Abstract
Background
National data show unexplained racial disparity in albuminuria. We assessed whether low serum vitamin D status contributes to racial disparity in albuminuria.
Methods
We examined the association between race and albuminuria (spot urinary albumin/creatinine ratio (ACR) ≥30) among non-Hispanic black and white nonpregnant adults who were free of renal impairment in the National Health and Nutrition Examination Survey (NHANES) from 2001–2006. We conducted analyses without and with serum 25(OH)D. We adjusted for age, sex, education level, smoking, body mass index (BMI), diabetes, diagnosis of hypertension, and use of antihypertensive medication.
Results
Albuminuria was present in 10.0% of non-Hispanic blacks and 6.6% in non-Hispanic whites. Being black (odds ratio (OR) 1.46; 95% confidence interval (CI) 1.23–1.73) was independently associated with albuminuria. There was a graded, inverse association between 25(OH)D level and albuminuria. Notably, the association between race and albuminuria was no longer significant (OR 1.19; 95% CI 0.97–1.47) after accounting for participants' serum 25(OH)D. Similar results were observed when participants with macroalbuminuria (ACR ≥300 mg/g) or elevated parathyroid hormone (>74 pg/ml) were excluded or when a continuous measure of 25(OH)D was substituted for the categorical measure. There were no interactions between race and vitamin D status though racial disparity in albuminuria was observed among participants with the highest 25(OH)D levels.
Conclusion
Suboptimal vitamin D status may contribute to racial disparity in albuminuria. Randomized controlled trials are needed to determine whether supplementation with vitamin analogues reduces risk for albuminuria or reduce racial disparity in this outcome.
Keywords: albuminuria, blood pressure, health status disparities, hypertension, vitamin D
African Americans have higher prevalence of chronic kidney disease than non-Hispanic whites.1 Higher prevalence of hypertension and diabetes contribute to, but do not appear to fully explain, this disparity.2,3 Recently published national data confirm that blacks with both hypertension and prehypertension have higher rates of early kidney disease as indicated by microalbuminuria.4 The reasons for racial disparities in microalbuminuria are not known.
Emerging data link low serum vitamin D status to hypertension,5,6,7,8 albuminuria,9,10 and chronic renal disease.11,12 Potential mechanisms include stimulation of renin production,13 vascular smooth muscle proliferation,14 inflammation,13 thrombosis,15 and impairment in endothelial function.16,17
Given unexplained racial disparities in microalbuminuria and well documented disparities in serum vitamin D status,18 we examined the hypothesis that low vitamin D might account for racial differences in albuminuria and also microalbuminuria.
Methods
Participants. We examined data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2001–2006. Because of our focus on racial disparity in albuminuria, we confined our analysis to participants who self-reported their ethnicity and race as non-Hispanic white or black.
Our sample included 7,651 adult participants (20 years and older) who participated in the interview and examination and for whom blood pressure (BP), vitamin D status, urinary albumin, and creatinine values were available. We excluded participants with chronic kidney disease (defined as estimated glomerular filtration rate <60) and females who were pregnant (positive urine pregnancy test or self-reported pregnancy). In secondary analyses, we excluded participants with macroalbuminuria and also those with elevated parathyroid hormone (PTH) (>74 pg/ml).19
Blood pressure. BP was measured by physicians trained in assessment using mercury sphygmomanometers and appropriately sized arm cuffs.20,21,22 Readings were taken from participants while sitting after 5 min of rest. In determining mean BP, the first reading was used if only one measurement was obtained; the second reading was used if two readings were taken; and if more than two readings were taken, values were averaged over the last two when available.20,21,22
Participants were defined as having treated hypertension if they reported taking antihypertensive medications and untreated if they did not. Among untreated participants, BP was classified using the Seventh Joint National Committee for Prevention, Detection, Evaluation, and Treatment of High BP (JNC-7) guidelines23: normal (systolic BP (SBP) was <120 mm Hg and the diastolic BP (DBP) was <80 mm Hg), prehypertension (SBP of 120–139 mm Hg or DBP of 80–89 mm Hg), stage 1 hypertension (SBP of 140–159 mm Hg or DBP of 90–99 mm Hg), and stage 2 hypertension (SBP of ≥160 mm Hg or DBP of ≥100 mm Hg). Participants taking antihypertensive drugs were considered to have controlled BP if their SBP was <140 mm Hg and their DBP was <90 mm Hg.
Vitamin D status. Serum 25(OH)D was measured using a radioimmunoassay kit (DiaSorin, Stillwater, MN). The coefficient of variation for the years 2001–2006 ranged from 10–13% and the sensitivity for the 25(OH)D assay was 1.5 ng/ml).24 The serum 25(OH)D was adjusted for assay drift using methods recommended by the National Center for Health Statistics.25 Although 1,25-dihydroxyvitamin D is the biologically active form of vitamin D, serum 25(OH)D is regarded as the best indicator of vitamin D status in individuals without kidney disease.26 We grouped 25(OH)D into the following categories: <25, 25–49, 50–74, 75–99, and ≥100 nmol/l.
Albuminuria. The untimed urinary albumin/creatinine ratio (ACR) was classified as normal if ACR <30 mg/g, microalbuminuria if ACR is between 30–299 mg/g, and macroalbuminuria if ACR ≥300 mg/g.27 In addition to predicting progression of renal disease, albuminuria (both micro and macro) predicts cardiovascular disease.28 The definition of ACR was based on the criteria of the National Kidney Foundation.29 The rationale for using ACR of 30 mg/g and above is based on evidence that microalbuminuria is a predictor of cardiovascular disease and end organ damage.30
In our primary analysis, we combined participants with micro and macroalbuminuria into a single category: albuminuria. In secondary analyses, we focused on microalbuminuria by excluding participants with macroalbuminuria from the analysis.
Race. Race and Hispanic ethnicity were assessed by self-report and were categorized as white (non-Hispanic) or black (non-Hispanic). Hispanics, Asians, Native Americans, and participants who self-identified with more than one race/ethnic group were not included in this study.
Control variables. We adjusted for potential confounding by including variables associated with albuminuria and vitamin D. These include age, sex, smoking, body mass index (BMI), BP, and diabetes.31,32,33,34,35,36 This represents a conservative approach since some of these variables may be affected by serum 25(OH)D levels.
Age at screening was grouped into categories of 20–34, 35–44, 45–54, 55–64, and those 65 years and older. Smoking status was defined as never smoked, current smoker, and former smoker. BMI was determined based on participants assessed weight (kg) and height (m) and categorized (<25.0; 25.0–29.9; ≥30 kg/m2). Diabetes mellitus was defined by self-report of physician diagnosis or current use of medications for diabetes mellitus.
Statistical analyses. Analyses were conducted with SUDAAN (version 10.01) and Stata (version 10.1, College Station, TX), adjusting for the complex survey design of NHANES to yield appropriate standard errors and population parameter estimates. We examined the independent association of race with albuminuria (present or not) using logistic regression models. We compared the black-white differences in log odds of albuminuria before and after adjustment for 25(OH)D. We assessed interactions between 25(OH)D and race and between 25(OH) D and BMI. We also conducted analyses stratified by serum 25(OH)D levels to examine possible differences in effects by race. Finally, we conducted several secondary analyses. For this purpose, we repeated our analyses using microalbuminuria as a dependent variable, and excluding those with macroalbuminuria. We also repeated the analyses excluding those with elevated PTH and also using 25(OH)D as a continuous variable.
The model was checked for multicollinearity and no problem existed (all variance inflation factors <2). Since all of our independent variables are categorical, logit plots for linearity assessment were not needed. Independence is assumed due to the strict sampling methodology used by the NHANES group. Inspection of residuals did not reveal any influential outliers. In addition, a Hosmer–Lemeshow Goodness-of-fit χ2 statistic of 4.06, P = 0.8516 indicates that our model fits the data well.
Results
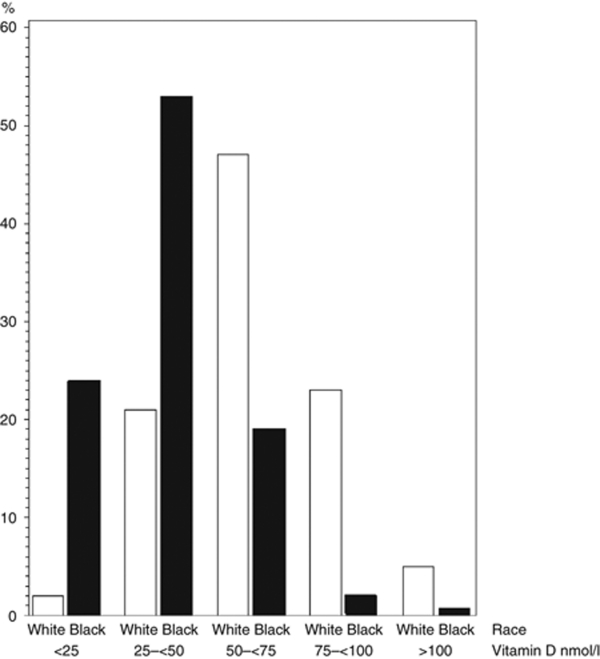
Our final sample included 7,651 non-Hispanic blacks and non-Hispanic whites, ages 20 years and older. Rates of missing data ranged from 0.01% for diabetes to 0.65% for estimated glomerular filtration rate. Albuminuria was missing for 3.5% of participants. Age, BP, and smoking were independently associated with missing albuminuria data. Twenty five percent of blacks had 25(OH)D levels below 25 nmol/l compared to only 2% of whites.
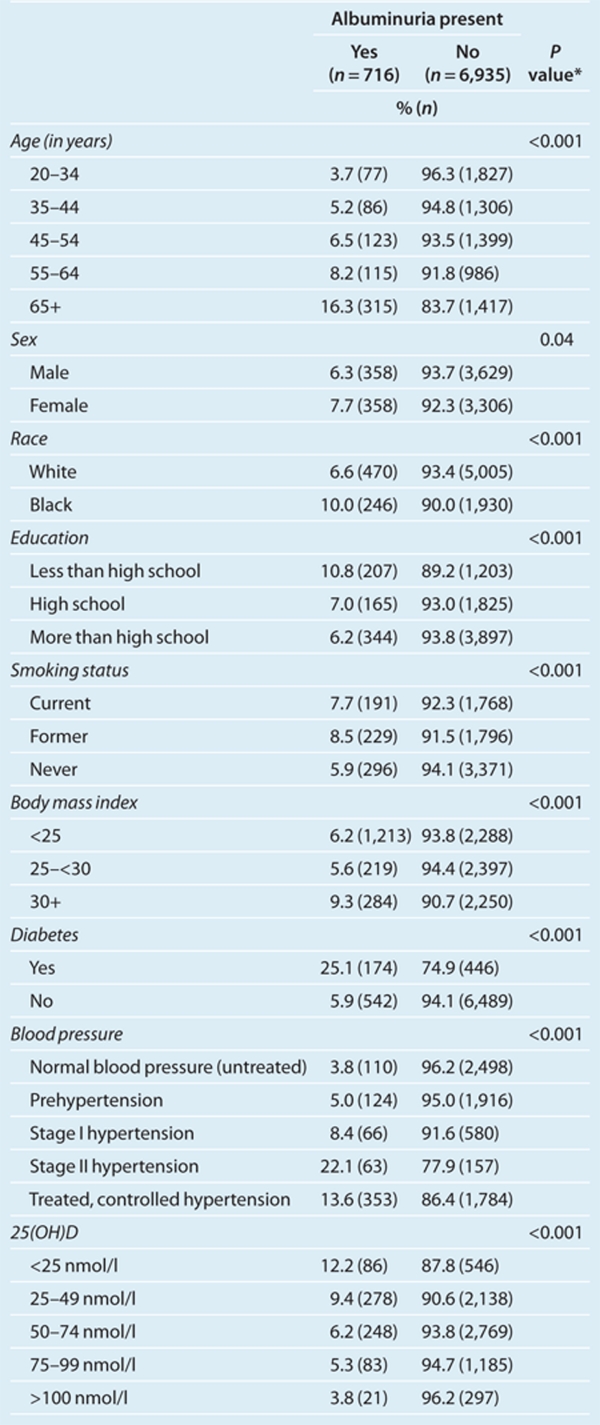
The overall prevalence of albuminuria in the total sample was 7.0%. Its prevalence was 10.0% among blacks and 6.6% among whites. Table 1 compares the characteristics of participants with and without albuminuria. As expected, those with hypertension, older than 55, less education, obese, diabetes, and those with lower serum 25(OH)D levels had higher prevalence of albuminuria. Figure 1 compares 25(OH)D levels by race. Blacks clustered in the lowest levels of 25(OH)D.
Table 1. Characteristics of participants by presence of albuminuria.
Figure 1.
Percentage of participants by race by serum 25(OH)D level.
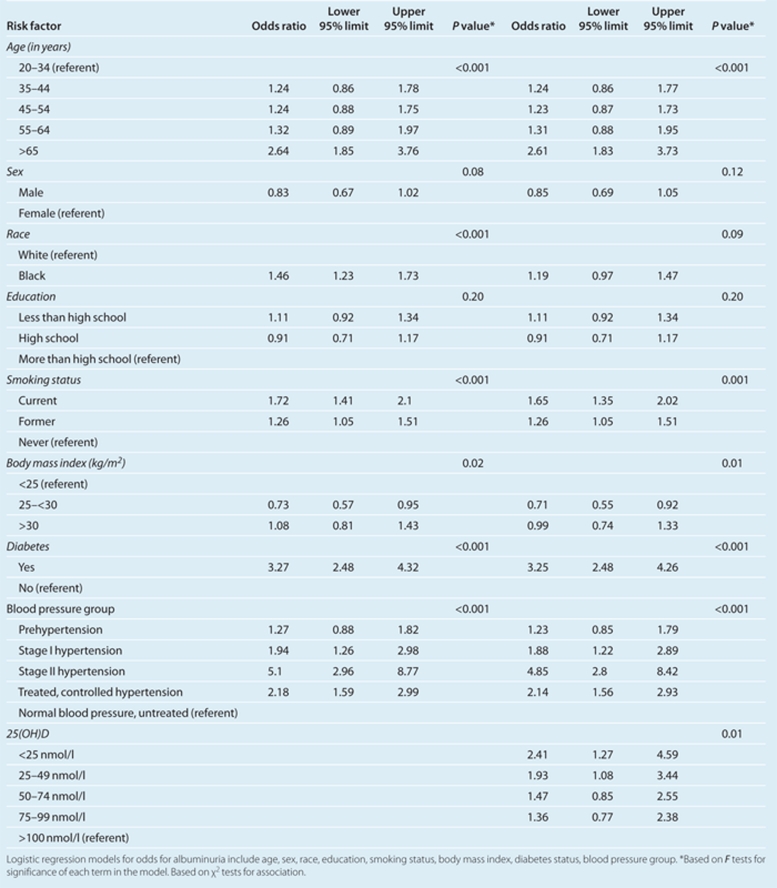
Race is a significant risk factor for albuminuria (Table 2). Blacks had a 46% greater odds of albuminuria (odds ratio (OR) 1.46; 95% confidence interval (CI) 1.23–1.73) than whites after adjusting for age, sex, education, smoking, BMI, diabetes, BP, and hypertension treatment status. Other risk factors for albuminuria besides race included older age, diabetes, BP, and smoking.
Table 2. Logistic regression results for albuminuria with and without adjustment for Vitamin D.
When vitamin D categories were added to the model, it showed a graded, inverse relationship with albuminuria. The lowest level was associated with a 2.4 higher odds for albuminuria compared to levels >100 nmol/l. Notably, the association between race and albuminuria was no longer statistically significant after accounting for serum 25(OH)D (OR 1.19; 95% CI 0.97–1.47). We observed similar effects when we used a continuous measure of 25(OH)D. Each nmol/l unit increase in 25(OH)D is associated with a 1% decreased odds for albuminuria (P < 0.01).
When we excluded participants with macroalbuminuria, we observed a similar relationship between race and microalbuminuria (OR 1.37; 95% CI 0.97–1.47) and a similar attenuation with adjustment for 25(OH)D status (OR 1.11; (95% CI 0.90–1.37). Similar results were also observed for the subsample (N = 2,375) where those with elevated PTH were excluded. Vitamin D status continued to show a graded inverse relationship with albuminuria. Similarly, the adjusted OR for race declined from 1.56 to 1.41. However, CIs were wider and overlapped 1.0 for these analyses based on a smaller subsample (N = 2,355).
There was no statistically significant interactions between race and 25(OH)D (measured either as categorical or continuous measure) or between race and BMI or BP. However, when we stratified the analyses by 25(OH)D level, blacks showed statistically significant higher rates of albuminuria only at 25(OH)D levels ≥75 nmol/l (P < 0.03). Among participants with 25(OH)D levels ≥75 nmol/l, blacks showed higher crude prevalence than whites for albuminuria (13.3% vs. 4.9%). This effect persisted after adjusting for age, sex, education, smoking, BMI, BP, and diabetes (P < 0.03). However, only nine blacks were in this category, i.e., had 25(OH)D levels ≥75 nmol/l.
Discussion
Reasons for disparity in albuminuria between blacks and whites are not fully known. Given emerging data linking low serum vitamin D status to albuminuria,9,10 and development of kidney disease,11,12 we examined the hypothesis that vitamin D explains the association between race and albuminuria in a national sample of black and white adults. The findings reported in this study confirm our hypothesis that vitamin D contributes to the noted racial disparity in albuminuria. Consistent with a previous report,4 we observed significantly higher prevalence of albuminuria among non-Hispanic blacks compared with non-Hispanic whites. We confirmed previous risk factors for albuminuria including older age, diabetes, BP, and smoking.31,32 Unexpectedly, the association of BMI and albuminuria was not statistically significant. This could represent adjustment for factors in the causal path between BMI and albuminuria (e.g., diabetes and BP) and/or inadequate statistical power.
As reported in a previous study,9 we also observed a graded, inverse response between 25(OH)D levels and albuminuria. The lowest levels were associated with the highest prevalence of albuminuria. Our findings did not change when participants with macroalbuminuria or elevated PTH were excluded from the analyses.
Notably, when we adjusted for serum 25(OH)D, the relationship between race and albuminuria was no longer statistically significant. This is consistent with the hypothesis that 25(OH)D may partly mediate the association between race and albuminuria. This finding is broadly consistent with a growing body of evidence suggesting that suboptimal vitamin D status is associated with vascular-related conditions that are more prevalent among blacks. These include hypertension,37 diabetic nephropathy,10 peripheral vascular disease,38 kidney disease progressing to renal failure,11 and cardiovascular diseases39 and mortality.40
Endothelial dysfunction represents a possible underlying mechanism of the effects of low vitamin D levels on albuminuria. Emerging data suggest that low vitamin D levels are associated with endothelial dysfunction and its correction alleviates this dysfunction.16,41,42,43,44 Albuminuria is associated with endothelial dysfunction including impaired vascular function.45,46 Intriguingly, blacks have higher rates of endothelial dysfunction and small vessel disease than whites.47,48 Thus, it is conceivable that suboptimal vitamin D status among blacks might contribute to disparities in vascular-related conditions via endothelial dysfunction as manifested in part through albuminuria.
Anthropological data suggest an evolutionary link between race and vitamin D consistent with an interaction between health outcomes, skin color, and latitude offering further plausibility for this provocative hypothesis. Vitamin D levels in response to ultraviolet (UV) exposure from the sun may have played a pivotal role in the evolution of differences in skin pigment that emerged based on continental ancestry.49 At low latitudes where UV exposure is high, dark skin pigment from higher concentrations of melanin may protect the skin and circulating blood folate.50 As human populations moved north with less intense UV exposure, selection pressures from vitamin D deficiency resulted in lighter skin pigment. Reductions in UV exposure from northern residence and less exposure (more in door activity and greater use of sun protection) appear to be associated with rising prevalence of vitamin D deficiency.51 Consistent with the findings from our study sample, vitamin D levels are lowest among blacks.52
These findings are subject to several important caveats. First, because our findings are based on cross-sectional data, we cannot determine the temporal relationship between 25(OH)D levels and albuminuria. A previous study showed that low vitamin D levels at baseline predicts progression of kidney disease up to 16 years later.11 Such results are conceivable given the relative stability of serum 25(OH)D levels over time.53,54 Second, we cannot exclude the potential for confounding; it is possible that vitamin D is simply a marker for other factors associated with albuminuria. However, findings from a small randomized controlled trial suggest the possibility of a causal relationship, such that supplementation with the vitamin D analog, paricalcitol, reduced albuminuria among persons with chronic kidney disease.44 Third, the use of a single untimed ACR sample may underestimate albumin excretion among blacks (due to higher creatinine excretion).55,56 Thus, use of ACR to assess kidney disease provides a conservative estimate of this racial disparity. Last, although we did not observe a statistically significant interaction between race and 25(OH)D, we observed a statistically significant effect of race and albuminuria only at 25(OH)D levels equal to or above 75 nmol/l. Whether this represents a chance finding or a differential effect by race, it has implications for the extent to which vitamin D status might contribute to this disparity. There is some evidence for vitamin D economy among blacks for bone turnover and formation, meaning that blacks maintain bone health at lower levels of vitamin D.57 Further study is needed to determine whether this holds for other outcomes. In particular, interventional trials are needed to both confirm a causal role of vitamin D status in albuminuria for both whites and blacks and determine whether there is a threshold effect.
In conclusion, vitamin D status may contribute to racial disparity in albuminuria. Randomized controlled trials are needed to determine whether supplementation with vitamin analogues will prevent albuminuria or mitigate racial disparity in albuminuria.
The authors declared no conflict of interest.
References
- Burrows NR, Li Y, Williams DE. Racial and ethnic differences in trends of end-stage renal disease: United States, 1995 to 2005. Adv Chronic Kidney Dis. 2008;15:147–152. doi: 10.1053/j.ackd.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J Am Soc Nephrol. 2007;18:1299–1306. doi: 10.1681/ASN.2006050524. [DOI] [PubMed] [Google Scholar]
- Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122:672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunniyi MO, Croft JB, Greenlund KJ, Giles WH, Mensah GA. Racial/ethnic differences in microalbuminuria among adults with prehypertension and hypertension: National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Hypertens. 2010;23:859–864. doi: 10.1038/ajh.2010.77. [DOI] [PubMed] [Google Scholar]
- Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–1954. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Diaz VA, Mainous AG, 3rd, Carek PJ, Wessell AM, Everett CJ. The association of vitamin D deficiency and insufficiency with diabetic nephropathy: implications for health disparities. J Am Board Fam Med. 2009;22:521–527. doi: 10.3122/jabfm.2009.05.080231. [DOI] [PubMed] [Google Scholar]
- Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20:2631–2639. doi: 10.1681/ASN.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG, Norris KC. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977–983. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- Beer TM, Venner PM, Ryan CW, Petrylak DP, Chatta G, Dean Ruether J, Chi KN, Curd JG, DeLoughery TG. High dose calcitriol may reduce thrombosis in cancer patients. Br J Haematol. 2006;135:392–394. doi: 10.1111/j.1365-2141.2006.06322.x. [DOI] [PubMed] [Google Scholar]
- Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, Toprak A, Yazici D, Sancak S, Deyneli O, Akalin S. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey Laboratory Procedure Manual: Parathyroid Hormone 2005. Available from: < : < http://www.cdc.gov/nchs/data/ nhanes/nhanes_03_04/l11pth_c_met.pdf >, accessed 5th October 2011.
- National Health and Nutrition Examination Survey National Health and Nutrition Examination Survey Physician examination procedures manual (original 1999, revised 2000) . < http://www.cdc.gov/nchs/nhanes/ nhanes_01_02/physician_year_3.pdf >, accessed 5th October 2011.
- National Health and Nutrition Examination Survey. National Health and Nutrition Examination Survey Physician examination procedures manual (original 1999, revised 2000) . < http://www.cdc.gov/nchs/nhanes/ nhanes_03_04/physician_year_3.pdf >, accessed 5th October 2011.
- National Health and Nutrition Examination Survey. National Health and Nutrition Examination Survey Physician examination procedures manual (original 1999, revised 2000) . < http://www.cdc.gov/nchs/nhanes/ nhanes_05_06/physician_year_3.pdf >, accessed 5th October 2011.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN, Hui SL, Lensmeyer GL, Massaro J, Peacock M, Rosner B, Wiebe D, Bailey RL, Coates PM, Looker AC, Sempos C, Johnson CL, Picciano MF, Vitamin D Roundtable on the NHANES Monitoring of Serum 25(OH)D: Assay Challenges and Options for Resolving Them NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140:2030S–2045S. doi: 10.3945/jn.110.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. Revised Analytical Note for NHANES 2000-2006 and NHANES III (1988-1994) 25-Hydroxyvitamin D Analysis (Revised November 2010)2010 Available from: < : < http://www.cdc.gov/nchs/data/ nhanes/nhanes3/VitaminD_analyticnote.pdf >, accessed 5th October 2011.
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansevoort RT, Nauta FL, Bakker SJ. Albuminuria: all you need to predict outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:513–518. doi: 10.1097/MNH.0b013e32833e4ce1. [DOI] [PubMed] [Google Scholar]
- Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, Jardine M, Gallagher M, Turnbull F, Chalmers J, Craig J, Huxley R. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008;5:e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:Suppl-266. [PubMed] [Google Scholar]
- Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, HOPE Study Investigators Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- Böhm M, Thoenes M, Danchin N, Bramlage P, La Puerta P, Volpe M. Association of cardiovascular risk factors with microalbuminuria in hypertensive individuals: the i-SEARCH global study. J Hypertens. 2007;25:2317–2324. doi: 10.1097/HJH.0b013e3282ef1c5f. [DOI] [PubMed] [Google Scholar]
- Yuyun MF, Khaw KT, Luben R, Welch A, Bingham S, Day NE, Wareham NJ. Microalbuminuria, cardiovascular risk factors and cardiovascular morbidity in a British population: the EPIC-Norfolk population-based study. Eur J Cardiovasc Prev Rehabil. 2004;11:207–213. doi: 10.1097/01.hjr.0000133070.75016.1d. [DOI] [PubMed] [Google Scholar]
- Benjamin A, Moriakova A, Akhter N, Rao D, Xie H, Kukreja S, Barengolts E. Determinants of 25-hydroxyvitamin D levels in African-American and Caucasian male veterans. Osteoporos Int. 2009;20:1795–1803. doi: 10.1007/s00198-009-0873-6. [DOI] [PubMed] [Google Scholar]
- Chan J, Jaceldo-Siegl K, Fraser GE. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control. 2010;21:501–511. doi: 10.1007/s10552-009-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kositsawat J, Freeman VL, Gerber BS, Geraci S. Association of A1C levels with vitamin D status in U.S. adults: data from the National Health and Nutrition Examination Survey. Diabetes Care. 2010;33:1236–1238. doi: 10.2337/dc09-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Ford ES, Li C, Kris-Etherton PM, Etherton TD, Balluz LS. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. J Hypertens. 2010;28:1821–1828. doi: 10.1097/HJH.0b013e32833bc5b4. [DOI] [PubMed] [Google Scholar]
- Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Reis JP, Michos ED, von Mühlen D, Miller ER., 3rd Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88:1469–1477. doi: 10.3945/ajcn.2008.26447. [DOI] [PubMed] [Google Scholar]
- Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB, Intermountain Heart Collaborative (IHC) Study Group Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2010;8:11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Mëtivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. Does vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations. Am J Med. 2010;123:335–341. doi: 10.1016/j.amjmed.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- Cosson E, Pham I, Valensi P, Pariès J, Attali JR, Nitenberg A. Impaired coronary endothelium-dependent vasodilation is associated with microalbuminuria in patients with type 2 diabetes and angiographically normal coronary arteries. Diabetes Care. 2006;29:107–112. doi: 10.2337/diacare.29.1.107. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction–the Hoorn Study. Kidney Int Suppl. 2004. pp. S42–S44. [DOI] [PubMed]
- Houghton JL, Philbin EF, Strogatz DS, Torosoff MT, Fein SA, Kuhner PA, Smith VE, Carr AA. The presence of African American race predicts improvement in coronary endothelial function after supplementary L-arginine. J Am Coll Cardiol. 2002;39:1314–1322. doi: 10.1016/s0735-1097(02)01781-3. [DOI] [PubMed] [Google Scholar]
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol. 2002;40:754–760. doi: 10.1016/s0735-1097(02)02015-6. [DOI] [PubMed] [Google Scholar]
- Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci USA. 2010;107 Suppl 2:8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478–2485. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101:916–923. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19:927–931. doi: 10.1158/1055-9965.EPI-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155:1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–1039. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. J BoneMinl Res. 2007;22:Suppl-8. doi: 10.1359/jbmr.07s220. [DOI] [PubMed] [Google Scholar]