Abstract
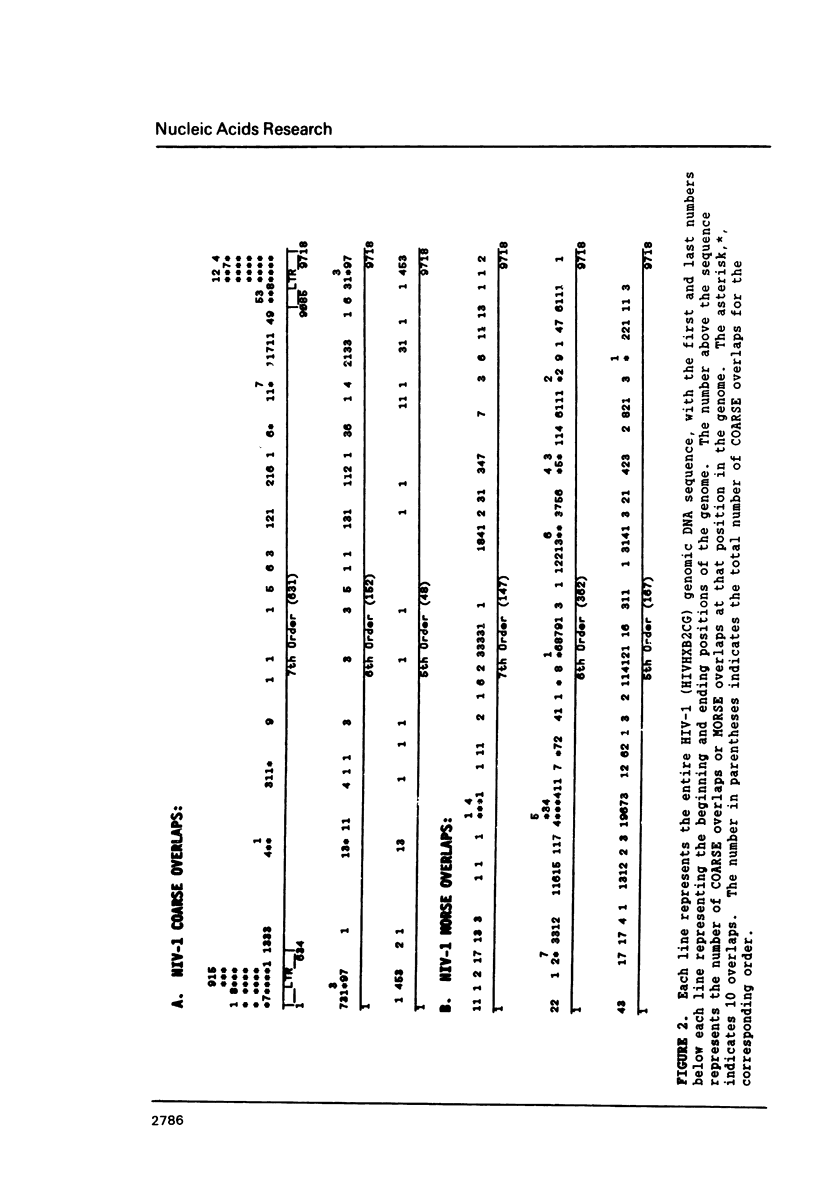
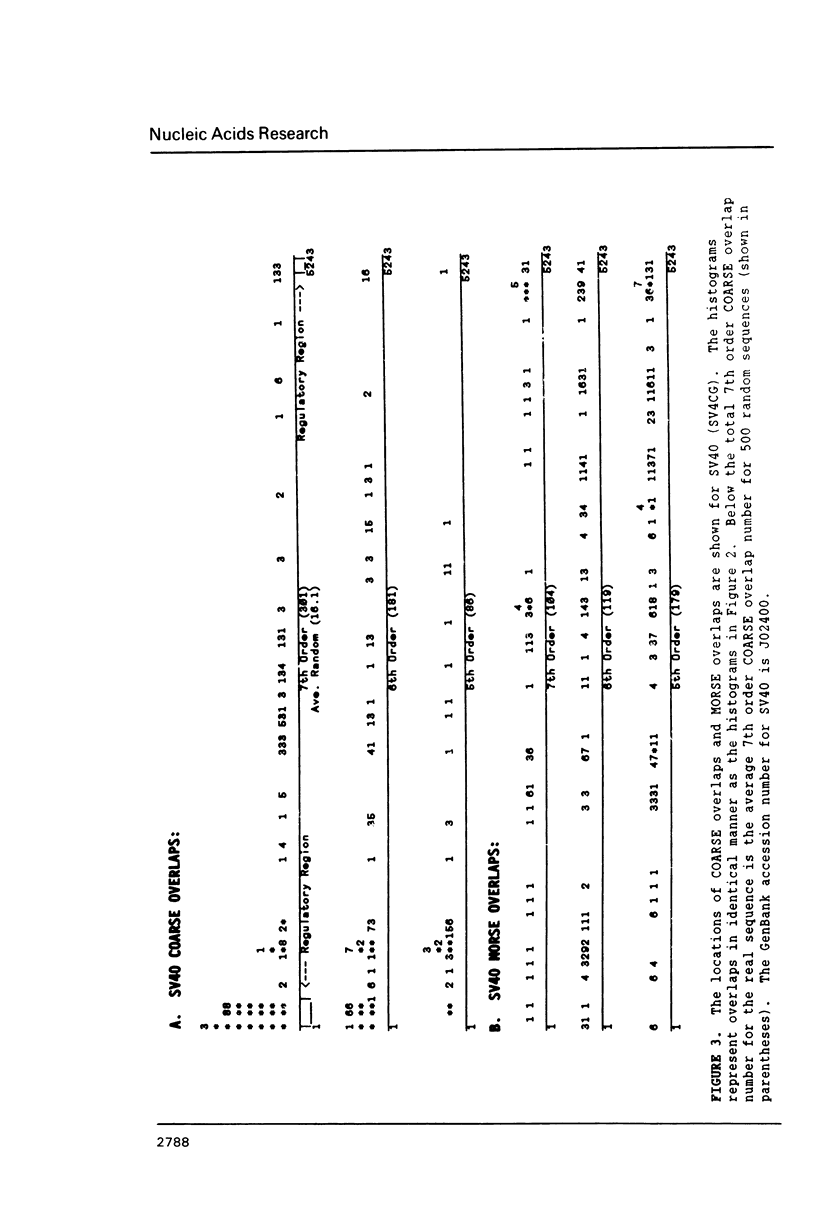
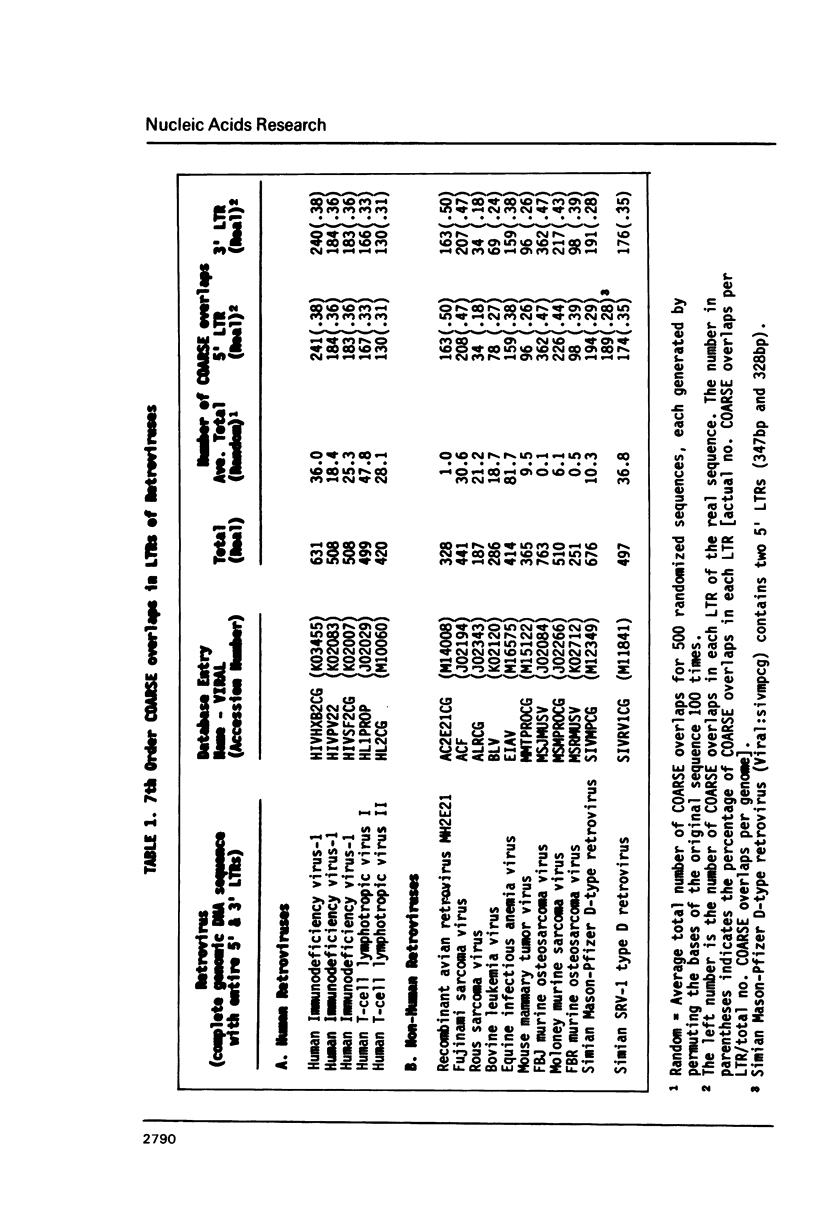
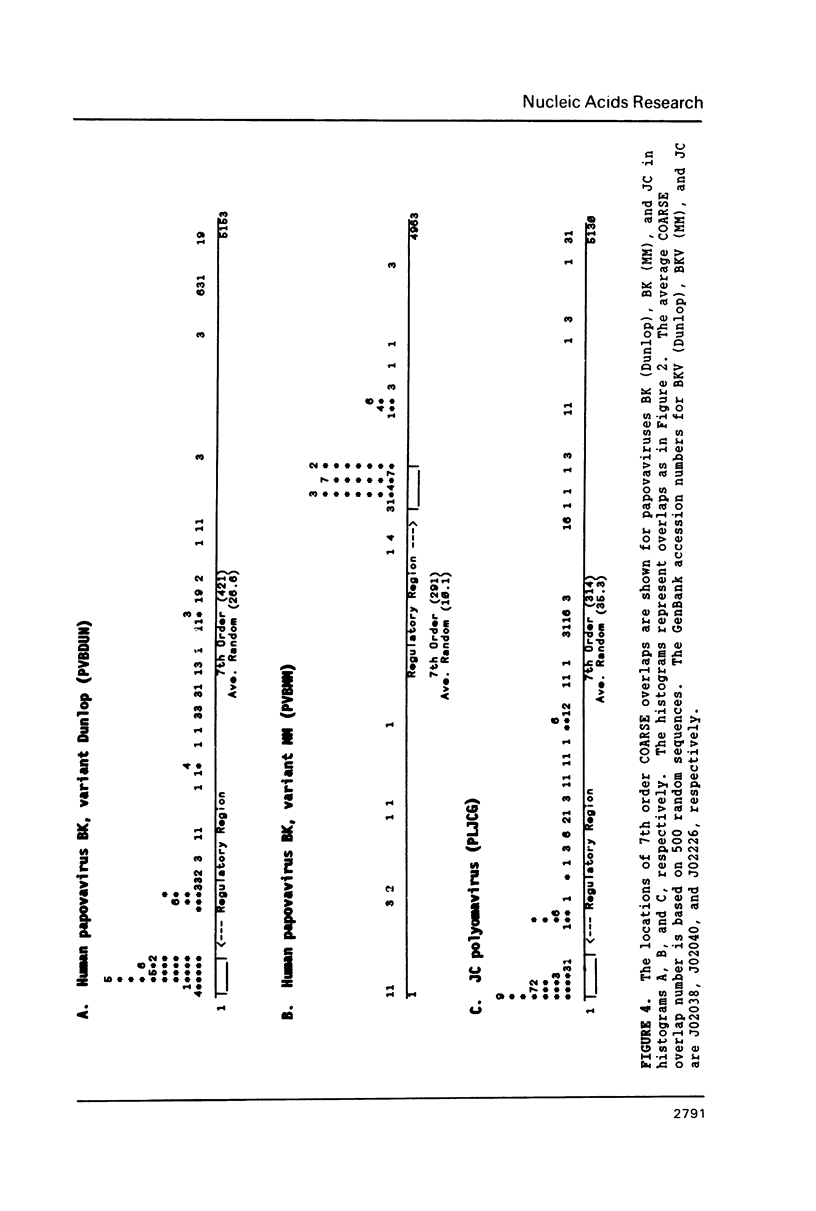
Overlapping redundant short oligomers in DNA sequences of retroviruses and papovaviruses have been identified. For each sequence, a search procedure determines the 5% short oligomers of the same length with the highest ratios of observed to expected occurrences based on singlet composition of the sequence. These short oligomers are referred to as compositionally-assessed redundant sequence elements (COARSEs). A pair of COARSEs overlapping by at least one base is considered to be a COARSE overlap. Most COARSE overlaps of the 7th order (overlapping septuplets) are found in long terminal repeats of retroviruses and in the regulatory control regions of papovaviruses SV40, BK and JC. Many of the 7th order COARSE overlaps in HIV-1 and SV40 are identical with regulatory elements determined experimentally. On the contrary, very few of the most frequently occurring oligomer overlaps, which are defined differently from COARSE overlaps, are present in the regulatory regions of retroviruses and papovaviruses. Examining DNA sequences of other genomes by the COARSE overlap method may identify putative regulatory regions.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilofsky H. S., Burks C. The GenBank genetic sequence data bank. Nucleic Acids Res. 1988 Mar 11;16(5):1861–1863. doi: 10.1093/nar/16.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernoult-Lange M., Omilli F., May E. Contribution of different GC-motifs to the control of simian virus 40 late promoter activity. Nucleic Acids Res. 1987 Oct 26;15(20):8177–8193. doi: 10.1093/nar/15.20.8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Holland E. C. HIV-1 tat trans-activation requires the loop sequence within tar. Nature. 1988 Jul 14;334(6178):165–167. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]
- Goodchild J., Agrawal S., Civeira M. P., Sarin P. S., Sun D., Zamecnik P. C. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Kenney S., Natarajan V., Strike D., Khoury G., Salzman N. P. JC virus enhancer-promoter active in human brain cells. Science. 1984 Dec 14;226(4680):1337–1339. doi: 10.1126/science.6095453. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Loeber G., Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988 May;62(5):1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz R. B., Dynan W. S. Binding of cellular proteins to the regulatory region of BK virus DNA. J Virol. 1988 Sep;62(9):3388–3398. doi: 10.1128/jvi.62.9.3388-3398.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., King D. M., Slauch J. M., Frisque R. J. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985 Jan;53(1):306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Miyamura T., Furuno A., Yoshiike K. DNA rearrangement in the control region for early transcription in a human polyomavirus JC host range mutant capable of growing in human embryonic kidney cells. J Virol. 1985 Jun;54(3):750–756. doi: 10.1128/jvi.54.3.750-756.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Wong-Staal F. Demonstration of virus-specific transcriptional activator(s) in cells infected with HTLV-III by an in vitro cell-free system. Cell. 1986 Oct 10;47(1):29–35. doi: 10.1016/0092-8674(86)90363-6. [DOI] [PubMed] [Google Scholar]
- Ondek B., Shepard A., Herr W. Discrete elements within the SV40 enhancer region display different cell-specific enhancer activities. EMBO J. 1987 Apr;6(4):1017–1025. doi: 10.1002/j.1460-2075.1987.tb04854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca R., Heath C., Goldenberg G. J., Rosen C. A., Sodroski J. G., Haseltine W. A., Hansen U. M. Transcription directed by the HIV long terminal repeat in vitro. AIDS Res Hum Retroviruses. 1987 Spring;3(1):41–55. doi: 10.1089/aid.1987.3.41. [DOI] [PubMed] [Google Scholar]
- Pomerantz B. J., Hassell J. A. Polyomavirus and simian virus 40 large T antigens bind to common DNA sequences. J Virol. 1984 Mar;49(3):925–937. doi: 10.1128/jvi.49.3.925-937.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Ryder K., DeLucia A. L., Tegtmeyer P. Binding of SV40 a protein to the BK virus origin of DNA replication. Virology. 1983 Aug;129(1):239–245. doi: 10.1016/0042-6822(83)90412-9. [DOI] [PubMed] [Google Scholar]
- Thornell A., Hallberg B., Grundström T. Differential protein binding in lymphocytes to a sequence in the enhancer of the mouse retrovirus SL3-3. Mol Cell Biol. 1988 Apr;8(4):1625–1637. doi: 10.1128/mcb.8.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Soeda E., Uchida S., Yoshiike K. DNA rearrangement affecting expression of the BK virus transforming gene. J Virol. 1984 Jul;51(1):1–6. doi: 10.1128/jvi.51.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. K., Garcia J. A., Harrich D., Gaynor R. B. Purification of the human immunodeficiency virus type 1 enhancer and TAR binding proteins EBP-1 and UBP-1. EMBO J. 1988 Jul;7(7):2117–2130. doi: 10.1002/j.1460-2075.1988.tb03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



