Abstract
Nuclear factor-κB (NF-κB) is a ubiquitously expressed transcription factor for genes involved in cell survival, differentiation, inflammation, and growth. This study examined the role of NF-κB pathway in stress-induced PTSD-like behavioral response patterns in rats. Immunohistochemical technique was used to detect the expression of the NF-κB p50 and p65 subunits, I-κBα, p38, and phospho-p38 in the hippocampal subregions at 7 days after exposure to predator scent stress. Expression of p65 nuclear translocation was quantified by western blot as the level of NF-κB activation. The effects of intraperitoneally administered corticosterone or a selective NF-κB inhibitor (pyrrolidine dithiocarbamate (PDTC)) at 1 h post exposure on behavioral tests (elevated plus-maze and acoustic startle response) were evaluated 7 days later. Hippocampal expressions of those genes were subsequently evaluated. All data were analyzed in relation to individual behavior patterns. Extreme behavioral responder animals displayed significant upregulation of p50 and p65 with concomitant downregulation of I-κBα, p38, and phospho-p38 levels in hippocampal structures compared with minimal behavioral responders and controls. Immediate post-exposure treatment with high-dose corticosterone and PDTC significantly reduced prevalence rates of extreme responders and normalized the expression of those genes. Stress-induced upregulation of NF-κB complex in the hippocampus may contribute to the imbalance between what are normally precisely orchestrated and highly coordinated physiological and behavioral processes, thus associating it with stress-related disorders.
Keywords: animal model, post-traumatic stress disorder, nuclear factor-κB (NF-κB), corticosterone, pyrrolidine dithiocarbamate, p38 receptor
INTRODUCTION
The nuclear factor-κB (NF-κB), or Rel, family of eukaryotic transcription factors (TFs) positively regulates a broad range of genes and plays a pivotal role in immune and inflammatory responses, neuronal plasticity, memory formation, and cell death and survival (Chen et al, 1999; Freudenthal et al, 2005; Kaltschmidt et al, 2005; Li et al, 2008; Li and Verma, 2002; Mattson, 2005; Mattson and Meffert, 2006; Meffert and Baltimore, 2005; O'Mahony et al, 2006; Yeh et al, 2002). The NF-κB family consists of five members: p50, p52, p65, c-Rel, and RelB (Meffert and Baltimore, 2005). All family members share a Rel homology domain, which contains the crucial functional regions for DNA binding, dimerization, and nuclear localization, and for interaction with the inhibitory I-κB proteins (Baeuerle and Baltimore, 1988), which inhibit NF-κB activity when it is not required. In the CNS, the most widely NF-κB subunits are p50 and p65 (Denk et al, 2000).
Under basal conditions, heterodimers of NF-κB, typically p50 and p65, are sequestered in the cytosol, where they are constitutively bound by members of the NF-κB inhibitor family of proteins, mainly I-κB (I-κBα and I-κBβ) (Schmitz et al, 2001; Yu et al, 1999). Upon stimulation, I-κB is phosphorylated by the I-κB kinase complex Iκκ, ubiquitinated, and consequently degraded by the 26S proteasome (Ben-Neriah, 2002). The released NF-κB then translocates to the nucleus, binds to its cognate DNA element, and activates the transcription of numerous target genes (Baeuerle and Baltimore, 1996; Clemens et al, 1997). A variety of factors can stimulate the activation of NF-κB, including proinflammatory cytokines such as tumor necrosis factor-α and interleukin-1, physical or oxidative stress, bacterial or viral proteins, nerve growth factor, lipopolysaccharides, and reactive oxygen species (Barger et al, 1995; Kaltschmidt et al, 1995; Maggirwar et al, 1998; Schreck and Baeuerle, 1994; Webster et al, 2002; Yu et al, 1999). Depending on the activating stimulus, NF-κB may be subject to posttranslational modifications that can enhance transcriptional activation of NF-κB-dependent genes (De Bosscher and Haegeman, 2009). Besides the classical NF-κB activation pathway, additional regulatory mechanisms have been identified that fine-tune nuclear NF-κB responses in a gene-specific way (Beck et al, 2008).
The role of NF-κB in the brain is presently unclear; because the activation of NF-κB is associated with cell injury and death in many different pathological settings (eg, ischemia and Parkinson), it has been proposed that the activation of NF-κB contributes to excitotoxic damage and to the cell death process (Clemens et al, 1997; Grilli et al, 1996; Yu et al, 1999). On the other hand, the activation of NF-κB has been reported to promote neuronal survival in various models (Barger et al, 1995; Bhakar et al, 2002; Guo et al, 1998; Mattson et al, 1997; Taglialatela et al, 1997; Yu et al, 1999), and a compete blockade of neuronal NF-κB activity resulted in a loss of neuroprotection in kainite-treated hippocampal slices (Fridmacher et al, 2003).
Evidence from pharmacological and genetic studies of the NF-κB complex in relation to anxiety-related behaviors and stress suggests that the NF-κB complex is indeed involved in emotional behavior and stress response (Koo et al, 2010; Kubera et al, 2011). For instance, Kassed and Herkenham (2004) reported that mice with a null mutation of the p50 subunit exhibited a distinctive behavioral phenotype characterized by decreased anxiety-like responses, elevated exploratory behavior, and reduced tendency to establish dominant–subordinate relationships among their cage mates. Koo et al (2010) reported that depressive-like behaviors caused by exposure to chronic stress are mediated by NF-κB signaling. It was recently reported that acute stress increases the DNA-binding activity of NF-κB in peripheral blood mononuclear cells (Bierhaus et al, 2003; Richlin et al, 2004; Vider et al, 2001). Moreover, male major depression patients with increased early life stress exhibit enhanced NF-κB to psychosocial stress, providing a preliminary indication of a link between major depression, early life stress, and adverse health outcomes in diseases associated with inflammation (Pace et al, 2006).
The NF-κB complex has been reported to be involved in a confounding array of processes, including cell growth and cell death, and it appears to be important in many stress-related processes. This study examined the relationship between the expression of the NF-κB complex response to stress in the hippocampal subareas and patterns of behavioral response. This study design involved the inescapable exposure of rodents to predator-scent stress (PSS) and behavioral testing on the elevated plus-maze (EPM) and acoustic startle response (ASR) tests. Biomolecular and physiological parameters were studied shortly after behavioral testing was completed. The key factor of the method was the approach to data analysis, entitled the cutoff behavioral criteria (CBC) method. In this approach, the behaviors of individual animals within each study group on both the EPM and ASR are used as a tool to classify individuals into groups corresponding to extreme behavioral response (EBR), minimal behavioral response (MBR), and partial behavioral response (PBR). Behavioral classification enables the assessment of physiological and biomolecular parameters in relation to the magnitude or severity of stress-induced behavioral disruption based on prevalence rates of each of the above within each study group, that is, under the various study conditions.
We examined the relationship between stress-induced PTSD-like behavioral response patterns in rats and levels of the NF-κB subunits p50 and p65, I-κBα, p38, and phospho-p38 in the hippocampus subareas. Two distinct NF-κB inhibitors, pyrrolidine dithiocarbamate salt (PDTC) and corticosterone (25 mg/kg) were administrated 1 h post-PSS exposure to block NF-κB activation. PDTC is a highly interesting compound with a dual mechanism of action as an antioxidant and a specific inhibitor of the NF-κB (Nurmi et al, 2006; Soerensen et al, 2009). PDTC reversibly inhibits the nuclear translocation of NF-κB by decreasing the concentration of metal ions and hydroxyl radicals that are required for the release of IκB from NF-κB (Hayakawa et al, 2003; Liu et al, 1999; Schreck et al, 1992; Ziegler-Heitbrock et al, 1993). Glucocorticoids (GCs) are among the most used anti-inflammatory and immunosuppressive drugs. GCs induce IκBα expression, preventing nuclear translocation of NF-κB (Aljada et al, 1999; Munhoz et al, 2006; Quan et al, 2000), and/or interact with the NF-κB p65 subunit, thereby blocking NF-κB-binding activity (Aljada et al, 1999; De Bosscher et al, 2000; Munhoz et al, 2006; Quan et al, 2000; Unlap and Jope, 1997). The associations between behavioral responses and NF-κB subunits and p38/phospho-p38 expression were also evaluated.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats weighing 175–225 g were habituated to housing conditions for at least 7 days, and they were housed four per cage in a vivarium with stable temperature and a reversed 12-h light/dark cycle (lights off at 0800 h) where they had unlimited access to food and water. Animals were handled once daily.
Experimental Design
Three experiments were conducted. In the first, the expression of protein levels for the p50 and p65 submits of the NF-κB complex, I-κBα, p38, and phospho-p38 were evaluated in selected areas of harvested brains from animals classified according to CBC at day 7 post-PSS exposure. Expression of p65 nuclear translocation as the level of NF-κB activation was quantified using western blot. In the second, animals were exposed to PSS, treated with a high dose (25 mg/kg) of corticosterone and assessed behaviorally in the EPM and ASR tests at day 7. Local levels of p50, p65, I-κBα, p38, and phospho-p38 in selected brain areas were then evaluated. The last experiment assessed the effects of a PDTC administration 1 h after PSS on behavioral responses and hippocampal expression of p50, p65, I-κBα, p38, and phospho-p38.
Predator Scent Stress
Test animals were placed on well-soiled cat litter (in use by the cat for 2 days, sifted for stools) in a plastic cage (inescapable exposure) placed on the paving of a yard, for 10 min in a closed environment. Control animals were exposed to fresh, unused litter for the same amount of time.
Behavioral Measurements
Behavioral responses were assessed in the EPM and the ASR paradigm based on previous data (Cohen et al, 2003, 2004, 2006, 2008b).
The CBC Model
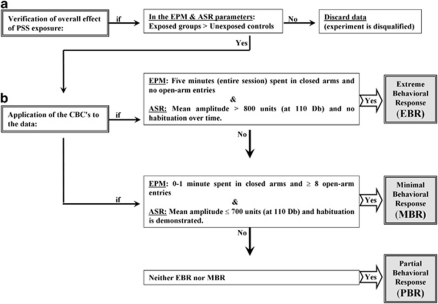
The classification of individuals according to the degree to which their individual behavior was affected by a stressor is based on the premise that extremely compromised behavior in response to the priming trigger is not conducive to survival and is thus inadequate and maladaptive, representing a pathological degree of response (Cohen et al, 2003, 2004, 2005; Cohen and Zohar, 2004). The procedure is detailed in Figure 1.
Figure 1.
The cutoff behavioral criteria (CBC) algorithm. In order to approximate the approach to understanding animal behavioral models more closely to contemporary clinical conceptions of PTSD, we use an approach that enables the classification of study animals into groups according to degree of response to the stressor, that is, the degree to which individual behavior is altered or disrupted. In order to achieve this, behavioral criteria were defined and then complemented by the definition of cutoff criteria reflecting severity of response; this parallels inclusion and exclusion criteria applied in clinical research. The procedure requires the following steps: (a) Verification of global effect: The data must demonstrate that the stressor had a significant effect on the overall behavior of exposed vs unexposed populations at the time of assessment. (b) Application of the CBCs to the data: In order to maximize the resolution and minimize false positives, extreme responses to both elevated plus-maze (EPM) and acoustic startle response (ASR) paradigms, performed in sequence, were required for ‘inclusion' into the EBR group, whereas a negligible degree of response to both was required for inclusion in the MBR group.
Tissue Preparation
At 24 h after the behavioral tests, animals were deeply anesthetized (ketamine and xylazine mixture) and perfused transcardially with cold 0.9% physiological saline followed by 4% paraformaldehyde (Sigma-Aldrich) in 0.1 M phosphate buffer (pH 7.4). Brains were quickly removed, postfixed in the same fixative for 12 h at 4 °C, and were cryoprotected overnight in 30% sucrose in 0.1 M phosphate buffer at 4 °C. Brains were frozen on dry ice and stored at −80 °C. Serial coronal sections (10 μm) at the level of dorsal hippocampus were collected from each animal, using a cryostat (Leica CM 1850) and mounted on coated slides.
Immunohistochemical
Sliced sections were air dried and incubated in frozen methanol (2 min) and in 4% paraformaldehyde (4 min). After three washes in phosphate-buffer saline (PBS) containing Tween 20 (PBS/T) (Sigma-Aldrich), the sections were incubated for 60 min in a blocking solution in (normal goat or horse serum in PBS) and then overnight at 4 °C with the primary antibodies against p50, p65, I-κBα, p38, and phospho-p38 (1 : 250 each; Santa Cruz Biotechnology, Santa Cruz, CA). After three washes in PBS/T, sections were incubated in DyLight-488 labeled goat-anti-rabbit IgG or Dylight-594 goat anti-mouse IgG (1 : 250; KPL, Gaithersburg, MD) in PBS containing 2% normal goat or house serum for 2 h. Sections were washed, mounted with mounting medium (Vectrastain Vector Laboratories, Burlingame, CA). Control staining was performed in the absence of the primary antibodies. Additionally, secondary fluorescent labels were swapped to check crossreactivity and sections were incubated without any primary antibodies to check for any non-specific binding of the secondary antibodies.
Quantification
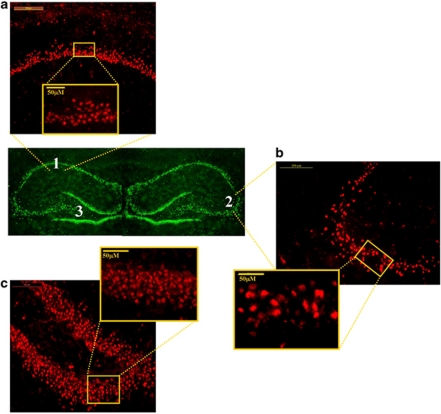
A computer-assisted image analysis system (Leica Application Suite V3.6, Leica, Germany) was used for quantitative analysis of the immunostaining and × 50 objective lens were employed to assess the number of cells positive for p50-IR, p65-IR, I-κBα-IR, p38-IR, and phospho-p38-IR in the hippocampus, divided into three (counted separately) areas: CA1 subfield, CA3 subfield, and DG. The regions of interest were outlined and computer-aided estimation was used to calculate the number of p50-IR, p65-IR, I-κBα-IR, p38-IR, and phospho-p38-IR cells in the pyramidal layer of CA1 and CA3, and in the granular layer of DG (Figure 2). Six representative sections of the hippocampus were chosen (between Bregma −2.30 and Bregma −3.60) from each animal, from each group (Paxinos and Watson, 2005). The sections were analyzed by two observers blinded to the treatment protocol. Standard technique was used to estimate the number of p50-IR, p65-IR, I-κBα-IR, p38-IR, and phospho-p38-IR cell profiles per unit area for each investigated hippocampal structure.
Figure 2.
Representative photomicrographs showing regions of interest for the immunohistochemical staining analysis: (a) hippocampal subregions of ammons horn (cornu ammonis) 1 (CA1), (b) CA3, and (c) DG. Coronary sections, light microscope ( × 10). The locations of the section regions used to analyze are shown by white rectangles, light microscope ( × 50).
Western Blotting
Brains were immediately removed from the decapitated rats. The DG, CA1, and CA3 subregions of the hippocampus from each rat were dissected and frozen at −70 °C for analysis.
Cytosolic and Nuclear Extracts
Brain samples extracted from the hippocampal CA1, CA3, and DG were homogenized in 300 μl lysis buffer by 20 strokes in a Dounce homogenizer (Pastele A) at 4 °C. Homogenates were centrifuged for 10 min at low speed (2500 g at 4 °C). The resulting supernatant and pellet were further processed to generate cytosol and nuclear extract, respectively. For cytosol preparation, supernatant was centrifuged (40 000 g at 4 °C) for 30 min. The final supernatant was used as the cytosolic tissue fraction. For nuclear extract preparation, the pellet from low-speed centrifugation of homogenate was washed twice by resuspension in 0.5 ml of homogenization buffer, followed by additional low-speed centrifugation (5 min, 2000 g at 4 °C). The washed pellet was then resuspended in 100 μl of homogenization buffer and sonicated for 15 s, 4 °C at 50% power capacity (Sonics and Materials, Newtown, CT). After centrifugation (14 000 g, 4 °C, 20 min), the supernatant was removed to a fresh tube and was kept as the nuclear fraction. Protein concentrations for each cytosolic and nuclear sample were determined according to the Bradford method.
Western Blotting
Western blot assays were performed according to a method modified from the procedure of Spencer et al (2000). For electrophoretic analysis, 2 μg of protein from the different samples was boiled for 5 min in loading buffer and incubated for 5 min at 4 °C. Samples were separated on 10% SDS-PAGE at 180 V for 40 min, electroblotted for 2 h (200 mA) on PVDF membrane and blocked with TBS/T containing 5% non-fat dry milk (Spartan, Grand Rapids, MI). For immunodetection, PVDF membranes were incubated overnight at 4 °C with a specific antibody against p65 (1 : 400, Santa Cruz Biotechnology), washed three times with TBS/T, and incubated for 1 h with the secondary antibody (1 : 2000 goat anti-rabbit IgG-HRP, Santa Cruz Biotechnology). The immunoreactive p65 bands were detected with enhanced chemiluminescence ECL Plus kit (Amersham Bioscience, Piscataway, NJ) and exposed to a sensitive film (Kodak). Quantification of the immunoblots was performed by El Logic 100 imaging system (Kodak) and molecular imaging software (Kodak). Exposure times were adjusted so that the darkest bands did not saturate the film. Quantitated values for p65 gene expression were converted into percent values of total protein (cytosolic and nuclear extracts protein levels). To minimize the effect of interblot variability, groups were counterbalanced across gels and a single batch of protein of rat brain in two different concentrations was used for normalization in each gel.
Injections
Rats were injected intraperitoneally (i.p.) with saline (NaCl: 0.9%), 25.0 mg/kg of corticosterone (Sigma), or 150 mg/kg of PDTC (Sigma-Aldrich Israel) at 1 h after stress exposure or sham exposure. Control groups were given 0.9% saline solution. The corticosterone dose was determined according to our previous study (Cohen et al, 2008a) and the PDTC dose according to Soerensen et al (2009).
Statistical Analyses
Molecular data were analyzed using one-way analysis of variance (ANOVA). Behavioral data were analyzed using two-way ANOVA and the post hoc Bonferroni test for multiple comparisons. The prevalence of affected rats as a function of rat group was tested using cross-tabulation and nonparametric χ2 tests.
RESULTS
Control sections incubated without the primary antibody were generated and demonstrated no staining.
NF-κB p50 and p65 Expression at Day 7 Post-PSS Exposure
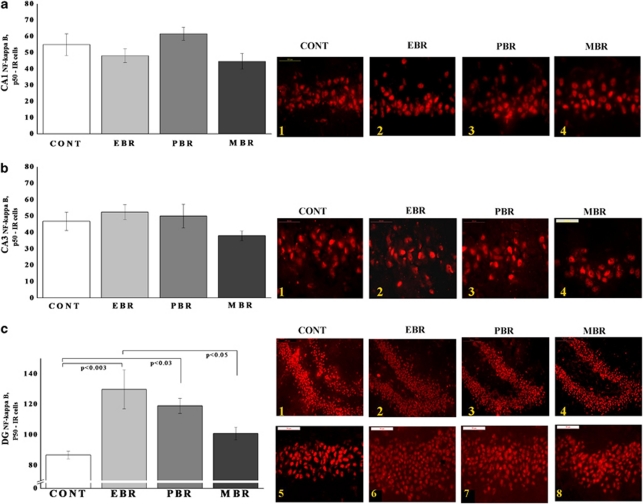
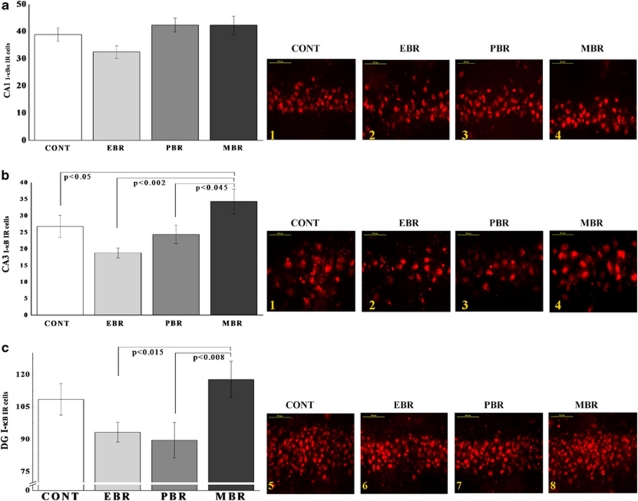
In the hippocampal subregion DG (Figure 3c), there were significant differences in p50-IR cells between groups (F(3, 23)=4.2, p<0.02). Post hoc Bonferroni test revealed that PSS significantly increased the expression of p50 in EBR animals compared with controls (p<0.003) and MBR animals (p<0.05). PSS significantly increased DG p50 expression in PBR animals compared with controls (p<0.03). In the CA1 and CA3 subregions (Figure 3a and b), no significant differences were found between exposed animals and controls.
Figure 3.
Effect of PSS exposure on NF-κB p50 immunoreactivity in the hippocampus subregions. Quantification of NF-κB p50 cells in the hippocampus CA1 (a), CA3 (b), and DG (c) subregions of naive unexposed rats and rats exposed to predator scent stress. Representative photographs of p50 immunoreactivity in the hippocampal CA1, CA3, and DG of animals in the naive control (1) (n=6), EBR (2) (n=9), PBR (3) (n=6), and MBR (4) (n=6) groups. Photographs were acquired at × 50 magnification. Scale bar, 50 μm. The cells in red were p50 positive. A single 10-min exposure to PSS significantly increased NF-κB p50 levels in the DG in EBR and PBR groups compared with unexposed controls. In the CA1 and CA3 subregions, no significant differences were found between exposed animals and controls. Results displayed as mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress. The color reproduction of this figure is available at the Neuropsychopharmacology journal online.
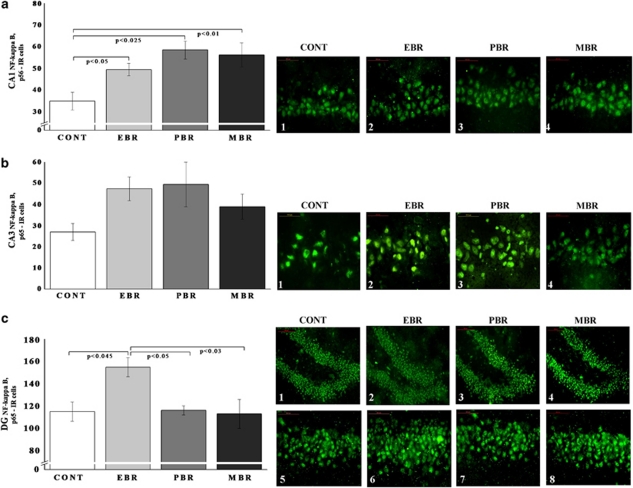
In the CA1 (Figure 4a) and DG (Figure 4c) subregions, there were significant differences in p65-IR cells between groups (F(3, 32)=4.25, p<0.02 and F(3, 32)=8.8, p<0.0006, respectively). Bonferroni test revealed that PSS significantly increased p65 expression in EBR, PBR, and MBR animals compared with controls (p<0.05, p<0.025, and p<0.01, respectively). In the DG subregion, PSS exposure significantly increased p65 expression in EBR rats compared with PBR (p<0.05), MBR (p<0.03), and naive controls (p<0.045). In the CA3 subregion (Figure 4b), no significant differences were found between the groups.
Figure 4.
Effect of PSS exposure on NF-κB p65 immunoreactivity in the hippocampus subregions. Quantification of NF-κB p65 cells in the hippocampus CA1 (a), CA3 (b), and DG (c) subregions of naive unexposed rats and rats exposed to predator scent stress. Representative photographs of p65 immunoreactivity in the hippocampal CA1, CA3, and DG of animals in the naive control (1) (n=6), EBR (2) (n=9), PBR (3) (n=6), and MBR (4) (n=6) groups. Photographs were acquired at × 50 magnification. Scale bar, 50 μm. The cells in green were p65 positive. PSS significantly increased CA1 NF-κB p65 levels in all exposed animals compared with controls and increased DG p65 levels in EBR animals compared with controls, MBR, and PBR animals. In the CA3 hippocampal subregions, no significant differences were found between exposed animals and controls. All data represent group mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress. The color reproduction of this figure is available at the Neuropsychopharmacology journal online.
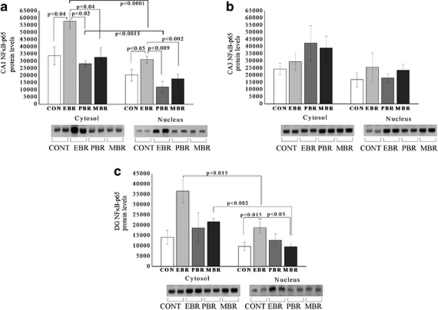
NF-κB p65 Translocation to the Nucleus 7 Days Post-PSS Exposure
In the DG subregion, two-way ANOVA revealed a significant effect for groups (F(3, 28)=8.1, p<0.0005) and a significant effect for cellular fraction (F(1, 28)=17.6, p<0.0003; Figure 5a–c). In the cytosolic fraction, p65 protein levels were not significantly different between groups. In the nuclear fraction, p65 protein levels at 1 week post-stress exposure were higher in the EBR individuals compared with controls (p<0.015) and MBR individuals (p<0.05). Nuclear p65 protein levels were significantly lower in the EBR and MBR individuals compared with their cytosolic fractions (p<0.015 and p<0.002, respectively).
Figure 5.
PSS exposure increased p65 translocation to the nucleus 7 days post-PSS exposure. Western blot analysis of p65 protein levels in densitometry values and representative gels (lower panels) in the cytosolic and nuclear fractions of hippocampal CA1 (a), CA3 (b), and DG subregions (c) of naive control (n=6), EBR (2) (n=6), PBR (3) (n=6), and MBR (4) (n=6) groups. Cytosolic fraction: in the CA1 subregion, p65 protein levels were significantly higher in the EBR animals when compared with controls, PBR, and MBR animals. Nuclear fraction: In the CA1 and DG subregions, p65 protein levels were significantly higher in the EBR animals when compared with controls and MBR animals. Each group contains duplicate lanes. Densitometry values are means of 3–5 gels. All data represent group mean±SEM. CON, control; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response.
I-κBα Expression at Day 7 Post-PSS Exposure
In the CA3 (Figure 6b) and DG (Figure 6c) subregions, there were significant differences between groups (F(3, 32)=3.9, p<0.02 and F(3, 32)=3.4, p<0.03, respectively). Bonferroni test revealed that PSS significantly increased CA3 I-κBα expression in MBR animals compared with control, EBR, and PBR animals (p<0.05, p<0.002, and p<0.045, respectively). In the DG subregion (Figure 6c), PSS exposure significantly increased I-κBα expression in MBR rats compared with EBR and PBR animals (p<0.015 and p<0.008, respectively). In the CA1 subregion, no significant differences were found between the groups.
Figure 6.
Effect of PSS exposure on I-κBα immunoreactivity in the hippocampus subregions. Quantification of I-κBα cells in the hippocampus CA1 (a), CA3 (b), and DG (c) subregions of naive unexposed rats and rats exposed to predator scent stress. Representative photographs of I-κB immunoreactivity in the hippocampal CA1, CA3, and DG of animals in the naive control (1) (n=10), EBR (2) (n=9), PBR (3) (n=10), and MBR (4) (n=9) groups. Photographs were acquired at × 50 magnification. Scale bar, 50 μm. The cells in red were I-κBα positive. A single 10-min exposure to PSS significantly increased CA3 and DG expression of I-κBα levels in MBR animals compared with EBR and PBR animals. All data represent group mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress. The color reproduction of this figure is available at the Neuropsychopharmacology journal online.
P38/phospho-p38 Expression at Day 7 Post-PSS Exposure
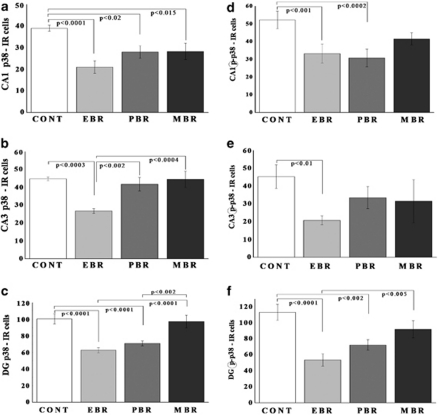
In the CA1 (Figure 7a), CA3 (Figure 7b), and DG (Figure 7c) subregions, there were significant differences in p38-IR cells between groups (F(3, 28)=6.8, p<0.0015; F(3, 28)=7.8, p<0.0007; and F(3, 28)=12.5, p<0.0001, respectively). Post hoc Bonferroni test revealed that PSS significantly decreased CA1 p38 expression in all exposed groups compared with controls (Bonferroni test: p<0.0001, p<0.02, and p<0.015). In CA3, PSS significantly decreased p38 expression in EBR animals compared with controls, PBR, and MBR animals (p<0.0003, p<0.002, and p<0.0004, respectively). In DG, PSS significantly decreased p38 expression in EBR and PBR animals compared with controls (p<0.0001 for both) and MBR animals (p<0.0001 and p<0.0002, respectively).
Figure 7.
Effect of PSS exposure on p38 and phospho-p38 immunoreactivity in the hippocampus subregions. Quantification of p38 and phospho-p38 cells in the hippocampus CA1 (a, d), CA3 (c, e), and DG (e, f) subregions of naive unexposed rats (n=8), EBR (n=8), PBR (n=8), and MBR (n=8) groups. PSS exposure significantly decreased CA1 and DG expression of p38 and phospho-p38 in all the exposed animals when compared with unexposed controls. In the CA3 subregion, PSS decreased expression of p38 and phospho-p38 only in the EBR animals. Results displayed as mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress.
In the CA1 (Figure 7d), CA3 (Figure 7e), and DG (Figure 7f) subregions, there were significant differences in phospho-p38-IR cells between groups (F(3, 28)=9.7, p<0.0002; F(3, 28)=4.0, p<0.02; and F(3, 28)=19.4, p<0.0001, respectively). Bonferroni test revealed that PSS significantly decreased CA1, CA3, and DG phospho-p38 expression in the EBR group compared with controls (p<0.001, p<0.01, and p<0.0001). In DG, PSS also decreased phospho-p38 expression in EBR compared with MBR animals (p<0.005). In CA3, PSS also decreased phospho-p38 expression in PBR animals compared with controls (p<0.0002 and p<0.002, respectively).
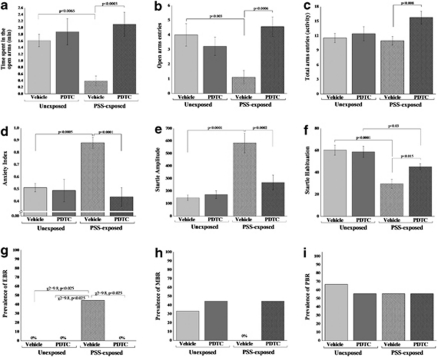
Behavioral Effects of Corticosterone Administration Immediately after PSS Exposure
Elevated plus-maze
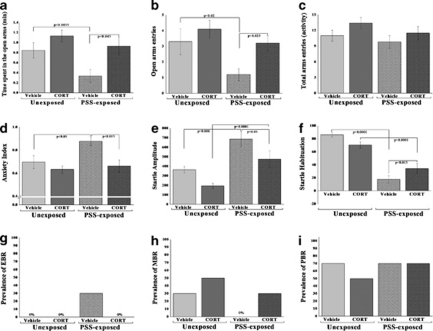
Two-way ANOVA revealed a significant PSS exposure and treatment effects in terms of time spent in open arms (F(1, 36)=4.8, p<0.035 and F(1, 36)=7.7, p<0.009, respectively; Figure 8a). No effects were observed for PSS exposure–treatment interaction. In terms of open arm entries, two-way ANOVA revealed significant effects of PSS exposure and treatment (F(1, 36)=6.4, p<0.016 and F(1, 36)=5.6, p<0.024, respectively; Figure 8b). No effects were observed for PSS exposure–treatment interaction. In terms of anxiety index, two-way ANOVA revealed significant effects of exposure and treatment (F(1, 36)=5.3, p<0.03 and F(1, 36)=9.4, p<0.004, respectively; Figure 8d). No effects were observed for PSS exposure–treatment interaction. Bonferroni test confirmed that exposed group treated with saline exhibited a significant decrease in overall time spent in the open arms and in open arm entries and a significantly increased anxiety index compared with unexposed vehicle-treated rats and exposed rats treated with corticosterone (Bonferroni test: time open: p<0.0035 and p<0.045; open entries: p<0.02 and p<0.025; anxiety index: p<0.05 and p<0.015, respectively). No differences were observed in overall time spent in the open arms of the maze or in open arm entries or in the anxiety index between PSS-exposed animals treated with corticosterone and unexposed vehicle or corticosterone controls. No differences were observed in total exploration on the maze among groups (Figure 8c).
Figure 8.
Effect of early post-stressor intervention with high-dose corticosterone on behavioral stress responses. A single 10-min exposure to PSS followed by immediate administration of high dose (25.0 mg/kg) corticosterone significantly increased the time spent in the open arms (a) and the number of entries to the open arms of the maze (b) and decreased anxiety index (d) when compared with vehicle treatment. Immediate corticosterone administration reduced the response to the stimulus (startle amplitude) (e) and significantly reversed the stress-induced habituation (f) deficit found in vehicle-treated rats, exposure–treatment interaction. Early treatment with high-dose corticosterone reduced the prevalence of PTSD-like behavioral responses (EBR) (g) relative to vehicle control treatment. No differences were observed in total exploration on the maze (c) and in the prevalence of minimal behavioral responders (h) or partial behavioral responses (i). All data represent group mean±SEM.
Startle response
Two-way ANOVA revealed a significant effect for PSS exposure (F(1, 36)=22.2, p<0.0001) and a treatment effect (F(1, 36)=8.9, p<0.005). Bonferroni test confirmed that exposed group treated with vehicle showed a significantly increased mean startle amplitude compared with unexposed controls (p<0.0001) and exposed rats treated with corticosterone (p<0.05; Figure 8e) When treated with corticosterone, members of the exposed group exhibited significant increases in mean startle amplitude compared with unexposed rats treated with corticosterone (p<0.0001).
Startle habituation
Two-way ANOVA revealed a significant effect for PSS exposure (F(1, 36)=104.5, p<0.0001) and an exposure–treatment interaction effect (F(1, 36)=14.2, p<0.0006; Figure 8f). Bonferroni test confirmed that PSS exposure caused a significant deficit in habituation in exposed animals treated with vehicle compared with unexposed groups (p<0.0001) and exposed rats treated with corticosterone (p<0.015) PSS exposure caused a significant deficit in habituation in exposed animals treated with corticosterone compared with unexposed rats treated with corticosterone (p<0.0001).
Relative prevalence rates according to CBC
There were significant differences in the prevalence rates of individuals displaying EBR among groups (Pearson's χ2=9.73, df=3, p<0.025). The prevalence of EBR individuals among PSS-exposed rats injected with vehicle was 30% of the total population, a marked difference from the rates observed in the unexposed and in the PSS-exposed groups treated with corticosterone, both of which lacked EBR individuals (Figure 8g). There were no significant differences in the prevalence of either MBR (Figure 8h) or PBR among groups (Figure 8i).
Effects of Immediate Post-Exposure Corticosterone Administration on NF-κB Expression
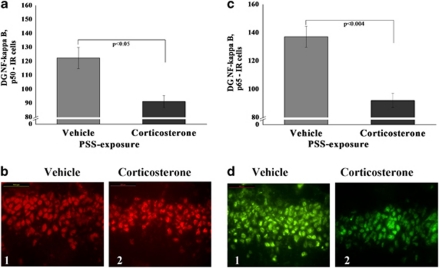
Administration of corticosterone immediately after exposure to stress significantly decreased expression of p50 (F(1, 18)=5.9, p<0.0025; Figure 9a) and p65 (F(1, 18)=12.8, p<0.002; Figure 9b) subunits in the DG areas compared with exposed animals treated with vehicle (Bonferroni test: p<0.05 and p<0.004, respectively) No differences were observed between the exposed groups in either the CA1 or CA3 area.
Figure 9.
Effect of early post-stressor intervention with high-dose corticosterone on NF-κB p50 and p65 immunoreactivity in the hippocampus. (a) The quantitative analysis of p50 subunit immunostaining in the DG area of exposed rats treated with high-dose corticosterone or vehicle. (b) Representative photographs of NF-κB p50 immunoreactivity in the DG of exposed animals treated with high-dose corticosterone (2) or vehicle (1). Photographs were acquired at × 40 magnification. Scale bar, 50 μm. The cells in red were p50 positive and in green were p65 positive. (c) The quantitative analysis of p65 subunit immunostaining in the DG area of exposed rats treated with high-dose corticosterone or vehicle. (d) Representative photographs of NF-κB p65 immunoreactivity in the DG of exposed animals treated with high-dose corticosterone (2) or vehicle (1). Photographs were acquired at × 40 magnification. Scale bar, 50 μm. The cells in white were p50 positive. Administration of high-dose corticosterone immediately post exposure significantly decreased expression of NF-κB p50 and p65 subunits in the DG when compared with exposed animals treated with vehicle. All data represent group mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress. The color reproduction of this figure is available at the Neuropsychopharmacology journal online.
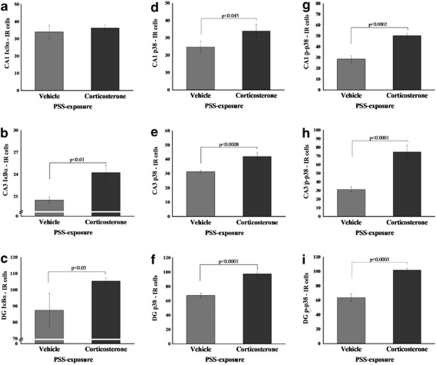
Effects of Corticosterone Administered Immediately after Exposure on I-κBα, p38/phospho-p38 Expression
Administration of corticosterone post exposure significantly increased expression of I-κBα in the CA3 (F(1, 12)=9.8, p<0.01) and DG (F(1, 12)=4.6, p<0.05) compared with exposed animals treated with vehicle (Figure 10a–c). Administration of corticosterone significantly increased expression of p38 and phospho-p38 in the CA1 (F(1, 18)=8.5, p<0.045 and F(1, 18)=22.7, p<0.0002, respectively), CA3 (F(1, 18)=16.4, p<0.0008 and F(1, 18)=35.7, p<0.0001, respectively), and DG (F(1, 18)=37.4, p<0.0001 and F(1, 18)=31.4, p<0.00035, respectively) compared with exposed animals treated with vehicle (Figure 10d–i).
Figure 10.
Effect of early post-stressor intervention with high-dose corticosterone on I-κBα, p38, and phospho-p38 immunoreactivity in the hippocampal subregions. Quantification of I-κBα, p38, and phospho-p38 cells in the hippocampus CA1 (a, d, g), CA3 (b, e, h), and DG (c, f, i) subregions of exposed rats treated with saline (n=10), and exposed rats treated with high-dose corticosterone (n=10). Administration of high-dose corticosterone immediately after exposure significantly increased expression of p38 and phospho-p38 in the hippocampal CA1 area, CA3, and DG when compared with exposed animals treated with vehicle. Administration of high-dose corticosterone significantly increased expression of I-κBα in the CA3 and DG when compared with exposed animals treated with vehicle. Scale bar, 10 μm. All data represent group mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress.
Behavioral Effects of PDTC Administration Immediately after PSS Exposure
Elevated plus-maze
Two-way ANOVA revealed significant treatment and exposure–treatment interaction effects in terms of time spent in open arms (F(1, 32)=11.3, p<0.025 and F(1, 32)=6.1, p<0.02, respectively; Figure 11a). No effects were observed for PSS exposure. In terms of open arm entries, two-way ANOVA revealed significant effects of PSS exposure and treatment (F(1, 32)=4.4, p<0.056 and F(1, 32)=11.0, p<0.002, respectively; Figure 11b). No effects were observed for PSS exposure–treatment interaction. In terms of total activity on the maze, two-way ANOVA revealed significant effects of treatment (F(1, 32)=5.8, p<0.025; Figure 11c). No effects were observed for exposure and exposure–treatment interaction. In terms of anxiety index, two-way ANOVA revealed significant effects of exposure, treatment, and exposure–treatment interaction (F(1, 32)=5.6, p<0.025; F(1, 32)=12.4, p<0.001; and F(1, 32)=10.0, p<0.003, respectively; Figure 11d). Bonferroni test confirmed that exposed group treated with saline exhibited a significant decrease in overall time spent in the open arms and in open arm entries and a significantly increased anxiety index compared with unexposed vehicle-treated rats and exposed rats treated with PDTC (Bonferroni test: time open: p<0.0065 and p<0.0033; open entries: p<0.003 and p<0.0006; anxiety index: p<0.0005 and p<0.0001, respectively). No differences were observed in overall time spent in the open arms of the maze or in open arm entries or in the anxiety index between PSS-exposed animals treated with PDTC and unexposed vehicle or PDTC controls.
Figure 11.
Effect of early post-stressor intervention with PDTC on behavioral stress responses. A single exposure to PSS followed by immediate administration of PDTC significantly increased the time spent in the open arms (a), the number of entries to the open arms (b), and decreased anxiety index (d) when compared with vehicle treatment. PDTC administration reduced the response to the stimulus (startle amplitude) (e) and reversed the stress-induced habituation (f) deficit found in vehicle-treated rats, exposure–treatment interaction. Early treatment with TDTC reduced the prevalence of PTSD-like behavioral responses (EBR) (g) relative to vehicle control treatment. No differences were observed in the prevalence of minimal behavioral responders (h) or partial behavioral responses (i). All data represent group mean±SEM.
Startle response
Two-way ANOVA revealed a significant effect for PSS exposure (F(1, 32)=25.5, p<0.0001), a treatment effect (F(1, 32)=7.5, p<0.0001), and an exposure–treatment interaction effect (F(1, 32)=10.3, p<0.003). Bonferroni test confirmed that exposed group treated with vehicle showed a significantly increased mean startle amplitude compared with unexposed controls (p<0.0001; Figure 11e). Exposed animals treated with PDTC exhibited significantly decreased mean startle amplitude compared with exposed animals treated with vehicle (p<0.0002).
Startle habituation
Two-way ANOVA revealed a significant effect for PSS exposure (F(1, 32)=27.7, p<0.0001) and an exposure–treatment interaction effect (F(1, 32)=4.2, p<0.05; Figure 11f). Bonferroni test confirmed that exposed animals treated with PDTC exhibited significantly increased startle habituation compared with exposed animals treated with vehicle (p<0.015).
Relative prevalence rates according to CBC
There were significant differences in the prevalence rates of individuals displaying EBR among groups (Pearson's χ2=9.8, df=3, p<0.025). The prevalence of EBR individuals among PSS-exposed rats injected with vehicle was 44.4% of the total population, a marked difference from the rates observed in the unexposed and in the PSS-exposed groups treated with PDTC, both of which lacked EBR individuals (Figure 11g). There were no significant differences in the prevalence of either MBR (Figure 11h) or PBR among groups (Figure 11i).
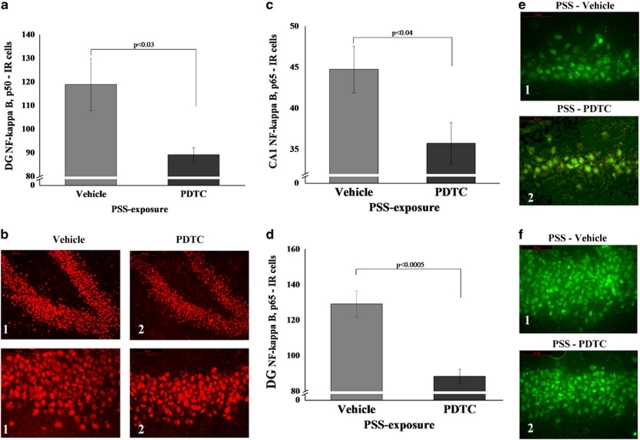
Effects of Immediate Post-Exposure PDTC Administration on NF-κB Expression
Administration of PDTC immediately after PSS exposure significantly decreased expression of p50 in the DG subregion (F(1, 18)=5.7, p<0.03; Figure 12b) and significantly decreased expression of p65 in the CA1 (F(1, 18)=18.0, p<0.0005; Figure 12c) and DG (F(1, 18)=4.9, p<0.04; Figure 12e) subregions compared with exposed animals treated with vehicle. No differences were observed between the exposed groups in the CA3 subregion.
Figure 12.
Effect of early post-stressor intervention with PDTC on NF-κB p50 and p65 immunoreactivity in the hippocampus. (a) The quantitative analysis of p50 subunit immunostaining in the DG area of exposed rats treated with PDTC or vehicle. (b) Representative photographs of NF-κB p50 immunoreactivity in the DG of exposed animals treated with PDTC (2) or vehicle (1). Photographs were acquired at × 20 (Scale bar, 100 μm) and × 40 magnification (Scale bar, 50 μm). The cells in red were p50 positive. (c) The quantitative analysis of p65 subunit immunostaining in the CA1 (c) and DG (d) areas of exposed rats treated with PDTC or vehicle. (e) Representative photographs of NF-κB p65 immunoreactivity in the CA1 (e) and DG (f) of exposed animals treated with PDTC (2) or vehicle (1). Photographs were acquired at × 40 magnification. Scale bar, 50 μm. The cells in green were p65 positive. Administration of high-dose corticosterone immediately post exposure significantly decreased expression of NF-κB p50 and p65 subunits in the DG when compared with exposed animals treated with vehicle. All data represent group mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress. The color reproduction of this figure is available at the Neuropsychopharmacology journal online.
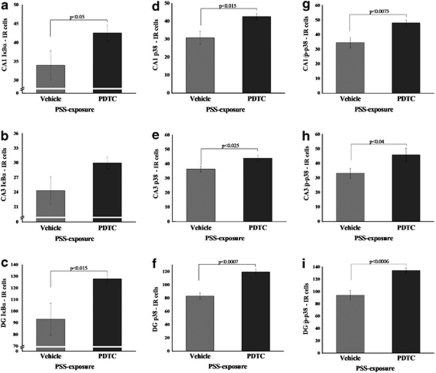
Effects of PDTC Administered Immediately after Exposure on I-κBα, p38/phospho-p38 Expression
Administration of PDTC significantly increased expression of I-κBα in the CA1 (F(1, 13)=4.7, p<0.05) and DG (F(1, 13)=8.5, p<0.015) compared with exposed animals treated with vehicle (Figure 10a–c). Administration of PDTC significantly increased expression of p38 and phospho-p38 in the CA1 (F(1, 16)=7.6, p<0.015 and F(1, 16)=9.5, p<0.0075, respectively), CA3 (F(1, 16)=6.7, p<0.025 and F(1, 16)=5.2, p<0.04, respectively), and DG (F(1, 16)=29.5, p<0.0007 and F(1, 16)=18.7, p<0.0006, respectively) compared with exposed animals treated with vehicle (Figure 13d–i).
Figure 13.
Effect of early post-stressor intervention with PDTC on I-κBα, p38, and phospho-p38 immunoreactivity in the hippocampal subregions. Quantification of I-κBα, p38, and phospho-p38 cells in the hippocampus CA1 (a, d, g), CA3 (b, e, h), and DG (c, f, i) subregions of exposed rats treated with saline (n=10) and exposed rats treated with PDTC (n=10). Administration of PDTC immediately after exposure significantly increased expression of I-κBα, p38, and phospho-p38 in the hippocampal CA1 area, CA3, and DG when compared with exposed animals treated with vehicle. All data represent group mean±SEM. DG, dentate gyrus; CA1, cornu ammonis 1; EBR, extreme behavioral response; MBR, minimal behavioral response; PBR, partial behavioral response; PSS, predator scent stress.
DISCUSSION
We looked at critical signaling molecules (NF-κB, I-κBα, and p38/phospho-p38) to obtain an integrated picture of neuro-immune-endocrine modulation in stress-related responses. The development of an extreme (PTSD-like) long-term behavioral response pattern at 1 week after exposure to stress was found to be associated with a distinct pattern of long-term excessive expression of the NF-κB complex with concomitant downregulation in the expression of I-κBα, and p38 and phospho-p38 mitogen-activated protein kinase (MAPK) molecular signaling in hippocampal structures. Pharmacological manipulation of NF-κB complex by administration of a single high dose of corticosterone or a selective NF-κB inhibitor immediately after stress exposure significantly reduced behavioral disruption, and concomitantly normalized the molecular pathway response, that is, downregulated NF-κB subunits and upregulated I-κBα and p38/phospho-p38 MAPK molecular signaling. Therefore, NF-κB complex is actively involved in the neurobiological response to PSS.
The initial stage of the study examining local brain levels of p50 and p65 subunits of NF-κB in stress-exposed animals revealed that at 7 days after exposure, p50 and p65 levels in the DG subregion were upregulated in animals whose behavior was severely affected by the stressor (EBR), whereas MBR animals and controls displayed no change in either p50 or p65 expression. This NF-κB upregulation was accompanied by translocation of the p65 subunit into the nucleus. Thus, in the EBR animals NF-κB is activated within the neurons in the hippocampus subregions. The implications of these finding were unclear. Depending on context and sequential factors, the NF-κB complex has been associated with both neurotoxic and neuroprotective properties. Hence, the significance of the localized findings characterizing extreme responders require further elucidation. They could be a marker (or a component) of required changes in neural plasticity and synaptic function in response to stresses or a marker of pathological processes.
Previously, we reported that at 7 days after PSS exposure, EBR animals displayed significantly higher circulating corticosterone levels and a significant elevation in glucocorticoid receptor (GR) translocation to the nucleus in the hippocampal CA3 and DG regions compared with MBR individuals and controls (Kozlovsky et al, 2009). GR is a hormone-dependent transcription factor superfamily critically involved in mediating the immunosuppressive function of GCs by repressing the expression of major cytokine genes and their pleiotropic actions on target cells (Almawi and Melemedjian, 2002; Auphan et al, 1995; Majdalawieh and Ro, 2010; Scheinman et al, 1995). This immunosuppressive and anti-inflammatory action involves negative cross-talk between GR and other transcription factors such as NF-κB. In other words, the relationship between NF-κB and GR is mutually antagonistic in that NF-κB can suppress the function of GR that, in turn, effectively inhibits NF-κB activation (McKay and Cidlowski, 1999; Scheinman et al, 1995; Szatmary et al, 2004). The goal of this regulatory interplay is to maintain homeostasis by avoiding excessive destruction and inflammation (Liberman et al, 2009). Hence, we expected excessive GCs to inhibit NF-κB signaling in the hippocampus in the EBR animals. Thus, we did not expect the findings, rejecting our hypothesis, of the significantly increased levels of DG NF-κB subunits we observed in the EBR individuals but not in control or MBR rats.
A proposed molecular mechanism of NF-κB/GR antagonism is via stimulation of I-κB synthesis (Auphan et al, 1995; Scheinman et al, 1995). In our investigation of the expression of I-κBα in the hippocampal subregions at 7 days post exposure, we showed that the MBR animals displayed significantly higher levels of I-κBα in the CA3 and DG subregions compared with EBR and PBR animals. Increased I-κB availability would result in its binding to, and sequestration of, NF-κB in the cytosol, which reduces NF-κB nuclear translocation and attenuates NF-κB-driven transcriptional activities (Almawi and Melemedjian, 2002). In the DG, MBR animals displayed significantly higher levels of I-κBα than controls. Our findings suggest that I-κBα downregulation in EBR animals may prevent repression of the NF-κB complex and thus diminish the inhibition of NF-κB transcriptional activities compared with MBR animals.
In addition to the classical I-κB kinase/I-κBα pathway, the transcriptional activation of NF-κB is also regulated by events that directly affect its activation (King et al, 2009). One family of kinases essential for signal transfer from the cell surface to the nucleus is MAPK. It has been shown that p38 and NF-κB are activated in response to similar stimuli and that p38 is required for NF-κB-dependent gene expression (Carter et al, 1999; Vanden Berghe et al, 1998). Karunakaran and Ravindranath (2009) demonstrated that phosphorylation of p38 is an important event upstream of NF-κB activation. p38 is known to phosphorylate I-κBα leading to its dissociation from the p65 subunit, thus facilitating the translocation of p65 to the nucleus (Calleros et al, 2006). Phosphorylated I-κBα was rapidly degraded by the proteasome (Finco and Baldwin, 1995). Moreover, it is now appreciated that at the cellular level the transcription factors NF-κB and GR have divergent effects (stimulatory vs inhibitory, respectively) in gene expression. It has also been found that activation of p38 inhibits the transcriptional activation of GR (Szatmary et al, 2004). In our study, the EBR animals displayed significantly lower p38 and Phospho-p38 expression in the CA1, CA3, and DG hippocampal subregions compared with controls. EBR animals also displayed significantly lower p38 and phospho-p38 expression in the DG compared with MBR animals. As p38/phospho-p38 are known to phosphorylate GR and inhibit its transcriptional activity (Szatmary et al, 2004), lower hippocampal levels of phospho-p38 should intensify GC action.
The finding that p38/phospho-p38 and I-κBα were upregulated in the DG subregion of minimally affected MBR rats, but not in PTSD-like EBR rats, suggests that the stress-induced upregulation of p38/phospho-p38 and I-κBα may be associated with adequate recovery processes and/or resilience, whereas a pattern of long-term excessive activation of the NF-κB complex and GR pathways in the hippocampus appear to be associated with stress-related behavioral responses.
Previous reports support our finding. Madrigal et al (2001) reported that stress exposure increased NF-κB activation and subsequent NF-κB-dependent gene expression in the cortex of rats exposed to immobilization. They reported that the release of excitatory amino acidergic components induced by stress triggers the process of iNOS induction by stimulating the translocation of NF-κB to the nucleus in brain cortex of rats exposed to acute restraint stress (Madrigal et al, 2001). Using an animal model of repeated trauma, Harvey et al (2005) investigated the effect of stress on the hippocampal NO-cGMP signaling pathway and its modulation using drugs selective for targets within the NMDA receptor cascade and NF-κB complex. Day 7 post stress revealed significantly increased hippocampal NO, which was blocked by NF-κB antagonist (Harvey et al, 2005). Munhoz et al (2006) tested whether chronic, unpredictable stress modulated the effects of lipopolysaccharides on NF-κB activity. They reported that CUS augmented the effect of LPS on NF-κB activation in the frontal cortex and hippocampus. Moreover, CUS augmented the effect of LPS on expression of the proinflammatory genes IL-β, TNF-α, and iNOS. Thus, stress or GCs can increase levels of proinflammatory cytokine in both the periphery and the brain. Increased NF-κB activation has also been described in the lymphocytes of women stressed by the experience of undergoing a breast biopsy (Bierhaus et al, 2003), a crucial step in diagnosing a life-threatening disorder but also a situation characterized by anxiety and desperation comparable with other forms of psychosocial stress.
In order to assess whether the above NF-κB patterns represented a marker of stress or a causal factor (indicating the direct involvement of NF-κB in the stress response), NF-κB levels were pharmacologically manipulated using two NF-κB inhibitors: a single high-dose corticosterone or a specific NF-κB inhibitor, PDTC, immediately following stress exposure. A single 25 mg/kg dose of corticosterone administered immediately after PSS resulted, relative to untreated and placebo-treated controls, in reduced prevalence of EBR individuals—that is,, a significant shift toward less extreme, stress-induced behavioral disruption—at 7 days with a concomitant increase in the prevalence of MBR individuals, compared with saline controls. The anxiolytic-like effect of high-dose corticosterone injected immediately after PSS exposure was accompanied by significant downregulation of the DG p50 and p65 subunits of the NF-κB complex with concomitant upregulation of region-specific I-κBα levels and their signaling pathways p38 and phospho-p38 compared with those given saline treatment.
One may speculate that high-dose corticosterone given immediately after PSS exposure activated the GC pathways. Activated GR is able to activate the p38/phospho-p38 MAPK signaling that may prevent the maladaptive influence of GCs on the brain via attenuation of GR function. Adequate GR signaling rescues the disrupted NF-κB complex via direct pathways (ie, elevated I-κBα expression) or indirect molecular pathways (ie, elevated p38/phospo-p38 MAPK signaling).
Our results demonstrated that PDTC given immediately after PSS exposure activated p38/phospho-p38 MAPK pathway, upregulated region-specific I-κBα levels, and downregulated NF-κB subunits when compared with vehicle treatment. These results confirm the bulk of evidence indicating that PDTC prevented degradation of IκB-α and NF-κB nucleus translocation and thus inhibited NF-κB activation (Cuzzocrea et al, 2002; Hayakawa et al, 2003; Liu et al, 1999; Schreck et al, 1992; Ziegler-Heitbrock et al, 1993). Pfeilschifter et al (2010) reported that PDTC potently reduces lesion volume in experimental animal model of stroke. The authors suggest that this effect may be mediated in part to a p38 MAPK pathway in brain endothelial cells (Pfeilschifter et al, 2010).
The use of the two NF-κB inhibitors elicited interesting results. The fact that PDTC not only corrected the NF-κB/p38 pathway but also the behavioral response pattern, combined with the fact that high-dose corticosterone not only predictably corrected behavior but also had a similar corrective effect to PDTC on the NF-κB/p38 pathway, is pivotal. The implication of these findings is that NF-κB/p38 pathway is integrated with steroid receptor signaling (Liberman et al, 2009) and its correlated molecular signaling (kinases and phosphatases) in the context of stress-related disorders. The final outcome of an adaptive stress response will depend on the satisfactory cross-talk between the NF-κB complex and steroid receptor signaling, whereas abnormal cross-talk or nonregulated NF-κB activation may cause several pathological states.
Taken together, our data led us to the speculation that stress-induced upregulation of NF-κB levels in the hippocampus may contribute to the imbalance between normally precisely orchestrated physiological and behavioral processes and are thus associated with stress-related disorders. Further studies will be required to examine whether pharmacological inhibition of NF-κB complex represents an avenue for secondary prevention or treatment of stress-related clinical disorders.
Acknowledgments
This study was supported by a grant from the National Institute for Psychobiology in Israel, funded by Charles E Smith Family, the Israel Academy of Science and Humanities (Grant no. 429/03), and by the Ministry of Health (Grant no. 6086) given to HC.
The authors declare no conflict of interest.
References
- Aljada A, Ghanim H, Assian E, Mohanty P, Hamouda W, Garg R, et al. Increased IkappaB expression and diminished nuclear NF-kappaB in human mononuclear cells following hydrocortisone injection. J Clin Endocrinol Metab. 1999;84:3386–3389. doi: 10.1210/jcem.84.9.6104. [DOI] [PubMed] [Google Scholar]
- Almawi W, Melemedjian O. Negative regulation of nuclear factor-kappaB activation and function by glucocorticoids. J Mol Endocrinol. 2002;28:69–78. doi: 10.1677/jme.0.0280069. [DOI] [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Baeuerle P, Baltimore D. NF-B: ten years after. Cell Death Differ. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Barger S, Horster D, Furukawa K, Goodman Y, Krieglestein J, Mattson M. Tumor necrosis factors alpha and beta protect neurons against amyloid-peptide toxicity: evidence for involvement of a B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck IM, Vanden Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, et al. Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-kappaB inhibition. EMBO J. 2008;27:1682–1693. doi: 10.1038/emboj.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, et al. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22:8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;1004:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleros L, Lasa M, Toro MJ, Chiloeches A. Low cell cholesterol levels increase NFkappaB activity through a p38 MAPK-dependent mechanism. Cell Signal. 2006;18:2292–2301. doi: 10.1016/j.cellsig.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gardi J, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappa B-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276:R1812–R1818. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- Clemens J, Stephenson D, Smalstig E, Dixon E, Little S. Global ischemia activates nuclear factor-B in forebrain neurons of rats. Stroke. 1997;28:1073–1080. doi: 10.1161/01.str.28.5.1073. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar M, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high dose corticosterone attenuates post traumatic stress response in an animal model of PTSD. Biol Psychiatry. 2008a;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high dose corticosterone attenuates post traumatic stress response in an animal model of PTSD. Biol Psychiatry. 2008b;15:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J. Animal models of post traumatic stress disorder: the use of cut off behavioral criteria. Ann NY Acad Sci. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of post-traumatic stress disorder. Biol Psychiatry. 2003;15:463–473. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB. Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2005;58:640–650. doi: 10.1016/j.biopsych.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, et al. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23:281–291. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: negative interference of activated glucocorticoid receptor with transcription factors. J Neuroimmunol. 2000;109:16–22. doi: 10.1016/s0165-5728(00)00297-6. [DOI] [PubMed] [Google Scholar]
- Denk A, Wirth T, Baumann B. NF-kappaB transcription factors: critical regulators of hematopoiesis and neuronal survival. Cytokine Growth Factor Rev. 2000;11:303–320. doi: 10.1016/s1359-6101(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Finco TS, Baldwin AS. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Freudenthal R, Boccia MM, Acosta GB, Blake MG, Merlo E, Baratti CM, et al. NF-kappa B transcription factor is required for inhibitory avoidance long-term memory in mice. Eur J Neurosci. 2005;21:2845–2852. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- Fridmacher V, Kaltschmidt B, Goudeau B, Ndiaye D, Rossi FM, Pfeiffer J, et al. Forebrain-specific neuronal inhibition of nuclear factor-kappaB activity leads to loss of neuroprotection. J Neurosci. 2003;23:9403–9408. doi: 10.1523/JNEUROSCI.23-28-09403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-B activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- Guo Q, Robinson N, Mattson MP. Secreted beta-amyloid precursor protein counteracts the proapoptotic action of mutant presenilin-1 by activation of NF-kappaB and stabilization of calcium homeostasis. J Biol Chem. 1998;273:12341–12351. doi: 10.1074/jbc.273.20.12341. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Bothma T, Nel A, Wegener G, Stein DJ. Involvement of the NMDA receptor, NO-cyclic GMP and nuclear factor K-beta in an animal model of repeated trauma. Hum Psychopharmacol. 2005;20:367–373. doi: 10.1002/hup.695. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, et al. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle P. Stimulation of ionotropic glutamate receptors activates transcription factor NF-B in primary neurons. Proc Natl Acad Sci USA. 1995;92:9618–9622. doi: 10.1073/pnas.92.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Ravindranath V. Activation of p38 MAPK in the substantia nigra leads to nuclear translocation of NF-kappaB in MPTP-treated mice: implication in Parkinson's disease. J Neurochem. 2009;109:1791–1799. doi: 10.1111/j.1471-4159.2009.06112.x. [DOI] [PubMed] [Google Scholar]
- Kassed CA, Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav Brain Res. 2004;154:577–584. doi: 10.1016/j.bbr.2004.03.026. [DOI] [PubMed] [Google Scholar]
- King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-kappaB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J Biol Chem. 2009;284:26803–26815. doi: 10.1074/jbc.M109.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. A distinct pattern of intracellular glucocorticoid-related responses is associated with extreme behavioral response to stress in an animal model of post-traumatic stress disorder. Eur Neuropsychopharmacol. 2009;19:759–771. doi: 10.1016/j.euroneuro.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Li H, Li X, Jia N, Cai Q, Bai Z, Chen R, et al. NF-kappaB regulates prenatal stress-induced cognitive impairment in offspring rats. Behav Neurosci. 2008;122:331–339. doi: 10.1037/0735-7044.122.2.331. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Liberman AC, Druker J, Garcia FA, Holsboer F, Arzt E. Intracellular molecular signaling. Basis for specificity to glucocorticoid anti-inflammatory actions. Ann NY Acad Sci. 2009;1153:6–13. doi: 10.1111/j.1749-6632.2008.03958.x. [DOI] [PubMed] [Google Scholar]
- Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- Madrigal J, Moro M, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem. 2001;76:532–538. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- Maggirwar S, Sarmiere P, Dewhurst S, Freeman R. Nerve growth factor-dependent activation of NF-B contributes to survival of sympathetic neurons. J Neurosci. 1998;18:10356–10365. doi: 10.1523/JNEUROSCI.18-24-10356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalawieh A, Ro HS. Regulation of IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages. Mediators Inflamm. 2010;2010:823821. doi: 10.1155/2010/823821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-B protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of Mn-SOD and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP. NF-kappa B in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappa B in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- McKay L, Cidlowski J. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kB and steroid receptor-signaling pathways. Endocrine Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmi A, Goldsteins G, Narvainen J, Pihlaja R, Ahtoniemi T, Grohn O, et al. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40:1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- O'Mahony A, Raber J, Montano M, Foehr E, Han V, Lu SM, et al. NF-kappa B/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol. 2006;26:7283–7298. doi: 10.1128/MCB.00510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2005The Rat Brain in Stereotaxic Coordinates5th edn.Elsevier Academic Press: Burlington, MA [Google Scholar]
- Pfeilschifter W, Czech B, Hoffmann BP, Sujak M, Kahles T, Steinmetz H, et al. Pyrrolidine dithiocarbamate activates p38 MAPK and protects brain endothelial cells from apoptosis: a mechanism for the protective effect in stroke. Neurochem Res. 2010;35:1391–1401. doi: 10.1007/s11064-010-0197-0. [DOI] [PubMed] [Google Scholar]
- Quan N, He L, Lai W, Shen T, Herkenham M. Induction of IB mRNA expression in the brain by glucocorticoids: a negative feedback mechanism for immune-to-brain signaling. J Neurosci. 2000;20:6473–6477. doi: 10.1523/JNEUROSCI.20-17-06473.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlin V, Arevalo J, Zack J, Cole S. Stress-induced enhancement of NF-B DNA-binding in the peripheral blood leukocyte pool: effects of lymphocyte redistribution. Brain Behav Immun. 2004;18:231–237. doi: 10.1016/j.bbi.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Scheinman RI, Cogswell PC, Lofquist AK, Baldwin S. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Bacher S, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186–190. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- Schreck R, Baeuerle P. Assessing oxygen radicals as mediators in activation of inducible eukaryotic transcription factor NF-kappa B. Methods Enzymol. 1994;234:151–163. doi: 10.1016/0076-6879(94)34085-4. [DOI] [PubMed] [Google Scholar]
- Schreck R, Meier B, Männel DN, Dröge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen J, Pekcec A, Fuest C, Nickel A, Potschka H. Pyrrolidine dithiocarbamate protects the piriform cortex in the pilocarpine status epilepticus model. Epilepsy Res. 2009;87:177–183. doi: 10.1016/j.eplepsyres.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Kalman BA, Cotter CS, Deak T. Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using western blot analysis. Brain Res. 2000;868:275–286. doi: 10.1016/s0006-8993(00)02341-6. [DOI] [PubMed] [Google Scholar]
- Szatmary Z, Garabedian MJ, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem. 2004;279:43708–43715. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- Taglialatela G, Robinson R, Perez-Polo J. Inhibition of nuclear factor-B (NF-B) activity induces nerve growth factor-resistant apoptosis in PC12 cells. J Neurosci Res. 1997;47:155–162. [PubMed] [Google Scholar]
- Unlap M, Jope R. Dexamethasone attenuates NF-kappa B DNA binding activity without inducing I kappa B levels in rat brain in vivo. Brain Res Mol Brain Res. 1997;45:83–89. doi: 10.1016/s0169-328x(96)00240-9. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, et al. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- Vider J, Laaksonen DE, Kilk A, Atalay M, Lehtmaa J, Zilmer M, et al. Physical exercise induces activation of NF-kappaB in human peripheral blood lymphocytes. Antioxid Redox Signal. 2001;36:1131–1137. doi: 10.1089/152308601317203639. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Lee CF, Gean PW. A requirement of nuclear factor-kappa B activation in fear-potentiated startle. J Biol Chem. 2002;277:46720–46729. doi: 10.1074/jbc.M206258200. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhou D, Bruce-Keller AJ, Kindy MS, Mattson MP. Lack of the p50 subunit of nuclear factor-kappaB increases the vulnerability of hippocampal neurons to excitotoxic injury. J Neurosci. 1999;19:8856–8865. doi: 10.1523/JNEUROSCI.19-20-08856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Sternsdorf T, Liese J, Belohradsky B, Weber C, Wedel A, et al. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J Immunol. 1993;151:6986–6993. [PubMed] [Google Scholar]