaptic excitability) and the magnitude of the resulting excitatory postsynaptic potentials (EPSPs) were unchanged in G72Tg mice (Figure 2a). We next evaluated short-term plasticity using paired-pulse facilitation. An increased response to the second of two closely spaced synaptic stimulations was observed in control animals (Figure 2b, empty symbols). This form of plasticity was significantly reduced in G72Tg mice (n=6 and 5 for G72Tg1 and WT mice, respectively; Figure 2b, significant differences indicated by asterisks, p=0.0047, 0.0112, and 0.01 for 10, 20, and 40 ms inter-pulse interval). At the MF-CA3 synapse, which exhibits a much more pronounced paired-pulse facilitation (Salin et al, 1996), a similar phenomenon was observed. Paired-pulse facilitation was significantly reduced in G72Tg mice (n=4 and 7 for G72Tg and WT mice, respectively; Figure 2c, significant differences indicated by asterisks, p=0.005 and 0.02 for 20 and 80 ms inter-pulse interval).
Figure 2.
Altered synaptic transmission in G72Tg mice. (a) Basal synaptic transmission is unaltered. The dependence of the normalized excitatory postsynaptic potential (EPSP) magnitude on the stimulation strength was unchanged in G72Tg mice. Likewise, the dependence of the presynaptic fiber volley size on stimulation strength was unchanged. Insets depict representative examples of EPSPs recorded from G72 wild-type (Wt) and G72Tg mice. (b, c) Paired-pulse presynaptic plasticity at path-dentate granule (PP-DG) (b) and mossy fiber-CA3 (MF-CA3) (C) synapses. The paired-pulse facilitation for control and G72 transgenic mice (empty and filled symbols, respectively) was calculated as the peak amplitude ratio of the second to the first of two consecutively elicited EPSPs. Representative examples in top panels and quantification in lower panels. (d) Response of PP-DG synapses to sustained synaptic stimulation. EPSPs were induced by stimulus trains at varying frequencies (1–30 Hz). A representative example for a G72Tg and a Wt mouse is shown. (e, f) Progressive reduction of the EPSP magnitude during 10 or 30 Hz stimulus trains. Average EPSP amplitudes are plotted normalized to the amplitude of the first EPSP in the train (e). The depression of EPSPs was significantly more pronounced in G72Tg mice for stimulation frequencies of both 10 and 30 Hz (f). (g, h) Frequency facilitation at MF-CA3 synapses at frequencies of 0.3 and 1 Hz. A pronounced facilitation of MF-EPSPs in both Wt and G72Tg mice (g) did not significantly differ between these groups (h). (i) Potentiation of MF-EPSPs by a high-frequency stimulus train. Tetanization resulted in a pronounced post-tetanic potentiation with a subsequent decline to stable potentiated levels for both Wt and G72Tg mice (empty and filled symbols, respectively) with no differences between groups. All data are expressed as mean±SEM. *p<0.05.
Because ATP production by synaptic mitochondria is important for sustained neurotransmitter release during repetitive synaptic activation (Verstreken et al, 2005), and because synaptic mitochondria are more vulnerable to Ca2+ overload than non-synaptic mitochondria (Brown et al, 2006), we next examined EPSPs induced by different stimulus trains (1–30 Hz). At frequencies >3 Hz, PP-DG synapses displayed a progressive reduction of the EPSP magnitude during the stimulus train (Figure 2d and e). This depression of EPSPs was significantly more pronounced in G72Tg mice for stimulation frequencies of both 10 and 30 Hz (Figure 2f, p=0.016 and 0.032, respectively, n=5 for both groups), indicating that G72Tg mice are less capable of sustained synaptic activity at high frequencies. To test if the same is true for synaptic short-term plasticity observed at low stimulation frequencies, we examined frequency facilitation at the MF-CA3 synapse. These synapses display short-term facilitation to low-frequency stimulation (Figure 2g). We stimulated MFs at frequencies of 0.3 and 1 Hz, resulting in a pronounced facilitation of MF-EPSPs in both Wt and G72Tg1 mice that did not differ significantly between these groups (Figure 2h). Finally, we assessed if potentiation of MF-EPSPs by a high-frequency stimulus train is altered in G72Tg1 mice. We found that tetanization resulted in a pronounced post-tetanic potentiation with a subsequent decline to stable potentiated levels (Figure 2i). The time course of this potentiation was not different in Wt and G72Tg mice with respect to the post-tetanic potentiation or the potentiation 15 min after application of the tetanus (Figure 2i). Our data indicate that synaptic short-term plasticity at low frequencies, or presynaptic plasticity induced by tetanization, are intact in G72Tg mice, but that there is a deficit in sustaining high-frequency transmission over time.
Pharmacological NAC Treatment Improves MWM Performance in G72Tg Mice
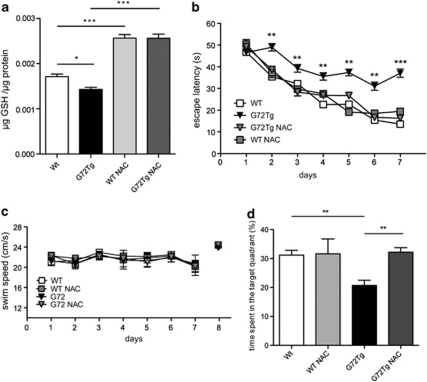
We next investigated if oral administration of NAC would ameliorate the GSH deficiency of G72Tg mice. Indeed, as shown in Figure 3a, a 5-week supplementation of NAC in the drinking water significantly increased GSH to similar levels in both genotypes (F3, 19=43.11, p<0.0001; Figure 3a). The time curve demonstrated that NAC treatment resulted in a significant increase of GSH content over time (time: effect F2, 16=7.63, p<0.01) without any difference between Wt and G72Tg mice (genotype: F1, 8=0.11, p<0.7501) and no genotype × time interaction (F2, 16=0.11, p<0.8975) (Supplementary Figure S5). We then evaluated if hippocampus-dependent memory formation is altered in G72Tg mice and affected by the NAC treatment in the MWM test (Figure 3b). Each group learned to locate the hidden platform, as indicated by decreasing escape latencies during consecutive trials (time: F6, 204=24.96, p<0.001). However, G72Tg mice showed longer escape latencies, indicating a striking deficit in spatial learning ability (genotype: F1, 34=7.071, p<0.05). This deficit was completely rescued by the NAC treatment, whereas NAC treatment did not influence the performance of Wt animals (genotype × treatment interaction: F1, 34=12.57, p<0.01; Figure 3c). Importantly, neither genotype nor treatment influenced the swim speed, suggesting that differences in their motor performance did not influence the results (genotype: F1, 36=0.702, p>0.05; time: F6, 216=1.53, p>0.05; genotype × time: F6, 216=0.559, p>005; Figure 3d).
Figure 3.
N-acetyl cysteine (NAC)-dependent rescue of spatial learning in G72Tg mice. (a) Brain glutathione (GSH) concentrations before and after NAC treatment (n=5). (b) Learning curves showing the average latency to find the platform in the Morris water maze (MWM) (n=8–12). (c) Velocity during training sessions (n=8–12). (d) Probe trial. Percent time spent in the target quadrant during the probe trial conducted on the final day of training (n=8–12). All results are shown as mean±SEM. *p<0.05, **p<0.01, and p***<0.001.
One day after completing the 7-day training period, the hidden platform was removed and the time searching the quadrant that formerly contained the platform was evaluated. G72Tg mice spent less time in the target quadrant during the probe trial compared with Wt mice. Again, this deficit was completely rescued by NAC treatment (F3, 36=56.06, p<0.0001; Figure 3d).
DISCUSSION
In this study, we propose a pathomechanism for G72-induced behavioral deficits: the mitochondrial flavin-binding protein LG72 reduces the activity of complex I of the mitochondrial respiratory chain, thereby increasing the oxidative stress in affected neurons. The increased oxidative stress results in a reduction of aconitase activity and an increase in lipid peroxidation, although antioxidant mechanisms are upregulated as evidenced by the increased expression of GSTs and the depleted pool of reduced GSH. Affected neurons show synaptic dysfunctions, including an impaired short-term plasticity and an inability to sustain higher frequency transmissions (Figure 4). These dysfunctions may contribute to the observed spatial learning deficit, because altered short-term plasticity would be expected to disturb the precise timing of firing in cortical circuits (Silberberg et al, 2004), and thus disrupt the dynamic synchronization of neuronal ensembles during cognitive processes.
Figure 4.
Suggested pathomechanism for G72 in the mitochondria. Mitochondrial LG72 attenuates the activity of the complex I of the respiratory chain, presumably through binding to the flavin-mononucleotide (FMN) group. Impaired complex I functions results in an increased production of superoxide (O2−.), which in turn leads to increased lipid/protein peroxidation and reduced aconitase activity. The detoxifying glutathione S-transferase (GST), which is upregulated in G72Tg mice, converts GSSH and lipid hydroperoxide (LO2H) into GSSG, water, and lipid alcohol (LOH). Augmented GST protein expression diminishes the glutathione pool in the cell.
Initial evidence for a putative function of LG72 was provided by biochemical studies, which demonstrated a binding of carbohydrates including flavin-adenine-dinucleotide and flavin-mononucleotide (FMN) (Chumakov et al, 2002; Pollegioni et al, 2007). Further evidence came from the demonstration that LG72 contains a mitochondrial localization signal at the N terminus and is located in the mitochondria (Kvajo et al, 2008). This is supported by our results showing a mitochondrial localization of LG72 upon expression in mouse neuroblastoma cells. These findings suggest that LG72 binds to flavin-containing mitochondrial proteins and possibly modulates their activity. Direct support for this idea is provided by our results showing that the activity of the FMN-containing oxidoreductase (complex I) of the mitochondrial respiratory chain is adversely affected by LG72 expression in G72Tg mice. It remains to be determined if this effect is due to a direct binding of LG72 to the FMN group, which could stabilize the flavosemiquinone moiety. This flavin-binding property of LG72 would explain the increased ROS generation, because the flavosemiquinone is the direct electron donor for the one-electron reduction of molecular oxygen to superoxide generated at respiratory chain complex I (Kudin et al, 2004; Kushnareva et al, 2002; Liu et al, 2002b).
Several lines of evidence indicate that elevated oxidative stress may also be a G72-related mechanism in schizophrenia. First, G72 expression is increased in brain samples from schizophrenic patients compared to control subjects (Korostishevsky et al, 2004). A number of recent studies have started to address the role of G72 in human brain connectivity and psychiatric disorders. One study found a strong genetic association between G72 and neurocognitive function as well as hippocampal activation (Goldberg et al, 2006). G72 was also identified as a specific genetic factor for the progression of prodromal syndromes to schizophrenia (Mossner et al, 2010). Moreover, it was shown that G72 genetic variation obviously modulates both the progressive brain changes that characterize schizophrenia (Hartz et al, 2010) and the reductions in temporal lobe and amygdala gray matter in people suffering from bipolar disorder (Zuliani et al, 2009). Furthermore, there is mounting evidence implicating mitochondrial defects and the resulting oxidative stress in the pathogenesis of schizophrenia (Altar et al, 2005; Herken et al, 2001; Mahadik and Mukherjee, 1996; Marchbanks et al, 2003; Prabakaran et al, 2004). Schizophrenic patients display a number of biomarkers indicative of increased oxidative stress, such as reduced GSH levels (Do et al, 2000), increased malondialdehyde levels (Zhang et al, 2006, 2007), and altered antioxidant enzyme activities (Gysin et al, 2007; Zhang et al, 2006). We observed an upregulation of the GSTM1 in both G72Tg mouse lines and additional an increased expression of the GSTP1 in G72Tg1 mice. GSTs are important endogenous antioxidant detoxification enzymes, which catalyze conjugation of oxidized products with the thiolate anion of GSH to form non-toxic and excretable products (Hayes et al, 2005; Sharma et al, 2004). Many studies have shown that an augmentation of GSTM1 expression leads to an increased tolerance of cells to toxic metabolites and elimination of ROS (Hayes and Pulford, 1995). The altered GST expression in G72Tg mice might reflect a compensatory mechanism to cope with higher cellular oxidative stress.
Mitochondrial deficits induced by G72 overexpression may contribute to a pathology that is common to several psychiatric diseases. For example, patients with psychiatric disorders that were also associated with G72 polymorphisms, such as depression and BPAD, showed mitochondrial abnormalities at multiple levels (Cataldo et al, 2010; Shao et al, 2008). A recent study revealed a decreased complex I activity with a concomitant oxidative damage of mitochondrial proteins in patients suffering from bipolar disorder (Andreazza et al, 2010). Mitochondrial deficits in psychiatric disorders were first suggested by PET analysis of the brain energy metabolism. Patients with depression exhibited reduced glucose utilization in the prefrontal cortex, anterior cingulate gyrus, and caudate nucleus (Videbech, 2000). In addition, mitochondrial dysfunctions in BPAD were suggested by magnetic resonance spectroscopy studies, which repeatedly showed that mitochondrial synthesis of N-acetyl-aspartate is reduced in the brains of BPAD patients compared to controls (Young, 2007). Furthermore, brain pH levels were decreased, ATP and phosphocreatine levels were reduced, and lactate levels were increased in BPAD patients. These findings are indicative of a decreased energy metabolism (Stork and Renshaw, 2005). Several independent investigators established expression profiles using DNA microarrays from post-mortal brain tissues of patients with BPAD (Iwamoto et al, 2005; Konradi et al, 2004; Sun et al, 2006). They consistently found a decreased expression of transcripts encoding components of the mitochondrial electron transport chain, thus supporting the notion of a reduced mitochondrial function in these patients.
G72 is highly expressed in granular cells of the dentate gyrus of the hippocampus (Otte et al, 2009). Here we show synaptic dysfunctions in the perforant path and MF neurons in the hippocampus of G72Tg mice. These dysfunctions are probably due to an inability to meet the high synaptic energy demand and to the known importance of synaptic mitochondria in maintaining transmitter release during high-frequency stimulation (Verstreken et al, 2005). These synaptic deficits most probably contribute to the spatial learning phenotype of G72Tg mice, because spatial learning relies on intact hippocampal function and oxidative damage to the hippocampus impairs spatial memory (Hopkins et al, 1995; Jackson-Smith et al, 1993; Liu et al, 2002a).
We hypothesized that the spatial learning deficit, if indeed caused by oxidative damage, might be ameliorated by increasing GSH via administration of the GSH precursor NAC. NAC releases cysteine after deacetylation, which in turn increases the formation of GSH within the intracellular pool of antioxidant molecules (Ferrari et al, 1991). It was previously shown that an impairment of hippocampus-dependent spatial memory caused by hypoxia and increased oxidative stress was significantly improved by NAC administration (Jayalakshmi et al, 2007). Moreover, acute NAC treatment restores the short-term spatial learning deficit in transiently GSH-depleted rats (Choy et al, 2010). We showed that an oral chronic supplementation of NAC completely rescued the spatial learning deficit of the G72Tg mice. These findings support the hypothesis that oxidative stress caused the spatial learning deficit of G72 transgenic mice. However, NAC also modulates levels of extrasynaptic glutamate (Odlaug and Grant, 2007) and thus stimulates metabotropic glutamate receptors (mGluRs) (Baker et al, 2003; Chen et al, 2010; Lafleur et al, 2006; LaRowe et al, 2006). Although we have not observed any treatment effect in Wt animals, we cannot exclude the possibility that an NAC-induced elevation of extracellular glutamate contributed to the cognitive improvement in G72Tg animals. In this context, it is also important to note that activation of mGluRs reduces the loss of cellular GSH (Sagara and Schubert, 1998). Interestingly, antioxidants including omega-3 fatty acids (Sivrioglu et al, 2007) and NAC have already demonstrated clinical efficacy in the treatment of schizophrenia symptoms (Berk et al, 2008a; Lavoie et al, 2008), obsessive-compulsive disease (Grant et al, 2009; Odlaug and Grant, 2007), and bipolar disorder (Berk et al, 2008b). Our findings provide a rationale for this treatment effect and suggest a therapeutic option for the therapy of G72-associated psychiatric disorders.
Acknowledgments
This work was supported by grants from the Federal Ministry of Education and Research (NGFN2 01G10474, NGFN-MOODS FKZ 01GS08144, and 01GW0511).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abou Jamra R, Schmael C, Cichon S, Rietschel M, Schumacher J, Nothen MM. The G72/G30 gene locus in psychiatric disorders: a challenge to diagnostic boundaries. Schizophr Bull. 2006;32:599–608. doi: 10.1093/schbul/sbl028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington AM, Gornick M, Sporn AL, Gogtay N, Greenstein D, Lenane M, et al. Polymorphisms in the 13q33.2 gene G72/G30 are associated with childhood-onset schizophrenia and psychosis not otherwise specified. Biol psychiatry. 2004;55:976–980. doi: 10.1016/j.biopsych.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol psychiatry. 2005;58:85–96. doi: 10.1016/j.biopsych.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Gawehn K.1970Methoden der enzymatischen Analyse 22. Aufl. edn.Akademie-Verlag: Berlin; XXXV, 834–1502 S., S. XXXVIII–LXIXpp. [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008a;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008b;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Board PG. The use of glutathione transferase-knockout mice as pharmacological and toxicological models. Expert Opin Drug Metab Toxicol. 2007;3:421–433. doi: 10.1517/17425255.3.3.421. [DOI] [PubMed] [Google Scholar]
- Bottas A, Cooke RG, Richter MA. Comorbidity and pathophysiology of obsessive-compulsive disorder in schizophrenia: is there evidence for a schizo-obsessive subtype of schizophrenia. J Psychiatry Neurosci. 2005;30:187–193. [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, McPhie DL, Lange NT, Punzell S, Elmiligy S, Ye NZ, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. 2010;177:575–585. doi: 10.2353/ajpath.2010.081068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Yin MC, Hsu CC, Liu TC. Antioxidative and anti-inflammatory effects of four cysteine-containing agents in striatum of MPTP-treated mice. Nutrition. 2007;23:589–597. doi: 10.1016/j.nut.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Chen HH, Stoker A, Markou A. The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology. 2010;209:343–350. doi: 10.1007/s00213-010-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy KH, Dean O, Berk M, Bush AI, van den Buuse M. Effects of N-acetyl-cysteine treatment on glutathione depletion and a short-term spatial memory deficit in 2-cyclohexene-1-one-treated rats. Eur J Pharmacol. 2010;649:224–228. doi: 10.1016/j.ejphar.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, et al. Genetic and physiological data implicating the new human gene G72 and the gene for -amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aniello A, D'Onofrio G, Pischetola M, D'Aniello G, Vetere A, Petrucelli L, et al. Biological role of -amino acid oxidase and -aspartate oxidase. Effects of D-amino acids. J Biol Chem. 1993;268:26941–26949. [PubMed] [Google Scholar]
- Detera-Wadleigh SD, McMahon FJ. G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry. 2006;60:106–114. doi: 10.1016/j.biopsych.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Ceconi C, Curello S, Cargnoni A, Alfieri O, Pardini A, et al. Oxygen free radicals and myocardial damage: protective role of thiol-containing agents. Am J Med. 1991;91:95S–105S. doi: 10.1016/0002-9343(91)90291-5. [DOI] [PubMed] [Google Scholar]
- Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe–S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci USA. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, et al. The G72/G30 gene complex and cognitive abnormalities in schizophrenia. Neuropsychopharmacology. 2006;31:2022–2032. doi: 10.1038/sj.npp.1301049. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- Grant SL, Shulman Y, Tibbo P, Hampson DR, Baker GB. Determination of -serine and related neuroactive amino acids in human plasma by high-performance liquid chromatography with fluorimetric detection. J Chromatogr. 2006;844:278–282. doi: 10.1016/j.jchromb.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci USA. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Ho BC, Andreasen NC, Librant A, Rudd D, Epping EA, et al. G72 influences longitudinal change in frontal lobe volume in schizophrenia. Am J Med Genet B. 2010;153B:640–647. doi: 10.1002/ajmg.b.31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Herken H, Uz E, Ozyurt H, Sogut S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiatry. 2001;6:66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain Cogn. 1995;27:180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14:241–253. doi: 10.1093/hmg/ddi022. [DOI] [PubMed] [Google Scholar]
- Jackson-Smith P, Kesner RP, Chiba AA. Continuous recognition of spatial and nonspatial stimuli in hippocampal-lesioned rats. Behav Neural Biol. 1993;59:107–119. doi: 10.1016/0163-1047(93)90821-x. [DOI] [PubMed] [Google Scholar]
- Jansen A, Krach S, Krug A, Markov V, Eggermann T, Zerres K, et al. A putative high risk diplotype of the G72 gene is in healthy individuals associated with better performance in working memory functions and altered brain activity in the medial temporal lobe. Neuroimage. 2009;45:1002–1008. doi: 10.1016/j.neuroimage.2008.12.054. [DOI] [PubMed] [Google Scholar]
- Jayalakshmi K, Singh SB, Kalpana B, Sairam M, Muthuraju S, Ilavazhagan G. N-acetyl cysteine supplementation prevents impairment of spatial working memory functions in rats following exposure to hypobaric hypoxia. Physiol Behav. 2007;92:643–650. doi: 10.1016/j.physbeh.2007.05.051. [DOI] [PubMed] [Google Scholar]
- Joyce E, Hutton S, Mutsatsa S, Gibbins H, Webb E, Paul S, et al. Executive dysfunction in first-episode schizophrenia and relationship to duration of untreated psychosis: the West London Study. Br J Psychiatry Suppl. 2002;43:s38–s44. doi: 10.1192/bjp.181.43.s38. [DOI] [PubMed] [Google Scholar]
- Kamencic H, Lyon A, Paterson PG, Juurlink BH. Monochlorobimane fluorometric method to measure tissue glutathione. Anal Biochem. 2000;286:35–37. doi: 10.1006/abio.2000.4765. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Lleonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22:85–90. doi: 10.14670/HH-22.85. [DOI] [PubMed] [Google Scholar]
- Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- Korostishevsky M, Kaganovich M, Cholostoy A, Ashkenazi M, Ratner Y, Dahary D, et al. Is the G72/G30 locus associated with schizophrenia? Single nucleotide polymorphisms, haplotypes, and gene expression analysis. Biol Psychiatry. 2004;56:169–176. doi: 10.1016/j.biopsych.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Kudina TA, Seyfried J, Vielhaber S, Beck H, Elger CE, et al. Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. Eur J Neurosci. 2002;15:1105–1114. doi: 10.1046/j.1460-9568.2002.01947.x. [DOI] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368 (Part 2:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, Dhilla A, Swor DE, Karayiorgou M, Gogos JA. Evidence implicating the candidate schizophrenia/bipolar disorder susceptibility gene G72 in mitochondrial function. Mol Psychiatry. 2008;13:685–696. doi: 10.1038/sj.mp.4002052. [DOI] [PubMed] [Google Scholar]
- Lafleur DL, Pittenger C, Kelmendi B, Gardner T, Wasylink S, Malison RT, et al. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology. 2006;184:254–256. doi: 10.1007/s00213-005-0246-6. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Karussis D, Misrachi-Koll R, Shezen E, Ovadia H, Abramsky O. Oral administration of the oxidant-scavenger N-acetyl--cysteine inhibits acute experimental autoimmune encephalomyelitis. J Neuroimmunol. 1994;50:35–42. doi: 10.1016/0165-5728(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Li D, He L. G72/G30 genes and schizophrenia: a systematic meta-analysis of association studies. Genetics. 2007;175:917–922. doi: 10.1534/genetics.106.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin CC, Yin MC. Effects of cysteine-containing compounds on biosynthesis of triacylglycerol and cholesterol and anti-oxidative protection in liver from mice consuming a high-fat diet. Br J Nutr. 2008;99:37–43. doi: 10.1017/S0007114507793881. [DOI] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl--carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci USA. 2002a;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002b;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophr Res. 1996;19:1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Marchbanks RM, Ryan M, Day IN, Owen M, McGuffin P, Whatley SA. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res. 2003;65:33–38. doi: 10.1016/s0920-9964(03)00011-2. [DOI] [PubMed] [Google Scholar]
- Mossner R, Schuhmacher A, Wagner M, Quednow BB, Frommann I, Kuhn KU, et al. DAOA/G72 predicts the progression of prodromal syndromes to first episode psychosis. Eur Arch Psychiatry Clin Neurosci. 2010;260:209–215. doi: 10.1007/s00406-009-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JB. Phencyclidine (PCP): a dangerous drug, but useful in schizophrenia research. J Psychol. 2002;136:319–327. doi: 10.1080/00223980209604159. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Shenton ME, Levitt JJ. Olfactory dysfunction in schizophrenia: a review of neuroanatomy and psychophysiological measurements. Harv Rev Psychiatry. 2010;18:279–292. doi: 10.3109/10673229.2010.511060. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Grant JE. N-acetyl cysteine in the treatment of grooming disorders. J Clin Psychopharmacol. 2007;27:227–229. doi: 10.1097/01.jcp.0000264976.86990.00. [DOI] [PubMed] [Google Scholar]
- Otte DM, Bilkei-Gorzo A, Filiou MD, Turck CW, Yilmaz O, Holst MI, et al. Behavioral changes in G72/G30 transgenic mice. Eur Neuropsychopharmacol. 2009;19:339–348. doi: 10.1016/j.euroneuro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Palay SL. Synapses in the central nervous system. J Biophys Biochem Cytol. 1956;2 (Suppl:193–202. doi: 10.1083/jcb.2.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G. Physiological functions of -amino acid oxidases: from yeast to humans. Cell Mol Life Sci. 2007;64:1373–1394. doi: 10.1007/s00018-007-6558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, et al. 2004Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress Mol Psychiatry 9684–697.643. [DOI] [PubMed] [Google Scholar]
- Prata D, Breen G, Osborne S, Munro J, St Clair D, Collier D. Association of DAO and G72(DAOA)/G30 genes with bipolar affective disorder. Am J Med Genet B. 2008;147B:914–917. doi: 10.1002/ajmg.b.30682. [DOI] [PubMed] [Google Scholar]
- Sacchi S, Bernasconi M, Martineau M, Mothet JP, Ruzzene M, Pilone MS, et al. pLG72 modulates intracellular -serine levels through its interaction with -amino acid oxidase: effect on schizophrenia susceptibility. J Biol Chem. 2008;283:22244–22256. doi: 10.1074/jbc.M709153200. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Schubert D. The activation of metabotropic glutamate receptors protects nerve cells from oxidative stress. J Neurosci. 1998;18:6662–6671. doi: 10.1523/JNEUROSCI.18-17-06662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Freudenberg J, Becker T, Ohlraun S, Otte AC, et al. Examination of G72 and -amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol Psychiatry. 2004;9:203–207. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, et al. Mitochondrial involvement in psychiatric disorders. Ann Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Wu C, Markram H. Synaptic dynamics control the timing of neuronal excitation in the activated neocortical microcircuit. J Physiol. 2004;556 (Part 1:19–27. doi: 10.1113/jphysiol.2004.060962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivrioglu EY, Kirli S, Sipahioglu D, Gursoy B, Sarandol E. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1493–1499. doi: 10.1016/j.pnpbp.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Sun X, Wang JF, Tseng M, Young LT. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–196. [PMC free article] [PubMed] [Google Scholar]
- Taylor MA, Berenbaum SA, Jampala VC, Cloninger CR. Are schizophrenia and affective disorder related? Preliminary data from a family study. Am J Psychiatry. 1993;150:278–285. doi: 10.1176/ajp.150.2.278. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Winokur G, Crowe RR. Morbidity risks of schizophrenia and affective disorders among first degree relatives of patients with schizophrenia, mania, depression and surgical conditions. Br J Psychiatry. 1980;137:497–504. doi: 10.1192/bjp.137.6.497. [DOI] [PubMed] [Google Scholar]
- Valles V, Van Os J, Guillamat R, Gutierrez B, Campillo M, Gento P, et al. Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophr Res. 2000;42:83–90. doi: 10.1016/s0920-9964(99)00117-6. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- Wiedemann FR, Vielhaber S, Schroder R, Elger CE, Kunz WS. Evaluation of methods for the determination of mitochondrial respiratory chain enzyme activities in human skeletal muscle samples. Anal Biochem. 2000;279:55–60. doi: 10.1006/abio.1999.4434. [DOI] [PubMed] [Google Scholar]
- Young LT. Is bipolar disorder a mitochondrial disease. J Psychiatry Neurosci. 2007;32:160–161. [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y, et al. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res. 2006;81:291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, Haile CN, et al. Disrupted antioxidant enzyme activity and elevated lipid peroxidation products in schizophrenic patients with tardive dyskinesia. J Clin Psychiatry. 2007;68:754–760. doi: 10.4088/jcp.v68n0513. [DOI] [PubMed] [Google Scholar]
- Zuliani R, Moorhead TW, Job D, McKirdy J, Sussmann JE, Johnstone EC, et al. Genetic variation in the G72 (DAOA) gene affects temporal lobe and amygdala structure in subjects affected by bipolar disorder. Bipolar Disord. 2009;11:621–627. doi: 10.1111/j.1399-5618.2009.00731.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.