Abstract
A series of cationic gold(I) complexes containing 1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea (1), [AuL(1)]n+ (where L is Cl−, Br-, SCN−, PEt3, PPh3, or 1), derived from a class of analogous platinum(II) antitumor agents, has been synthesized. Unlike platinum, gold does not form permanent adducts with DNA, and its complexes are two orders of magnitude less cytotoxic in non-small-cell lung cancer cells than the most active platinum-based agent. Instead, several gold analogues show submicromolar and selective antimicrobial activity against Mycobacterium tuberculosis.
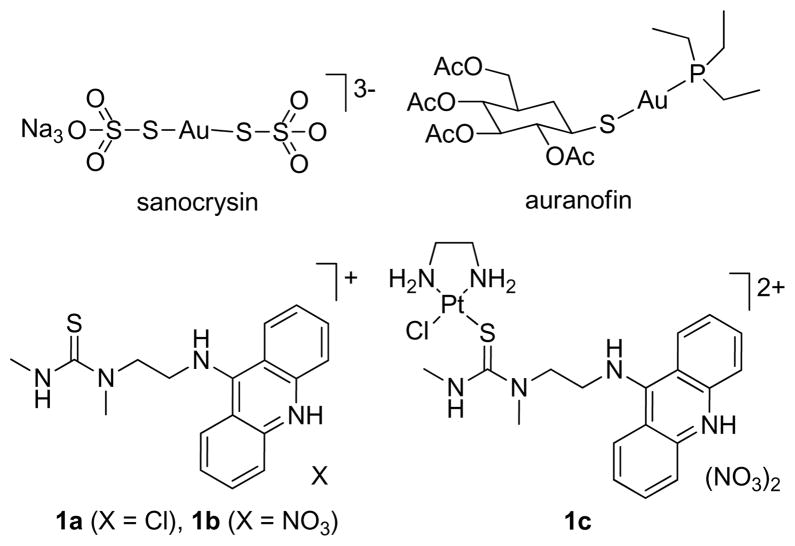
The growing incidence of drug resistance in life-threatening malignant neoplastic and infectious diseases has resulted in a pressing need for more effective chemotherapies.1, 2 Metallopharmaceuticals have enormous potential to help overcome the limitations of current therapies by virtue of their unique geometries and chemical reactivities.3 Gold(I)-based coordination compounds have been a major focus area in inorganic drug design since Robert Koch’s discovery of the bacteriostatic effects of dicyanoaurate(I), [Au(CN)2]−.4, 5 Complexes such as sodium bis(thiosulfato-S)aurate(I) (sanocrysin, Chart 1) have shown considerable promise as antimicrobial treatments for Mycobacterium tuberculosis (Mtb), the pathogen responsible for tuberculosis (TB).4 However, the high systemic toxicity of this agent, the lack of a statistically significant antitubercular effect at tolerable doses, and shortcomings in the design and evaluation of early clinical trials resulted in a complete abandonment of Mtb-directed gold therapy.4 On the other hand, the orally active drug triethylphosphine(2,3,4,6-tetra-O-acetyl-β-1-D-thiopyranosato-S)gold(I) (auranofin, Chart 1) has become an established second-line therapy in the treatment of severe rheumatoid arthritis and other autoimmune disorders.6 Recently, several classes of gold(I) complexes have been reported that efficiently inhibit cancer cell growth, most likely by interfering with mitochondrial thioredoxin reductase (TrxR), suggesting possible applications of gold(I) in oncology.7–9
Chart 1.
As part of a drug discovery approach targeted at chemoresistant cancers, a class of dual DNA platinating–intercalating hybrid agents has been developed that induce DNA adducts unlike those formed by cisplatin-type drugs. The prototypical agent derived from the DNA-affinic carrier ligand 1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea in its protonated form (1), [PtCl(en)(1)](NO3)2 (1c),10 contains a monofunctional platinum moiety linked to thiourea sulfur (Chart 1). The most active derivatives in this class have shown inhibition of cell proliferation at nanomolar concentrations and promising antitumor activity in mouse xenograft models.11
In ongoing structure–activity relationship (SAR) studies, systematic structural changes are made to critical parts of the molecule. The most radical structural alteration made to date is the replacement of the square planar platinum(II) moiety with linear gold(I). Here, we report the discovery that while this modification results in a severe loss of cytotoxicity in mammalian cells, several of the new complexes show potent and selective activity against Mtb.
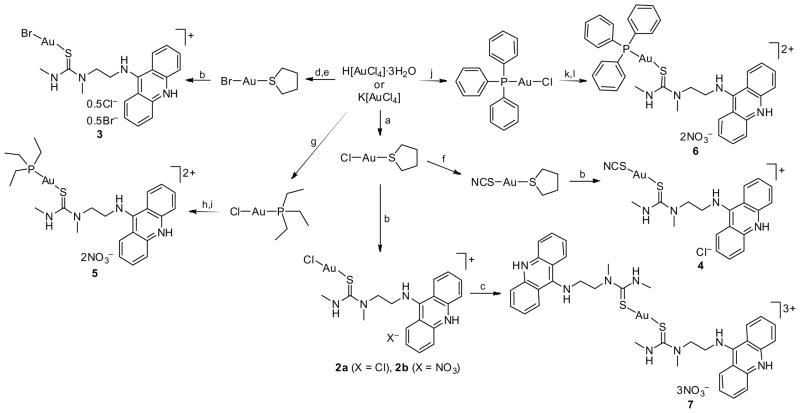
A set of cationic complexes [Au(1)L]n+ was developed, in which gold is linearly coordinated by the thiourea sulfur of the acridine ligand and a trans-ligand L, including (pseudo)halides, phosphines, or a second equivalent of acridine 1. The synthesis of the (pseudo)halo derivatives, [AuCl(1)]X (2a, X = Cl−; 2b, X = NO3−), [AuBr(1)]Br/Cl (3), and [AuSCN(1)]Cl (4) succeeded under mild conditions and with 70–80% yields via selective substitution of tetrahydrothiophene (tht) by acridinylthiourea 1 in the appropriate precursor complexes, [AuL(tht)] (Scheme 1). To generate the phosphine-modified complexes, [Au(1)PEt3](NO3)2 (5) and [Au(1)PPh3](NO3)2 (6), an alternate route was chosen, which exploited the lability of oxygen donors attached to gold(I) in addition to the trans-labilizing properties of the phosphorous ligands. The procedure involved removal of the chloro ligands in the appropriate precursor complexes [AuCl(PR3)] using silver nitrate, and subsequent reaction of the labile nitrato complexes (generated in situ) with acridine 1b (Scheme 1). It was necessary to perform reactions under argon and at low temperature to avoid unwanted redox chemistry and minimize the formation of elemental gold(0) subsequent to chloride abstraction. Finally, thallium nitrate was used to activate the chloro complex 2b for reaction with a second equivalent of 1b to form [Au(1)2](NO3)3 (7) (Scheme 1). Target complexes 2–7 were fully characterized by NMR and IR spectroscopies as well as elemental analyses. In addition, the molecular structures of 2–5 and 7 in the solid state were determined by single-crystal X-ray crystallography (Supporting Information). The solid state structures and IR data provide insight into the effect of the ligands L on the Au–S linkage. The trans-influence of L has a significant effect on the Au–Sthiourea coordinative bond. Bond lengthening increases in the following order: Cl− ≈ Br− < SCN− < 1-S < PR3, which correlates well with π-acceptor properties of the ligands (see Supporting Information for details). A recent study showed that the reactivity of complexes [AuCl(PR3)] with sulfur of the cysteine protease cathepsin can be tuned by changing the donor/acceptor properties of the phosphine ligands.12, 13 Similarly, the electronic effects of the ligand combinations used in complexes 2–7 may provide a means of modulating the reactivity of the metal in reactions with biological nucleophiles.
Scheme 1.
Synthesis of Gold–Acridine Derivatives 2–7.a
a Reagents and conditions: (a) Tht, EtOH/H2O, 15 min, rt; (b) 1a or 1b, MeOH, 1 h, rt; (c) 1b, 2b, TlNO3, DMF, 16 h, rt; (d,e) 1. excess 47% HBr, NaOH, 5 d, 2. tht, MeOH, 1 h; (f) KSCN, H2O/CH2Cl2, 3 h, rt; (g) PEt3, EtOH, 1 h, rt, argon; (h,i) 1. AgNO3, DMF, 18 h, −45 °C, 2. 1b, DMF, 4 h, −45 → 25 °C; (j) PPh3, MeOH, 8 h, rt, argon; (k,l) 1. AgNO3, DMF, 18 h, −45 °C, 2. 1b, DMF, 4 h, −45 → 25 °C.
Complexes 2–7, along with the metal-free acridine 1, were tested for their potential to produce a cytotoxic effect in the NCI-H460 non-small-cell lung cancer cell line using a colorimetric cell proliferation assay. All compounds show moderate activity with IC50 values in the micromolar range, with only little variation across the set of complexes, which proved to be only slightly more cytotoxic than the metal-free acridine 1b by approximately 2–3-fold (Table 1). A similar effect was observed in the human leukemia cell line, HL-60 (not shown). This contrasts the situation for conjugate 1c, which exhibits a 30–40-fold cytotoxic enhancement compared to 1b, rendering the platinum analogue an order of magnitude more active than the gold complexes in the lung cancer cell line.14, 15
Table 1.
Antimycobacterial Activity and Cytotoxicity of Compounds 1–7
| compd | IC50a H37Rvb |
IC90 H37Rv |
IC50 Veroc |
selectivity index (SI)d | IC50 H460e |
|---|---|---|---|---|---|
| 1b | 59.97 | 94.20 | 30.00 | 0.318 | 6.376±0. |
| 2a | 0.490 | 0.704 | 11.70 | 16.61 | 3.080±0. |
| 3 | < 0.290 | 0.409 | 7.070 | 17.28 | 3.077±0. |
| 4 | 0.652 | 1.141 | 27.01 | 23.66 | 3.940±0. |
| 5 | 0.645 | 1.050 | 5.233 | 4.983 | 2.376±0. |
| 6 | 0.817 | 1.204 | 8.803 | 7.310 | 1.763±0. |
| 7 | > 100 | > 100 | > 30 | N/A | 2.298±0. |
Inhibitory concentrations (IC) for antibacterial activity and cell cytotoxicity are given in μM.
Determined in Mtb (H37Rv strain) in a high-throughput screen by TAACF.
Determined in kidney epithelial cells (Vero line) by TAACF.
SI = IC50(Vero)/IC90(H37Rv).
Determined in NCI-H460 non-small cell lung cancer using colorimetric cell proliferation assays. Values are the mean of four independent experiments ± S.E.M.
To assess the utility of compounds 1–7 as selective antimicrobial agents, an initial high-throughput screen was conducted against Mtb (H37Rv strain) by the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) of the National Institute of Allergy and Infectious Diseases (NIAID). In parallel, the mammalian cell cytotoxicity of 1–7 was determined in kidney epithelial cells (Vero line) using a cell viability assay. Acridine 1b was inactive in H37Rv, based on inhibitory concentrations in the high micromolar range. Likewise, the compound showed relatively low cytotoxicity in Vero cells, but was slightly more toxic in mammalian cells than in Mtb (SI < 1; Table 1). Modification of thiourea sulfur in acridine 1 with a (pseudo)halogold(I) or phosphinegold(I) moiety, giving complexes 2–6, resulted in a dramatic enhancement of the antimycobacterial activity, with IC50 and IC90 values in the low-micromolar to submicromolar range (Table 1). All complexes in the above series were cytotoxic in Vero cells at considerably higher inhibitory concentrations than those determined in the Mtb strain. While the bromo complex 3 showed the best antimycobacterial activity, the thiocyanato complex 4 proved to be the least cytotoxic and the most Mtb-selective complex. In contrast, the phosphine-substituted analogues, 5 and 6, were less selective for Mtb, and the bisacridine complex 7 showed no activity in Mtb and, likewise, was noncytotoxic to Vero cells.
The current study demonstrates that replacement of monofunctional platinum(II) in conjugate 1c with a gold(I) moiety leads to greatly reduced toxicity in mammalian cells. In H460 non-small-cell lung cancer, the most potent platinum–acridine derivative identified to date11 proves to be two orders of magnitude more active than the gold analogues. Complex 1c and its derivatives were designed as DNA-targeted hybrid anticancer agents. In previous SAR studies, a correlation was established between a specific derivative’s ability to form permanent adducts with DNA nucleobases and its antiproliferative potential. Complexes that were shown to react sluggishly with DNA, or not at all, because of unfavorable sterics and/or electronics of the metal center, produced poor biological activity, indicating that intercalation alone is only moderately cytotoxic.16, 17 These findings suggest that rapid, irreversible DNA binding by this type of agent is a prerequisite for stalling rapid cell proliferation. To test the hypothesis that inefficient DNA metalation by the gold analogues might contribute to their relatively poor activity in cancer cells, we studied the interactions of complexes 2a, 4, and 5 with negatively supercoiled plasmid DNA. Gel mobility shift assays performed with the three compounds indicate that Au(I), unlike Pt(II),18 is unable to form permanent adducts with DNA nitrogen (see Supporting Information). Likewise, attempts to detect intact gold(I)–nucleobase adducts in digested samples of DNA treated with complex 2a were unsuccessful (unreported data). Previous DNA binding studies of gold(I) complexes have shown some degree of association of the metal with DNA constituents, but the coordinative bonds formed appear to be relatively labile.19, 20 These observations reinforce evidence that the cytotoxic effect of gold(I) complexes is not triggered at the nuclear DNA level, but is rather a consequence of mitochondrial poisoning.21 While contributions from non-DNA mediated mechanisms to the cytotoxicity of the gold–acridines cannot be ruled out, they would appear less efficient than platinum-mediated DNA damage.
Complexes 2a–6 are remarkably strong inhibitors of Mtb and are the only cases of water-soluble, cationic gold(I) complexes active against this pathogen reported thus far. It is noteworthy to mention that the 1+ charged complexes 2a–4 showed better selectivity for Mtb than the 2+ charged complexes 5 and 6 (see SI values in Table 1), which may indicate differential complex uptake by Mtb and mammalian cells, in addition to differences in complex reactivity. Compound 7, which contains two protonable acridine moieties, producing the highest (formally 3+) charged complex in this series, turns out to be the least active compound. It is unclear if this effect is caused by the excessive cationic charge, which may prevent efficient cross-membrane transport of gold, or altered reactivity due to the trans-thiourea sulfur ligation.
While details of the metabolism and possible pharmacological targets of antiarthritic gold(I) complexes are now emerging,12 the molecular basis for the antimicrobial effects of this metal remains elusive. In the present system, both the metal and the nature of the donor set appear to be important for antimycobacterial activity and selectivity. Based on the preliminary SAR and DNA binding data generated in this study, bacterial DNA would appear an unlikely target of the gold complexes. Alternatively, their mechanism of action may involve specific or nonspecific metalation of cysteine-containing enzymes and proteins, the classical targets of thiophilic gold(I), although (less common) binding to nitrogen-containing amino acid residues is also a possibility.22 The potential roles of acridine 1 as a carrier ligand for gold delivery or as a targeting agent depend on the ligand exchange chemistry in these complexes, which has yet to be investigated.
Compound 4, one of the three complexes characterized by a SI of greater than 10, the current criterion for further testing by the TAACF, was chosen for in vivo evaluation. Serum samples collected from mice treated at a maximum tolerated dose (MTD) of 300 mg/kg administered via oral gavage did not inhibit Mtb, which may indicate limited oral bioavailability of the complex. Future SAR studies and pharmacokinetic assays will address the relevance of the nature of the ligand set to improve Mtb selectivity and intestinal absorption of gold(I) in this system. In conclusion, the current set of complexes shows considerable potential as relatively nontoxic anti-Mtb agents. Given the urgent need for effective treatment options for multi-drug resistant forms of TB, novel gold(I) complexes based on improved prodrug design and delivery may represent a promising approach to combating this disease.
Supplementary Material
Acknowledgments
We thank Dr. Sam Ananthan (TAACF, Southern Research Institute, Birmingham, Alabama) and Dr. Scott G. Franzblau (Institute for Tuberculosis Research, University of Illinois at Chicago) for helpful discussions. This work was supported, in part, by a grant from the National Institutes of Health (CA101880).
Abbreviations
- Mtb
Mycobacterium tuberculosis
- SI
selectivity index
- TB
tuberculosis
- TrxR
thioredoxin reductase
Footnotes
Supporting Information Available: Details of experimental procedures and compound characterization, 1H NMR spectra of complexes, thermal ellipsoid crystallographic plots for complexes 2a–5, and 7, and results of the DNA binding experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Payne DJ. Microbiology. Desperately seeking new antibiotics. Science. 2008;321:1644–1645. doi: 10.1126/science.1164586. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S, Isab AA, Ali S, Al-Arfaj AR. Perspectives in bioinorganic chemistry of some metal based therapeutic agents. Polyhedron. 2006;25:1633–1645. [Google Scholar]
- 4.Benedek TG. The history of gold therapy for tuberculosis. J Hist Med Allied Sci. 2004;59:50–89. doi: 10.1093/jhmas/jrg042. [DOI] [PubMed] [Google Scholar]
- 5.Shaw IC. Gold-based therapeutic agents. Chem Rev. 1999;99:2589–2600. doi: 10.1021/cr980431o. [DOI] [PubMed] [Google Scholar]
- 6.Tiekink ER. Phosphinegold(I) thiolates - pharmacological use and potential. Bioinorg Chem Appl. 2003:53–67. doi: 10.1155/S1565363303000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickey JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price SJ, Filipovska A. Mitochondria-targeted chemotherapeutics: the rational design of gold(I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J Am Chem Soc. 2008;130:12570–12571. doi: 10.1021/ja804027j. [DOI] [PubMed] [Google Scholar]
- 8.Baker MV, Barnard PJ, Berners-Price SJ, Brayshaw SK, Hickey JL, Skelton BW, White AH. Cationic, linear Au(I) N-heterocyclic carbene complexes: synthesis, structure and anti-mitochondrial activity. Dalton Trans. 2006:3708–3715. doi: 10.1039/b602560a. [DOI] [PubMed] [Google Scholar]
- 9.Ott I, Qian X, Xu Y, Vlecken DH, Marques IJ, Kubutat D, Will J, Sheldrick WS, Jesse P, Prokop A, Bagowski CP. A gold(I) phosphine complex containing a naphthalimide ligand functions as a TrxR inhibiting antiproliferative agent and angiogenesis inhibitor. J Med Chem. 2009;52:763–770. doi: 10.1021/jm8012135. [DOI] [PubMed] [Google Scholar]
- 10.Martins ET, Baruah H, Kramarczyk J, Saluta G, Day CS, Kucera GL, Bierbach U. Design, synthesis, and biological activity of a novel non-cisplatin-type platinum-acridine pharmacophore. J Med Chem. 2001;44:4492–4496. doi: 10.1021/jm010293m. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z, Choudhury JR, Wright MW, Day CS, Saluta G, Kucera GL, Bierbach U. A non-cross-linking platinum-acridine agent with potent activity in non-small-cell lung cancer. J Med Chem. 2008;51:7574–7580. doi: 10.1021/jm800900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunatilleke SS, Barrios AM. Inhibition of lysosomal cysteine proteases by a series of Au(I) complexes: a detailed mechanistic investigation. J Med Chem. 2006;49:3933–3937. doi: 10.1021/jm060158f. [DOI] [PubMed] [Google Scholar]
- 13.Gunatilleke SS, Barrios AM. Tuning the Au(I)-mediated inhibition of cathepsin B through ligand substitutions. J Inorg Biochem. 2008;102:555–563. doi: 10.1016/j.jinorgbio.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Guddneppanavar R, Saluta G, Kucera GL, Bierbach U. Synthesis, biological activity, and DNA-damage profile of platinum-threading intercalator conjugates designed to target adenine. J Med Chem. 2006;49:3204–3214. doi: 10.1021/jm060035v. [DOI] [PubMed] [Google Scholar]
- 15.Hess SM, Mounce AM, Sequeira RC, Augustus TM, Ackley MC, Bierbach U. Platinum-acridinylthiourea conjugates show cell line-specific cytotoxic enhancement in H460 lung carcinoma cells compared to cisplatin. Cancer Chemother Pharmacol. 2005;56:337–343. doi: 10.1007/s00280-004-0987-7. [DOI] [PubMed] [Google Scholar]
- 16.Ackley MC, Barry CG, Mounce AM, Farmer MC, Springer BE, Day CS, Wright MW, Berners-Price SJ, Hess SM, Bierbach U. Structure-activity relationships in platinum-acridinylthiourea conjugates: effect of the thiourea nonleaving group on drug stability, nucleobase affinity, and in vitro cytotoxicity. J Biol Inorg Chem. 2004;9:453–461. doi: 10.1007/s00775-004-0541-4. [DOI] [PubMed] [Google Scholar]
- 17.Guddneppanavar R, Choudhury JR, Kheradi AR, Steen BD, Saluta G, Kucera GL, Day CS, Bierbach U. Effect of the diamine nonleaving group in platinum-acridinylthiourea conjugates on DNA damage and cytotoxicity. J Med Chem. 2007;50:2259–63. doi: 10.1021/jm0614376. [DOI] [PubMed] [Google Scholar]
- 18.Baruah H, Rector CL, Monnier SM, Bierbach U. Mechanism of action of non-cisplatin type DNA-targeted platinum anticancer agents: DNA interactions of novel acridinylthioureas and their platinum conjugates. Biochem Pharmacol. 2002;64:191–200. doi: 10.1016/s0006-2952(02)01107-3. [DOI] [PubMed] [Google Scholar]
- 19.Mirabelli CK, Sung CM, Zimmerman JP, Hill DT, Mong S, Crooke ST. Interactions of Gold Coordination-Complexes with DNA. Biochem Pharmacol. 1986;35:1427–1433. doi: 10.1016/0006-2952(86)90106-1. [DOI] [PubMed] [Google Scholar]
- 20.Blank CE, Dabrowiak JC. Absorption and Circular-Dichroism Studies of a Gold(I)-DNA Complex. J Inorg Biochem. 1984;21:21–29. doi: 10.1016/0162-0134(84)85036-9. [DOI] [PubMed] [Google Scholar]
- 21.McKeage MJ, Maharaj L, Berners-Price SJ. Mechanisms of cytotoxicity and antitumor activity of gold(I) phosphine complexes: the possible role of mitochondria. Coord Chem Rev. 2002;232:127–135. [Google Scholar]
- 22.Zou J, Taylor P, Dornan J, Robinson SP, Walkinshaw MD, Sadler PJ. First Crystal Structure of a Medicinally Relevant Gold Protein Complex: Unexpected Binding of. Angew Chem Int Ed Engl. 2000;39:2931–2934. doi: 10.1002/1521-3773(20000818)39:16<2931::aid-anie2931>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.