Abstract
Background
Proton-pump inhibitors (PPIs) and clopidogrel are frequently co-prescribed though the benefits and harms of their concurrent use are unclear.
Objective
To examine the association between concurrent PPI and clopidogrel use and the risks for gastroduodenal bleeding hospitalizations and serious cardiovascular disease.
Design
Retrospective cohort study that used automated data to identify patients who received clopidogrel between 1999 through 2005 following hospitalization for coronary heart disease.
Setting
Tennessee Medicaid Program
Patients
20,596 patients (including 7593 concurrent users of clopidogrel and PPIs) hospitalized for myocardial infarction, coronary artery revascularization, or unstable angina pectoris.
Measurements
Baseline and followup drug use assessed from automated records of dispensed prescriptions. Primary outcomes were hospitalizations for gastroduodenal bleeding and serious cardiovascular disease (fatal or nonfatal myocardial infarction/sudden cardiac death, stroke, or other cardiovascular death).
Results
Pantoprazole and omeprazole accounted for 62% and 9% of the concurrent PPI use. Adjusted gastroduodenal bleeding hospitalization incidence in concurrent PPI users was 50% lower than that in nonusers (HR, 0.50 [95% CI, 0.39 to 0.65]). For patients at highest risk of bleeding, PPI use was associated with an absolute reduction of 28 (12 to 37) gastroduodenal bleeding hospitalizations per 1000 person years. The hazard ratio associated with concurrent PPI use for risk of serious cardiovascular disease was 0.99 (CI, 0.82 to 1.19) and was 1.01 (CI, 0.77 to 1.30) among patients who had percutaneous coronary interventions with stenting.
Limitations
There was possible unmeasured confounding and misclassification of exposure (no information on adherence or over-the-counter use of drugs) and endpoints (not confirmed by medical record review). Because many patients entered the cohort from hospitals with relatively few cohort members, the analysis relied on the assumption that after adjusting for observed covariates, PPI users from one such hospital could be compared with nonusers from a different hospital.
Conclusion
Among patients with serious coronary heart disease treated with clopidogrel, concurrent PPI use was associated with reduced incidence of gastroduodenal bleeding hospitalizations. The corresponding point estimate for serious cardiovascular disease was not increased; however, the 95% confidence interval included a clinically important increased risk.
Funding
Agency for Healthcare Quality and Research, National Heart Lung and Blood Institute.
Although proton-pump inhibitors (PPIs) are commonly prescribed with clopidogrel to reduce the risk of serious gastroduodenal bleeding(1–4), there is growing concern that this practice decreases the efficacy of clopidogrel. The biotransformation of clopidogrel to its active metabolite requires the hepatic cytochrome P450 2C19 isoenzyme(5). Patients treated with clopidogrel who carry CYP2C19 loss-of-function alleles have reduced levels of the clopidogrel active metabolite, decreased inhibition of platelet aggregation, and increased risk of major cardiovascular disease(6,7). All presently available PPIs are CYP2C19 substrates and some inhibit CYP2C19 metabolism(8). Omeprazole cotherapy in patients undergoing a percutaneous coronary intervention with stenting resulted in decreased formation of the active metabolite of clopidogrel and attenuated platelet inhibition(9). A cohort and a case-control study have now reported that in patients with recent myocardial infarctions prescribed clopidogrel, concurrent PPI use was associated with poorer cardiovascular outcomes(10,11).
If PPIs do decrease clopidogrel efficacy, then the clinical decision to coprescribe a PPI will require balancing the benefits of reduced gastroduodenal bleeding versus the risks of increased adverse cardiovascular outcomes. This in turn will require quantification of the effect of PPIs on the occurrence of both clinical outcomes in the patient populations receiving clopidogrel. However, there is considerable uncertainty with regard to the magnitude of the PPI effect on both bleeding and cardiovascular outcomes.
Most of the evidence that PPIs prevent clopidogrel-related gastroduodenal bleeding is either mechanism-based or indirect. Clopidogrel-related gastroduodenal bleeding is thought to be due to impaired healing of asymptomatic gastrointestinal lesions(12). PPIs could reduce the risk of gastroduodenal bleeding by either decreasing the occurrence of spontaneous ulcers or promoting ulcer healing. PPIs reduce the risk of non-steroidal antiinflammatory drug-related peptic ulcers, which may have a similar etiology(13). The direct evidence that PPIs prevent clopidogrel-related gastroduodenal bleeding consists of two case-control studies with limited sample size(14,15).
Whether or not PPIs interfere with the cardiovascular benefits of clopidogrel is also controversial(16,17). The magnitude of the adverse effect reported in the cohort study(11) exceeds that of the beneficial clopidogrel effect found in many clinical trials(18). Thus, it has been suggested that the increased rates of adverse cardiovascular outcomes in this study were due, at least in part, to uncontrolled confounding(16,17). Indeed, a recent observational analysis of data from the TRITON clinical trial of clopidogrel(19) found no increased cardiovascular risk associated with concurrent PPI use.
We sought to quantify the effects of concurrent PPI use on the risk of both gastroduodenal bleeding hospitalizations and serious cardiovascular disease in a cohort of patients hospitalized for coronary heart disease who were prescribed clopidogrel.
Methods
Design Overview
We conducted a retrospective cohort study of patients hospitalized for acute myocardial infarction, coronary artery revascularization, or unstable angina pectoris and prescribed clopidogrel during the study period 1 January 1999 through 31 December 2005. We studied the effects of concurrent PPI use on the risk of both gastroduodenal bleeding hospitalizations and serious cardiovascular disease. The latter was defined as fatal or non-fatal myocardial infarction, stroke, or other cardiovascular death, a standard endpoint for measuring clopidogrel efficacy(3).
The study was conducted with automated data from the Tennessee Medicaid program(20). Study files included an enrollment file linked with death certificates as well as files recording prescriptions filled at the pharmacy, hospital admissions, outpatient visits, and long-term care residence. These files permitted cohort identification, tracking of study medication use, classification of baseline gastrointestinal and cardiovascular risk, and endpoint ascertainment(20,21).
The study was conducted according to a prespecified protocol that included provision for analysis of cardiovascular effects of individual PPIs, as these differ in their effects on 2C19 metabolism(22,23). The study was approved by the Vanderbilt Committee for the Protection of Human Subjects, which waived informed consent, as well as the State of Tennessee Bureau of TennCare and Department of Health.
Cohort, Medication Exposure and Followup
The cohort included Medicaid enrollees 30 years of age or older with at least one day of current clopidogrel use during the study period. Because clopidogrel generally is prescribed for serious coronary heart disease, cohort members had to have a qualifying hospitalization for such disease during the study period that preceded the clopidogrel use. Prior to the qualifying hospitalization, cohort members had to have ≥365 days of Medicaid enrollment in a plan with full medication benefits.
Serious coronary heart disease was defined as acute myocardial infarction, coronary artery revascularization or unstable angina pectoris. Myocardial infarctions were identified from the primary discharge diagnosis of hospitalizations with > 2 calendar day stay (to exclude diagnostic evaluations)(24). Coronary revascularization (percutaneous coronary intervention and coronary artery bypass graft surgery) was identified from codes for procedures, excluding those associated with valve replacement. Admissions for unstable angina were identified from hospital discharge diagnoses, using the definition of Shahi et al(25).
Other cohort eligibility criteria assured the availability of necessary study data and excluded patients likely to have events unrelated to medications. Because medical care encounters were used to classify patients according to baseline comorbidity, cohort members had to have evidence of regular medical care during the 365 days preceding the qualifying hospital admission, defined as at least one prescription or outpatient visit. They could not have a prior potentially life-threatening non-cardiovascular illness or a condition predisposing to chronic gastroduodenal bleeding. The exclusion illnesses were diagnosed cocaine use or alcohol abuse, cancer (excluding non-melanoma skin cancers), HIV, renal, hepatic or respiratory failure, organ transplant, liver cirrhosis, esophageal varices, bariatric or other surgery resulting in gastrojejunal anastomosis. The cohort also excluded nursing home residents, as the death certificate cause of death may be less reliable in this population. The cohort ultimately included 20,596 clopidogrel users. (See Figure A-1 for the study flow chart).
Cohort medication exposure for each day of followup was defined from Medicaid files of medications dispensed at the pharmacy. These include the dispensing date, drug, quantity, dose, and days of supply (edited to resolve occasional discrepancies with the quantity), but do not have patient-reported adherence. Current use was the period from the prescription fill date to the end of the days of supply, the time during which persons are likely to be taking the drug. Nonuse included person-time with at least 365 prior days with no current use. To reduce misclassification, other person-time was classified separately as indeterminate or former. For hospital stays of more than 7 days (14% of stays), medication use after hospital discharge was not considered as current until prescriptions were refilled, as our data suggested that medications often were changed during long hospital stays. For clopidogrel, ticlopidine, and aspirin, which have persistent antiplatelet effects, current use extended 7 days following the end of the days of supply. PPI use also was classified by dose according to standard clinical criteria(26).
Follow-up began the day after the qualifying hospital discharge closest to first clopidogrel use and continued until the last study day, endpoint occurrence, death, or loss of cohort eligibility. Follow-up was restricted to days with current use of clopidogrel and, to avoid misclassification of PPI use status, either current use or nonuse of a PPI. Follow-up excluded person-time in the hospital (day following admission to day of discharge) because we did not have information on in-hospital medications.
Endpoints
Serious gastroduodenal bleeding was defined as a hospital admission with diagnostic and procedure codes compatible with bleeding at a gastroduodenal site (excluding angiodysplasia of stomach/duodenum) using validated diagnostic codes with a positive predictive value of 91%(27). To check for residual bias or confounding, similarly validated codes(27) were used to define bleeding at sites unlikely to be affected by PPI use. These other sites were classified as other gastrointestinal or non-gastrointestinal.
Serious cardiovascular disease was defined as acute myocardial infarction/sudden cardiac death, nonfatal or fatal stroke, or other cardiovascular death. Acute myocardial infarctions were identified from hospital admissions using a definition(28,29) with a positive predictive value of 93%(24). Sudden cardiac deaths were identified from death certificates linked with prior medical care encounters using a definition that had a positive predictive value of 87%(30). Strokes were identified from hospital admissions with a definition that had a positive predictive value of 89%(31) and from death certificates. Other cardiovascular deaths were identified from death certificates with an underlying cause of death coded as due to cardiovascular disease.
Analysis
The statistical analysis compared the adjusted incidence of endpoints between clopidogrel patients who were either current users or nonusers of PPIs, where PPI use was a time-dependent variable. Relative risk was estimated with the hazard ratio, calculated from Cox regression models, using robust sandwich variance estimators to account for possible clustering by hospital for the qualifying admission(32).
Because PPIs may be preferentially prescribed to more severely ill patients, we utilized the Medicaid data to identify a large number of variables reflecting baseline risk of bleeding at gastroduodenal or other sites, cardiovascular comorbidity, and other comorbidities. The bleeding-related variables included past history of upper/lower gastrointestinal disease or bleeding, drugs used to treat gastrointestinal disease, and Helicobacter pylori eradication therapy. The cardiovascular variables included prescribed medications, diagnosed disease, and medical care utilization (frequency of inpatient admissions, emergency department visits, and outpatient encounters). A complete list of these variables is included in Appendix Table A-1.
We used these variables to calculate a propensity score, which facilitates parsimonious regression models and allows adjustment for the effect of all the covariates, even those only weakly associated with the endpoints(33). The propensity score--the probability that the patient was a PPI user on the first day of study followup—was converted to deciles and included as a variable in all regression models. There was no evidence that several key assumptions for use of the propensity score--overlap between users and nonusers of PPIs, balance, and ability to pool across deciles--were violated (Appendix: Figure A-2, Table A-2). For the primary findings, the propensity score models had essentially identical results to traditional regression models that included all of the covariates.
Regression models included both baseline and time-dependent variables. Baseline variables included demographic characteristics (age, gender, TennCare uninsured enrollment, race, calendar year), qualifying hospitalization diagnosis and procedures (coronary artery bypass graft, drug eluting stent, bare metal stent, none), and the propensity score. Time-dependent covariates included in all models were PPI use, change from baseline PPI use status (from user to nonuser or vice-versa), subsequent hospital readmissions (characterized according to diagnosis, length of stay, and time since discharge), emergency department visits, and current use of prescribed low-dose aspirin. Additional time-dependent variables for the bleeding endpoint models were drugs associated with bleeding--anticoagulants, COX-2 selective or nonselective nonsteroidal antiinflammatory drugs, systemic corticosteroids--and recent gastrointestinal symptoms. Additional time-dependent variables in the cardiovascular endpoint models were subsequent revascularization procedures, current use of statins, as well as newly prescribed cardiovascular drugs (calcium channel blockers, digoxin, loop diuretics, insulin) or new cardiovascular diagnoses (heart failure, stroke/other cerebrovascular disease, peripheral vascular disease).
We conducted several planned a priori subgroup analyses. For serious gastroduodenal bleeding, subgroups were defined according to specific risk factors for such bleeding: age 65 years or older, prior history of hospitalization for upper gastrointestinal disease or bleeding, recent use of anticoagulants, current use of other medications that increase bleeding risk (systemic corticosteroids, nonsteroidal antiinflammatory drugs), and any hospital discharge in the past year.(34,35) For serious cardiovascular disease, we performed an analysis for patients having percutaneous coronary interventions with stenting, for whom clopidogrel use is particularly important,(18) and for the first year of followup for such patients. Subgroups also were defined according to individual PPI (whose inhibition of CYP2C19 varies(23)) and PPI dose.
In some analyses, we calculated the adjusted absolute rate differences for endpoints and 95% confidence intervals. The difference was calculated as I0*(HR-1), where I0 was the rate in the nonusers of PPIs and HR the adjusted hazard ratio for current PPI use versus nonuse. Finally, using data from the study cohort, we constructed trade-off scenarios of potential benefits and harms of concurrent therapy for patients at low, moderate, and high risk of gastroduodenal bleeding and serious cardiovascular disease. All statistical analyses were performed with SAS 9.1.
Role of the Funding Source
The study was funded by grants from the Agency for Healthcare Quality and Research and the National Heart Lung and Blood Institute, which had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Cohort Characteristics
The cohort included 20,596 current clopidogrel users, of whom 13,003 initially had no PPI use and 7593 initially had concurrent PPI use. Pantoprazole was the most commonly used PPI (62%), whereas only 9% of the cohort used omeprazole. Baseline nonusers and current users of PPIs were of comparable age (Table 1); however, the latter were more often female, entered the cohort in a later calendar year, and had a longer qualifying hospital stay. Compared with nonusers, PPI users also more often had prior cardiovascular and gastrointestinal disease, as well as more frequent use of other medications that increase the risk of gastrointestinal bleeding.
Table 1.
Baseline characteristics of the cohort, according to concurrent use of proton-pump inhibitors (PPIs) on the first day of study followup.*
| No Concurrent PPI | Concurrent PPI | p value | p value, propensity-score adjusted | |
|---|---|---|---|---|
| N of patients | 13,003 | 7593 | ||
| Calendar year of cohort entry, mean | 2001.8 | 2002.7 | <.001 | .850 |
| Age in years, mean±std | 60.4±11.2 | 60.8±11.3 | .017 | .990 |
| Male, % | 53.1% | 45.6% | <.001 | .947 |
| White, % | 77.6% | 78.9% | .028 | .991 |
| Qualifying hospital admission** | ||||
| Acute myocardial infarction, % | 31.2% | 28.5% | <.001 | .983 |
| Percutaneous intervention with stent, % | 67.0% | 61.2% | <.001 | .913 |
| Stent not drug eluting, % | 52.0% | 38.6% | <.001 | .859 |
| Stent drug eluting, % | 15.0% | 22.6% | <.001 | .946 |
| Coronary artery bypass graft, % | 12.0% | 13.6% | <.001 | .987 |
| Hospital stay, days, mean | 3.9 | 4.5 | <.001 | .966 |
| Prior cardiovascular disease or medications*** | ||||
| Heart failure, % | 25.1% | 31.1% | <.001 | .868 |
| Cerebrovascular disease, % | 18.9% | 20.8% | <.001 | .781 |
| Peripheral vascular disease, % | 14.8% | 17.4% | <.001 | .978 |
| Hospitalization for cardiovascular disease, % | 29.4% | 40.9% | <.001 | .921 |
| Clopidogrel or ticlopidine, % | 17.0% | 23.3% | <.001 | .956 |
| Digoxin, % | 13.1% | 12.5% | .22 | .995 |
| Loop diuretic, % | 34.2% | 42.1% | <.001 | .950 |
| Insulin, % | 16.3% | 20.0% | <.001 | .973 |
| Oral hypoglycemic, % | 31.1% | 34.1% | <.001 | .982 |
| Prior gastrointestinal/bleeding disease or medications associated with increased risk of bleeding *** | ||||
| Peptic ulcer hospitalization, % | 2.0% | 6.7% | <.001 | .991 |
| Gastritis, % | 1.1% | 4.5% | <.001 | .951 |
| Esophageal disease, % | 7.8% | 29.8% | <.001 | .716 |
| Other upper gastrointestinal disease, % | 1.0% | 2.9% | <.001 | .997 |
| Diverticulitis/diverticulosis, % | 1.1% | 2.5% | <.001 | .618 |
| Other lower gastrointestinal disease, % | 3.1% | 6.3% | <.001 | .808 |
| Other gastrointestinal symptoms, % | 9.1% | 15.5% | <.001 | .951 |
| Gastrointestinal bleeding, % | 1.4% | 4.9% | <.001 | .982 |
| Other bleeding, % | 2.1% | 2.6% | .013 | .991 |
| NSAID, % | 66.5% | 69.7% | <.001 | .980 |
| Coxib, % | 15.4% | 29.2% | <.001 | .889 |
| Systemic corticosteroid, % | 23.4% | 35.8% | <.001 | .910 |
| Anticoagulant, % | 11.2% | 12.2% | .029 | .991 |
Baseline is defined as the day following the qualifying hospitalization discharge. Medical care encounters and filled prescriptions are either for the qualifying hospitalization or the 365 preceding days.
Patient may be in more than a single category.
Medical care encounters are either for the qualifying hospitalization or the 365 preceding days. NSAID = non selective nonsteroidal antiinflammatory drug; Coxib = COX-2-selective nonsteroidal antiinflammatory drug.
Hospitalization with Gastroduodenal or Other Bleeding
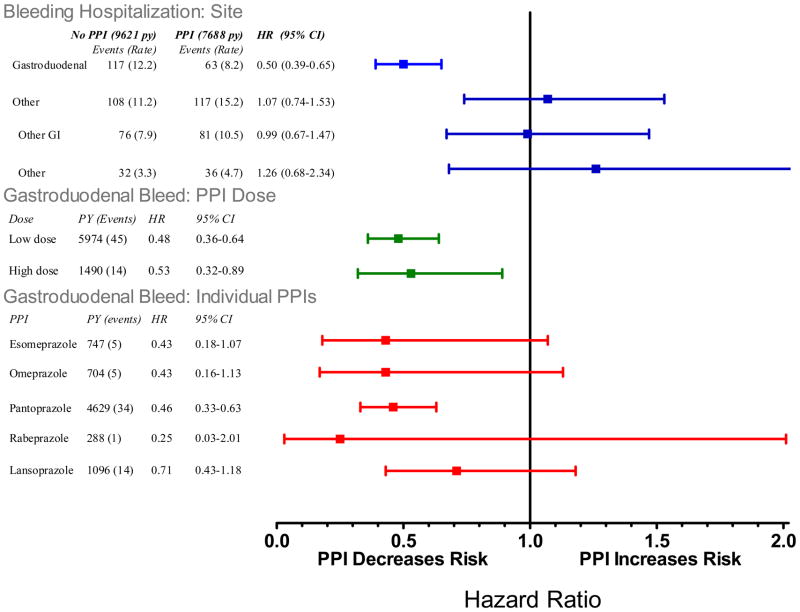
Current users of clopidogrel who were nonusers of PPIs had 12.2 gastroduodenal bleeding hospitalizations per 1000 person-years of follow-up (Figure 1). This incidence was reduced by 50% (HR, 0.50 [CI 0.39 to 0.65]) among concurrent users of PPIs and by 54% (HR 0.46, [CI 0.33 to 0.63]) among users of pantoprazole, the most commonly prescribed PPI. Concurrent PPI use was not associated with a statistically significant decreased incidence of bleeding at other sites, though these estimates had wide confidence bounds.
Figure 1.
Relative risk of bleeding endpoints for current clopidogrel users according to use of individual PPIs and PPI dose. Reference category is nonusers of any PPI. PY is person-years are person years of PPI use, events is number of cases of serious cardiovascular disease. The sum for individual drugs and doses does not equal total use because the former exclude persons with use of more than one PPI. HR is the adjusted hazard ratio, CI, confidence interval. The high dose cutpoints were: esomeprazole >40mg, omeprazole >20mg, pantoprazole >40mg, rabeprazole >20mg, and lanzoprazole >30mg.
For non-users of PPIs, the incidence of gastroduodenal bleeding hospitalizations increased according to the recorded number of recognized risk factors for such bleeding (Appendix Figure A-3). Patients with none of these factors had 3.2 hospitalizations per 1000 person-years of follow-up whereas those with 3 or more factors had 46.7 hospitalizations per 1000 person-years. For patients with 3 or more risk factors, current PPI users had an absolute reduction in gastroduodenal bleeding hospitalizations of 28.5 (CI, 11.7 to 36.9) per 1000 person-years. Those with 2 risk factors or 1 risk factor had respective reductions of 10.7 (CI, 5.1 to 14.0) and 4.9 (CI, 0.6 to 7.0) hospitalizations per 1000 person-years. PPI users with no risk factors had no reduction in the incidence of gastroduodenal bleeding hospitalizations; there were, however, few such hospitalizations in this group.
Serious Cardiovascular Disease
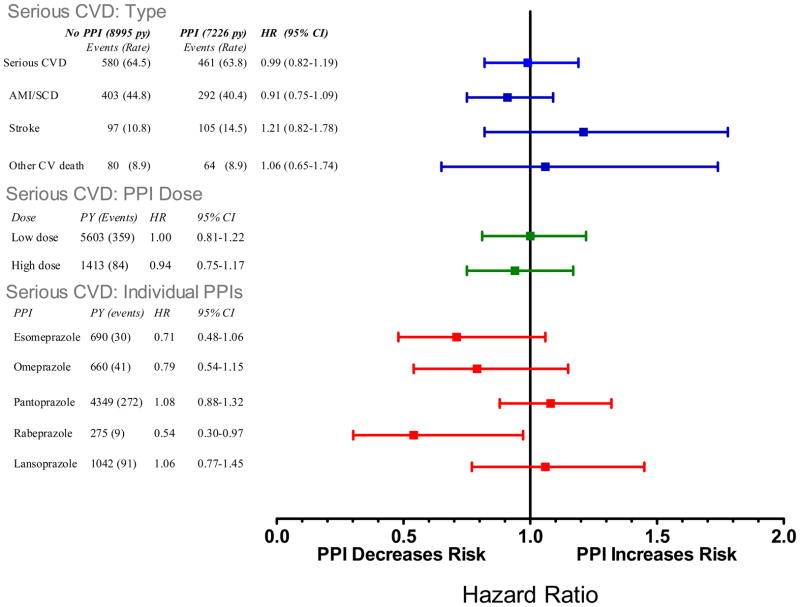
Among current users of clopidogrel, concurrent PPI use was not associated with a statistically significant increased risk of serious cardiovascular disease (Figure 2, HR, 0.99 [CI, 0.82 to 1.19]). Similar findings were present when the cohort was restricted to patients undergoing a baseline percutaneous coronary intervention with stenting, for either the entire period (HR, 1.01 [CI, 0.77 to 1.30]) or first year of follow-up (HR, 0.94 [CI, 0.69 to 1.29], see Appendix Table A-6, Figure A-4).
Figure 2.
Relative risk of serious cardiovascular disease endpoint (nonfatal or fatal myocardial infarction, stroke, or other cardiovascular death) for current clopidogrel users according to use of individual PPIs and PPI dose. Reference category is nonusers of any PPI. PY is person-years are person years of PPI use, events is number of cases of serious cardiovascular disease. The sum for individual drugs and doses does not equal total use because the former exclude persons with use of more than one PPI. HR is the adjusted hazard ratio, CI, confidence interval. The high dose cutpoints were: esomeprazole >40mg, omeprazole >20mg, pantoprazole >40mg, rabeprazole >20mg, and lanzoprazole >30mg.
Neither pantoprazole (HR, 1.08 [CI, 0.88 to 1.32], omeprazole (HR, 0.79 [CI, 0.54 to 1.15]) nor any of the other individual PPIs was associated with a statistically significant increased risk for serious cardiovascular disease (Figure 2). However, confidence bounds for several of the estimates were wide. Concurrent use of clopidogrel and high-dose PPIs also was not associated a statistically significant increased risk of serious cardiovascular disease (HR, 0.94 [CI, 0.75 to 1.17]).
We conducted several sensitivity analyses to explore the robustness of study findings (see Appendix, Table A-7). We restricted the cohort to persons more like clinical trial participants so that it included persons with no use of clopidogrel prior to the qualifying hospitalization who had clopidogrel started during their hospital stay and assessed the first year of followup for such patients, the period during which the benefit of clopidogrel is most well defined(18). The serious cardiovascular disease hazard ratios for all PPIs and pantoprazole were 0.91 (CI, 0.70 to 1.19) and 1.02 (CI, 0.71 to 1.46), respectively. For this group, we performed two additional analyses. First, time-dependent covariates (which may over adjust for factors on the causal pathway) were removed from regression models, thus fixing PPI use status on the first day of study follow-up (like intention-to-treat in clinical trials). Second, follow-up was censored when PPI use status changed (like efficacy analyses in clinical trials). Neither analysis found evidence of increased risk of serious cardiovascular disease, either for all PPIs or pantoprazole. We also modified cohort inclusion/exclusion criteria and endpoint definitions to make our study more comparable to the recent Veterans Affairs hospitals study that reported increased cardiovascular risk for concurrent PPI use(11). The hazard ratio for serious cardiovascular disease was 1.03 (CI, 0.68 to 1.56) for all PPIs and 1.04 (CI, 0.67 to 1.61) for pantoprazole.
Tradeoffs of Gastroduodenal Benefits and Cardiovascular Risks
Table 2 models the potential clinical tradeoff between the gastroduodenal benefits and cardiovascular risks of concurrent clopidogrel and PPI use. Given our estimate of the hazard ratio for serious cardiovascular disease less than 1, prescribing a PPI is always beneficial, preventing as many as 3 to 28 (per 1000 person-years) gastroduodenal bleeding hospitalizations, with benefit increasing according to baseline bleeding risk. In a “worst-case” scenario, in which the hazard ratio for serious cardiovascular disease is 1.19 (the upper bound of our study’s confidence interval), the risks of PPI use (adverse cardiovascular events) could exceed the benefits (prevented bleeds) in some patient groups. In this “worst case” scenario, a PPI is beneficial (assuming equal severity for both types of events) for patients with low or moderate cardiovascular risk and high gastrointestinal bleed risk.
Table 2.
Gastroduodenal (GD) bleed hospitalizations prevented versus cases of serious cardiovascular disease (CVD) caused under two scenarios. The “base case” assumes no adverse cardiovascular effect for PPIs, suggest by point estimate from study. The “worst case” assumes PPIs increase CV events by 19%, the upper bound of the study 95% CI for the CVD effect.
| Gastroduodenal bleed hospitalization rate, absent PPI* | ||||||
|---|---|---|---|---|---|---|
| Low: 7/1000 | Moderate: 12/1000 | High: 47/1000 | ||||
| Bleeds prevented | CV events caused | Bleeds prevented | CV events caused | Bleeds prevented | CV events caused | |
| Base case: | ||||||
| 3.5 | 0 | 6.0 | 0 | 28.5 | 0 | |
| Worst case: | ||||||
| CVD rate absent PPI** | ||||||
| Low: 30/1000 | 3.5 | 5.7 | 6.0 | 5.7 | 28.5 | 5.7 |
| Moderate: 50/1000 | 3.5 | 9.5 | 6.0 | 9.5 | 28.5 | 9.5 |
| High: 150/1000 | 3.5 | 28.5 | 6.0 | 28.5 | 28.5 | 28.5 |
The 12/1000 rate is that for the entire cohort, nonusers of PPIs. The 7/1000 and 47/1000 correspond to those for nonusers of PPIs with 1 or fewer or 3 or more GD bleeding risk factors. The number of hospitalizations prevented assumes a PPI relative risk of 0.50, the point estimate from the study.
The 50/1000 rate is that for all cohort members with PCI and stent implantation. The 30/1000 rate corresponds to those for cohort members who were <50 years of age with unstable angina only and no revascularization; the 150/1000 to cohort members >65 years of age with an acute myocardial infarction.
Discussion
Use of clopidogrel in clinical practice is complicated by the frequent occurrence of serious bleeding, commonly at gastroduodenal sites. We found that concurrent users of clopidogrel and a PPI had 50% fewer gastroduodenal bleeding hospitalizations than did current users of clopidogrel alone. The protective effect of PPIs appeared specific for bleeding at gastroduodenal sites, and the absolute magnitude of the protective effect increased with increasing numbers of bleeding risk factors.
Concurrent PPI use was not associated with a statistically significant increased risk of serious cardiovascular disease, either for the entire cohort or for patients having a percutaneous coronary intervention with stenting. Of note, however, the upper bounds of the confidence intervals for these estimates were compatible with a possible increased cardiovascular risk associated with concurrent PPI use. A “worst-case” analysis that assumed the hazard ratio for serious cardiovascular disease associated with concurrent PPI use was 1.19 (the upper bound of the study confidence interval) suggested that the risks of PPI use (adverse cardiovascular events) could exceed the benefits (prevented bleeds) in patient groups with high cardiovascular risk and low gastrointestinal bleed risk.
Our findings seemingly differ from those of two of three other observational studies found through a English language MEDLINE literature search in August 2009. One of these studies involved Canadian patients hospitalized with acute myocardial infarctions(10) while another included patients with acute coronary syndrome admissions to U.S. Veterans Affairs hospitals(11). In those studies, concurrent PPI users had greater risk for adverse cardiovascular outcomes than did users of clopidogrel alone. However, a cohort analysis of data from the TRITON trial of clopidogrel versus prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary interventions did not find increased risk for PPIs(19).
Differences in the pharmacokinetic properties of the PPIs most commonly used in the study cohorts may partially explain differences in findings. Omeprazole accounted for at least 60% of PPI use in the Veterans Affairs study(11), 37% of use in the TRITON study(19), and only 9% of use in our cohort. It is a potent inhibitor of CYP2C19 both in vitro and in vivo(22,23) and markedly inhibits clopidogrel’s anti-platelet activity(9). Pantoprazole, which accounted for 62% of PPI use in our study and 40% in the TRITON study, does not inhibit CYP2C19(23,36,37) and may not affect clopidogrel’s anti-platelet activity(38). In the Canadian study, it accounted for approximately 30% of PPI use and was not associated with increased cardiovascular risk (10). Other PPIs (29% of use in our Tennessee cohort) have some CYP2C19 inhibitory effect in vitro(37) but have not been shown to materially affect clopidogrel’s anti-platelet activity(38,39).
Several limitations of our study merit discussion, including residual confounding, misclassification of both medication exposure and clinical endpoints, and possible hospital effects not accounted for in the analysis. Although the study data included multiple variables that reflected both bleeding and cardiovascular risk, these were obtained retrospectively and inevitably will fail to capture some confounders. Given the probable channeling of PPIs to higher risk patients, the resulting bias is likely to cause underestimation of gastroduodenal benefits and overestimation of cardiovascular risk. Medication exposure, determined from computerized records of dispensed prescriptions (20,40–42), did not reflect patient adherence or over-the-counter medication use and thus is subject to misclassification that most probably would bias to the null. Similarly, misclassification of both gastroduodenal and cardiovascular endpoints identified from computer case definitions would bias to the null. Finally, many patients entered the cohort from hospitals that contributed relatively few cohort members. Thus, the analysis relied on exchangeability assumptions: that after adjusting for observed covariates, PPI users from one such hospital could be compared with nonusers from a different hospital.
In conclusion, in this large cohort of clopidogrel users hospitalized with serious coronary artery disease, concurrent PPI users had 50% fewer hospitalizations for gastroduodenal bleeding than did nonusers of PPIs. Patients with multiple risk factors associated with gastrointestinal bleeding had a large absolute reduction in such hospitalizations. Concurrent use of a PPI, which in this population was predominantly pantoprazole, was not associated with a statistically significant increased risk of serious cardiovascular disease though the upper bound of the confidence interval for that estimate did not rule out the possibility of such harm. Data from additional studies including trials will be important to clarify precise estimates of effects of concurrent PPI use on cardiovascular outcomes.
Supplementary Material
Acknowledgments
Support: Agency for Healthcare Research and Quality, Centers for Education and Research on Therapeutics cooperative agreement (grant # HS1-16974); National Heart Lung and Blood Institute (grant # HL081707).
We gratefully acknowledge the Tennessee Bureau of TennCare and Department of Health, which provided study data.
Reference List
- 1.Gurbel PA, Lau WC, Tantry US. Omeprazole. A possible new candidate influencing the antiplatelet effect of clopidogrel. J Am Coll Surg. 2008;51:261–63. doi: 10.1016/j.jacc.2007.07.090. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Fox KAA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Eng J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FKL, Furberg CD, et al. ACCF/ACG/AHA 2008 Expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008 Oct;15:1–16. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 5.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–47. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 6.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:1–9. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 7.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:1–13. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 8.Yu KS, Yim DS, Cho JY, Park SS, Park JY, Lee KH, et al. Effect of omeprazole on the pharmacokinetics of moclobemide according to the genetic polymorphism of CYP2C19. Clin Pharmacol Therap. 2001;69:266–73. doi: 10.1067/mcp.2001.114231. [DOI] [PubMed] [Google Scholar]
- 9.Gilard M, Arnaud B, Cornily JC, LeGal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin. J Am Coll Cardiol. 2008;51:256–60. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 10.Juurlink DN, Gomes T, Ko DT, Szmitko PE, Austin PC, Tu JV, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. Can Med Assoc J. 2009;180:1–5. doi: 10.1503/cmaj.082001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–44. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 12.Cryer B. Reducing the risks of gastrointestinal bleeding with antiplatelet therapies. N Engl J Med. 2005;352:287–89. doi: 10.1056/NEJMe048330. [DOI] [PubMed] [Google Scholar]
- 13.Ray WA, Chung CP, Stein CM, Smalley WE, Arbogast PG, Griffin MR. Risk of peptic ulcer hospitalizations in users of NSAIDs with gastroprotective cotherapy versus coxibs. Gastroenterol. 2007;133:790–798. doi: 10.1053/j.gastro.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Lanas A, Garcia-Rodriguez LA, Arroyo MT, Bujanda L, Gomollon F, Forne M, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507–15. doi: 10.1111/j.1572-0241.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Chin MWS, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: A case-control study. Am J Gastroenterol. 2007;102:2411–16. doi: 10.1111/j.1572-0241.2007.01460.x. [DOI] [PubMed] [Google Scholar]
- 16.Kwang Chae Y. Adverse outcomes associated with use of proton pump inhibitors and clopidogrel. JAMA. 2009;302:29. doi: 10.1001/jama.2009.896. [DOI] [PubMed] [Google Scholar]
- 17.Schneider-Linder V. Adverse outcomes associated with use of proton pump inhibitors and clopidogrel. JAMA. 2009;302:29–30. doi: 10.1001/jama.2009.897. [DOI] [PubMed] [Google Scholar]
- 18.Eshaghian S, Kaul S, Amin S, Shah P, Diamond GA. Role of clopidogrel in managing atherothrombotic cardiovascular disease. Ann Intern Med. 2007;146:434–41. doi: 10.7326/0003-4819-146-6-200703200-00008. [DOI] [PubMed] [Google Scholar]
- 19.O’Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;381:1–7. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 20.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–49. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- 21.Ray WA. Population-based studies of adverse drug effects. N Engl J Med. 2003;349:1592–94. doi: 10.1056/NEJMp038145. [DOI] [PubMed] [Google Scholar]
- 22.Freedman JE, Hylek EM. Clopidogrel, genetics, and drug responsiveness. N Engl J Med. 2009;360:411–13. doi: 10.1056/NEJMe0810513. [DOI] [PubMed] [Google Scholar]
- 23.Gerson LB, Triadafilopoulos G. Proton pump inhibitors and their drug interactions: an evidence-based approach. Eur J Gastroenterol Heaptol. 2001;13:611–16. doi: 10.1097/00042737-200105000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Choma NN, Griffin MR, Huang RL, Mitchel EF, Jr, Kaltenbach LA, Gideon P, et al. An algorithm to identify incident myocardial infarction using medicaid data. Pharmacoepidemiology Drug Safety. 2009 doi: 10.1002/pds.1821. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahi CN, Rathore SS, Wang Y, Thakur R, Wu WC, Lewis JM, et al. Quality of care among elderly patients hospitalized with unstable angina. Am Heart J. 2001;142:263–70. doi: 10.1067/mhj.2001.116477. [DOI] [PubMed] [Google Scholar]
- 26.Kahrilas PJ. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700–1707. doi: 10.1056/NEJMcp0804684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–62. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Ray WA, Stein CM, Hall K, Daugherty JR, Griffin MR. Non-steroidal anti-inflammatory drugs and the risk of serious coronary heart disease. Lancet. 2002;359:118–23. doi: 10.1016/S0140-6736(02)07370-1. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA, Stein M, Daugherty JR, Hall K, Arbogast P, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071–73. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 30.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmaco Drug Safety. 2008;17:20–26. doi: 10.1002/pds.1518. [DOI] [PubMed] [Google Scholar]
- 32.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. 1. London: Arnold; 2000. [Google Scholar]
- 33.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic & Clinical Pharmacology & Toxicology. 2006;98:253–59. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Diaz S, Garcia-Rodriguez LA. Epidemiologic assessment of the safety of conventional nonsteroidal anti-inflammatory drugs. Am J Med. 2001;110:20S–7S. doi: 10.1016/s0002-9343(00)00682-3. [DOI] [PubMed] [Google Scholar]
- 36.Gugler R, Hartmann M, Rudi J, Brod I, Huber R, Steinijans VW, et al. Lack of pharmacokinetic interaction of pantoprazole with diazepam in man. Br J Clin Pharmacol. 1996;42:249–52. doi: 10.1046/j.1365-2125.1996.40619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansorprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–27. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 38.Siller-Matula J, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J. 2009;157:E1–E5. doi: 10.1016/j.ahj.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Small DS, Farid NA, Payne CD, Weerakkody GJ, Li YG, Brandt JT, et al. Effects of the proton pump inhibitor lansoprozole on the pharmacokinetics and pharmacodynamics of prasurgrel and clopidogrel. J Clin Pharmacol. 2008;48:475–84. doi: 10.1177/0091270008315310. [DOI] [PubMed] [Google Scholar]
- 40.Landry JA, Smyer MA, Tubman JG, Lago DJ, Roberts J, Simonson W. Validation of two methods of data collection of self-reported medicine use among the elderly. Gerontologist. 1988;28:672–76. doi: 10.1093/geront/28.5.672. [DOI] [PubMed] [Google Scholar]
- 41.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–10. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 42.Johnson RE, Vollmer WM. Comparing sources of drug data about the elderly. J Am Geriatr Soc. 1991;39:1079–84. doi: 10.1111/j.1532-5415.1991.tb02872.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.