Abstract
We previously used the γ-H2AX assay as a biodosimeter for total-body-irradiation (TBI) exposure (γ-rays) in a rhesus macaque (Macaca mulatta) model. Utilizing peripheral blood lymphocytes and plucked hairs, we obtained statistically significant γ-H2AX responses days after total-body exposure to 1–8.5 Gy (60Co γ-rays at 55 cGy min−1). Here, we introduce a partial-body exposure analysis method, Qγ-H2AX, which is based on the number of γ-H2AX foci per damaged cells as evident by having one or more γ-H2AX foci per cell. Results from the rhesus monkey – TBI study were used to establish Qγ-H2AX dose-response calibration curves to assess acute partial-body exposures. γ-H2AX foci were detected in plucked hairs for several days after in vivo irradiation demonstrating this assay’s utility for dose assessment in various body regions. The quantitation of γ-H2AX may provide a robust biodosimeter for analyzing partial body exposures to ionizing radiation in humans.
Keywords: biological dosimetry, γ-H2AX, Qγ-H2AX, Fγ-H2AX, rhesus macaque (Macaca mulatta), lymphocytes, 60Co γ-rays, ionizing radiation, partial-body exposure
1. Introduction
Measurement of in vivo DNA damage responses to various treatments is providing useful information to clinicians for improving human health. Hundreds of H2AX molecules are rapidly phosphorylated on serine 139 (γ-H2AX) in response to the formation of each DNA double-strand break (DSB) (Rogakou et al., 1999; Rogakou et al., 1998). The most sensitive assays of γ-H2AX utilize immunocyto- and immunohisto-chemistry techniques to stain for γ-H2AX foci, intranuclear structures composed of the hundreds of γ-H2AX molecules, which form at each DSB site in cells and tissues (Bonner et al., 2008). Exposure of humans to ionizing radiation during various radiological diagnostic or therapeutic treatments (Kuefner et al., 2009; Lobrich et al., 2005; Porcedda et al., 2006; Sak et al., 2007) and space travel (Ohnishi et al., 2009) lead to DSB formation that can be measured by counting the γ-H2AX foci in many tissues including lymphocytes, buccal cells, and skin biopsies; see reviews by (Redon et al., 2010b; Rothkamm and Horn, 2009).
Thus, counting γ-H2AX foci in cells of persons exposed to irradiation may be useful in determining the extent of exposure and in providing optimum medical care. In addition to radiation exposure from medical procedures and/or diagnostics, the risk of accidental irradiation to the general public and emergency responders has also increased in recent years. We recently utilized a non-human primate (NHP) model to further validate the γ-H2AX assay following TBI with both non-lethal and lethal radiation doses (Redon et al., 2010a). Measuring γ-H2AX foci in NHP lymphocytes and plucked hairs enabled us to assess the subject’s exposure several days after irradiation at doses in the medical triage range, making γ-H2AX biodosimetry a robust tool for measuring TBI in macaques for several days after irradiation.
However, a serious consideration is that many radiation accidents are likely to involve inhomogeneous exposures or partial-body irradiation (PBI) (Prasanna et al.), the extent of which may be unknown both in terms of the fraction of the body irradiated and dose delivered to the irradiated fraction. In cases of acute exposure, the determination that a fraction of the blood volume had escaped irradiation would indicate that a portion of the body, and hence the bone marrow, may have also escaped lethal irradiation. Such knowledge may be critical for guiding decisions on optimal medical treatment for the victim, for example, whether a bone-marrow transplant is necessary for survival.
A method (Qdr) to evaluate PBI in individuals was originally demonstrated by Sasaki and Miyata (Sasaki and Miyata, 1968) utilizing the frequency of chromosomal aberrations (dicentric and ring chromosomes) in lymphocyte metaphase spreads taken from exposed subjects. It was later adapted to utilize the frequency of chromosome fragments present in lymphocytes subjected to premature chromosome condensation (Blakely et al., 1995; Prasanna, et al.). Here, we extend this method to utilize the levels of γ-H2AX foci in the lymphocyte population of the exposed subject to assess dose and fraction of body exposed to PBI.
We also briefly introduce the use of plucked hairs as a mean to assess PBI. It may take a few minutes for blood to make a complete bodily circuit, hence, the fraction of the blood volume exposed and the dose received by that fraction may differ considerably from that received by other portions of the body, depending in large part on the dose rate and length of exposure. Exposure of sufficient duration to any part of a body could lead to irradiation of the complete blood volume. However, in these cases, measurements of γ-H2AX foci levels in plucked hair bulbs could help determine the extent of partial-body exposure.
2. Materials and Methods
2.1 PBI Calculations
The data used to develop the method for PBI calculations were obtained from a previous study by Redon et al. (2010) using γ-H2AX foci levels in macaque lymphocytes for TBI biodosimetry (Redon, et al., 2010a). This method, based on the Sasaki and Miyata method (Sasaki and Miyata, 1968), may provide useful estimates of the fraction of the body exposed to radiation. Considering a γ-H2AX focus to denote a damaged chromosome, the term Qγ-H2AX, the mean number of γ-H2AX foci per cell in the cells containing foci, ignoring the foci-free cells, can be calculated. Similarly, we use the term Fγ-H2AX to refer to the fraction of lymphocyte population containing damaged cells. Fγ-H2AX and Qγ-H2AX can be calculated with the following formula:
∑fγ-H2AX is the total number of γ-H2AX foci in all cells observed; NγH2AX is the number of damaged cells (with at least one γ-H2AX focus); Nt, the total number of cells observed and N0 is the number of cells without foci.
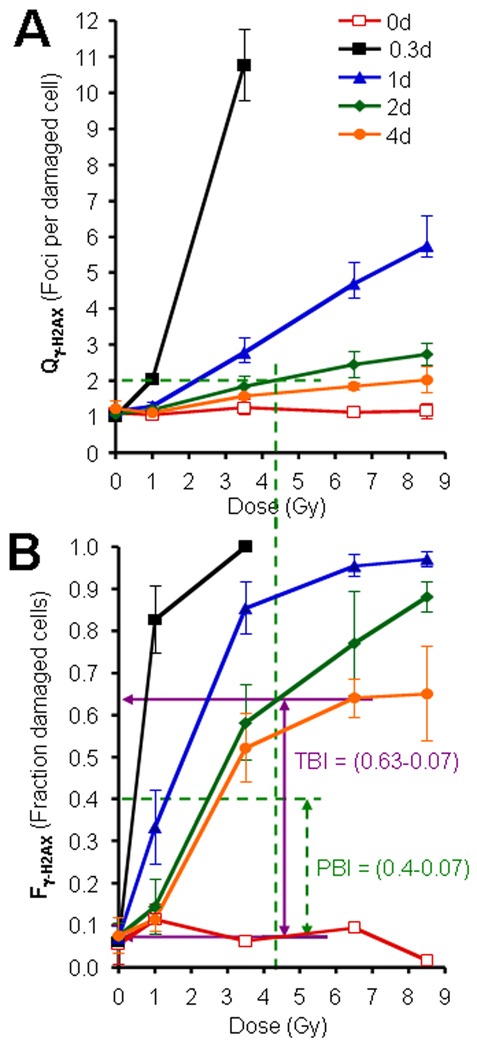
We determined both Fγ-H2AX and Qγ-H2AX values vs doses from the TBI data, values that allowed us to establish standard curves (Fig. 2A and 2B). Following PBI, both experimental Qγ-H2AX and experimental Fγ-H2AX can be determined after γ-H2AX examination in lymphocytes. Both experimental Qγ-H2AX and experimental Fγ-H2AX and the standard curves obtained from TBI will allow the estimate of the irradiation dose and of the fraction of the body that was exposed.
Fig.2. The use of Qγ-H2AX and Fγ-H2AX for partial-body irradiation (PBI).
(A) Graph of Qγ-H2AX (foci per damaged cell) vs TBI exposure. This value approximates the dose received by the lymphocytes in the fraction of the body subjected to irradiation, independent of the size of that fraction. (B) Graph of Fγ-H2AX (fraction of damaged cells) vs TBI exposure. This value is used to approximate the PBI fraction. For example, assume values of Qγ-H2AX = 2, and Fγ-H2AX = 0.4 were calculated from lymphocytes obtained from a subject 2 days after irradiation of unknown amount and bodily distribution. The Qγ-H2AX value indicates that the exposed portion of the subject received ~4.4 Gy (panel A, green dashed lines). If the subject had been exposed to TBI of 4.4 Gy, then an Fγ-H2AX of ~0.63 would be expected in lymphocytes taken 2 days after irradiation (Solid horizontal violet arrow in panel B). Thus, an Fγ-H2AX of ~0.4 (horizontal dashed green line) indicates that the subject received less than TBI. Taking into account the control values, the PBI fraction is estimated as (0.4–0.07)/(0.63–0.07) = ~59% of the body being irradiated. Graph in panel B uses the same legends as Graph in panel A. Errors bars represent standard deviations of the mean (n=6).
As an example, shown graphically in Fig. 2A and 2B and mathematically here, we consider a hypothetical case in which a blood sample collected 2 days after a possible PBI was found to yield the experimental values Qγ-H2AX =2 and Fγ-H2AX = 0.40. Using the standard curve for 2 days after TBI in Fig. 2A, the value of Qγ-H2AX yields an estimated dose of ~4.4 Gy to the exposed portion of the body. In Fig. 2B, a dose of 4.4-Gy TBI would yield an Fγ-H2AX value after 2 days of 0.63, considerably greater than the value in the hypothetical example of 0.4, a difference that would indicate PBI. PBI can be calculated by the ratios after subtracting the background values as follows:
-
-
“Experimental Fγ-H2AX” is determined after lymphocyte examinations (= 0.40)
-
-
“TBI Fγ-H2AX”” is determined using the standard curve Fγ-H2AX vs dose (Fig. 2B) (= 0.63)
-
-
0.07 is the Fγ-H2AX value in sham-irradiated macaques.
2.2 γ-H2AX detection in plucked hairs
Plucked hairs preparation for immunohistochemistry and γ-H2AX detection were performed as previously described (Redon, et al., 2010a).
3. Results
3.1 γ-H2AX foci in lymphocytes for acute partial-body irradiation (PBI) biodosimetry
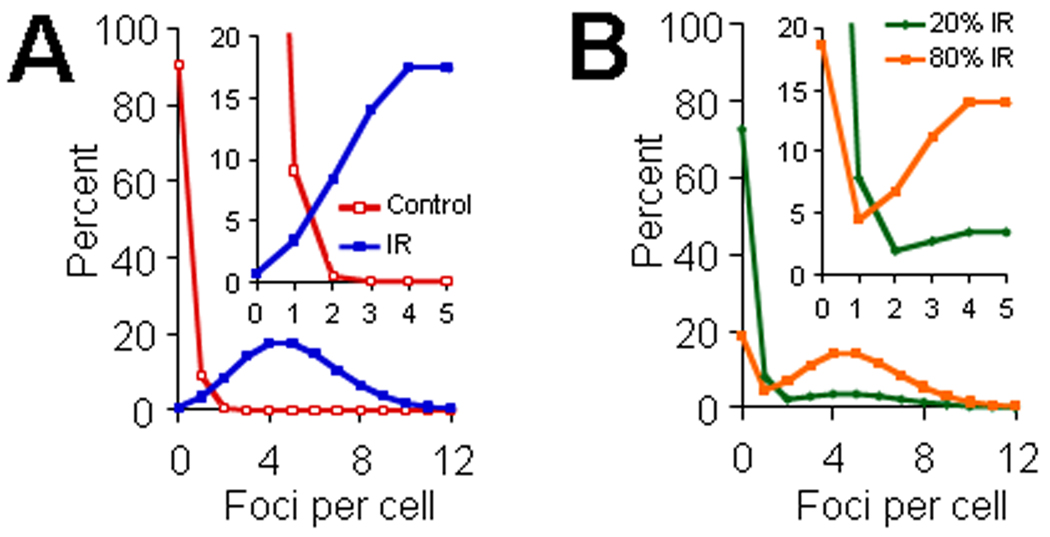
The method utilizing γ-H2AX foci distribution frequencies is modeled for TBI and simulated PBI in Fig. 1A. In an idealized acute PBI scenario, the lymphocyte population would have two components, one from the irradiated fraction exhibiting cells with foci (Fig 1A, blue line), and the other from the unirradiated fraction exhibiting cells with few if any foci (Fig 1A, red line). The fraction of each of these two components represented in the lymphocyte population would depend on the fraction of the blood volume irradiated. Figure 1B presents two modeled situations, one with 80% PBI (80% of cells from TBI samples and 20% of cells from unirradiated samples) and 20% PBI (20% of cells from TBI samples and 80% of cells from unirradiated samples). By first focusing on the damaged cell component of the TBI data, the number of foci per damaged cell, named Qγ-H2AX after the Sasaki and Miyata method, can be calculated. This term gives information on the dose received by the exposed body portion, largely independent of the fraction of the body exposed (Fig 2A). Second, the fraction of total cells with foci, Fγ-H2AX, when used in conjunction with Fγ-H2AX calibration curves determined for TBI, gives information on the fraction of the body exposed (Fig 2B). Calibration curves for the application of the Qγ-H2AX and Fγ-H2AX analysis method for the case of TBI are illustrated in Figure 2A and 2B.
Fig.1. Scheme of γ-H2AX foci distributions for TBI and PBI.
(A) The left panel illustrates foci distributions for samples from TBI (blue color) and sham-treatment (red line). Poisson distributions with averages of 0.1 and 5 fpc were used to simulate control and TBI populations. The insert panel in Fig. 1A represents the same data shown in the left panel but magnified to illustrate the distribution pattern for foci numbers less than 6 foci per cell. (B) Right panel illustrates foci distributions in samples from PBI. Two PBI conditions are illustrated based on data shown in Fig. 1A; PBI involving 20% (B, green line) or 80% (B, orange line) of the lymphocyte population irradiated. See manuscript for additional details. The insert panel in Fig. 1B represents the same data shown in the right panel but magnified to illustrate the distribution pattern for foci numbers less than 6 foci per cell.
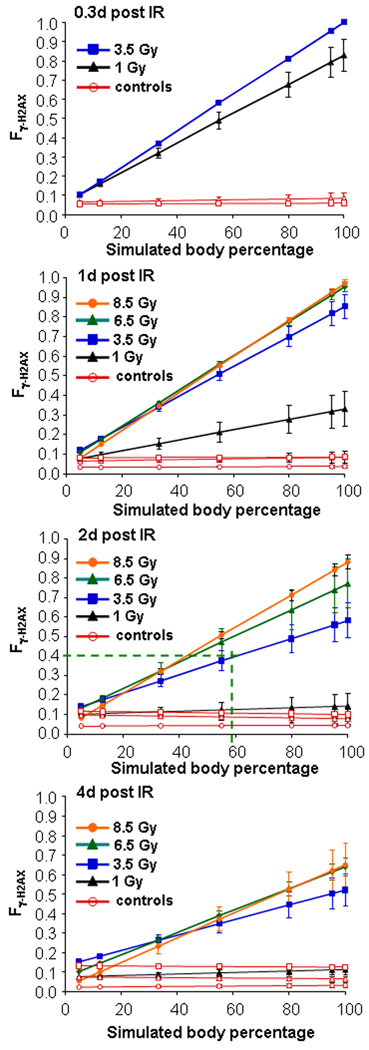
Results from an illustrative example, as described in the Methods paragraph above, is shown graphically utilizing the TBI calibration graphs for Qγ-H2AX and Fγ-H2AX (Figs. 2A and 2B). In this example, blood drawn from a putative acute PBI victim 2 days after exposure yielded values for Qγ-H2AX of 2 foci per cell, and a Fγ-H2AX of 0.4, which is significantly less than that a TBI value of 0.63. The resulting estimate from these input values is that 59% of the body received ~4.4 Gy, and more importantly, 41% of the body was not exposed to radiation (see Materials and Methods paragraph for details of the calculation). To check for internal consistency, computer PBI simulations of γ-H2AX foci distributions for 5, 12.5, 33, 55, 80 and 95% of total-body exposure to doses of 1, 3.5 6.5 and 8.5 Gy at 0.3, 1, 2 and 4 days post exposure were prepared as modeled in Fig. 3A. Fγ-H2AX values were calculated for the simulated γ-H2AX foci distributions and plotted vs the input PBI values (Fig. 3). When the result of the hypothetical example (59% of total body exposed to ~4.4 Gy) is plotted in the “2 d post-irradiation” panel (Fig. 3, 3rd panel from top), an Fγ-H2AX value of 0.4–0.45 is obtained, in good agreement with the input value of 0.40. The 41% of the body that received little or no irradiation exposure may contain sufficient undamaged bone marrow to replenish the blood cell population without the need for a transplant.
Fig.3. Graphs of Fγ-H2AX vs body percentage in a computer simulation of PBI.
To test the consistency of the PBI estimate, various mixtures of total body and sham exposed γ-H2AX foci distributions of lymphocytes were used to simulate situations in which 5, 12.5, 33, 55, 80, and 95% of the body was exposed to irradiation (as demonstrated for 20 and 80% in Fig 1B). Fγ-H2AX values were calculated for these mixtures for the different irradiation exposures and phlebotomy times. In the example given above, on the graph of 2 days post irradiation (third panel from top), the calculated PBI percentage of 59% and the Fγ-H2AX value of 0.4 intersect slightly above the line for 3.5 Gy and below the line for 6.5 Gy, consistent with the estimated value of 4.4 Gy (see text for further explanation). Closed symbols: irradiated macaques; open symbols: sham-irradiated controls. Errors bars represent standard deviations of the mean (n=6).
3.2 γ-H2AX in plucked hairs after TBI
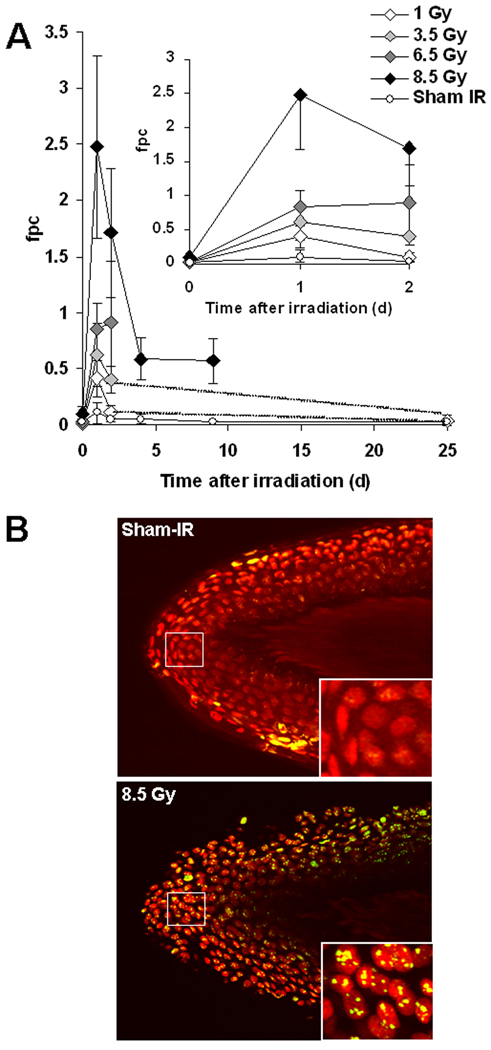
We previously showed that plucked hairs may be a useful surrogate tissue for assessing exposures to ionizing radiation (Redon, et al., 2010a). Other studies show the use of hair follicles can be applied to monitor radiation exposure (Kyoizumi et al., 1998; Sieber et al., 1992) as well as to use γ-H2AX as a marker for DNA damage in patients undergoing chemotherapy (Fong et al., 2009). γ-H2AX residual foci in plucked hairs are dose and time dependent (Fig. 4). The incidence of γ-H2AX foci after 1 Gy-TBI is low as signals disappear after 2 days TBI. In contrast γ-H2AX signals were still detected after 2 days for 3.5 and 6.5 Gy-, and 8.5 Gy-TBI, after 9 days for 8.5 Gy-TBI.
Fig.4. Kinetics for γ-H2AX foci in macaque plucked hairs after total-body irradiation.
(A) (Main graph) Kinetics for γ-H2AX foci formation and loss in plucked hairs for sham-irradiation (Sham IR) and at time points after the indicated TBI dose for up to 39 days. (Inset) Short-time course for γ-H2AX foci formation and loss. (B) Representatives images of plucked hairs obtained (eyebrow) from a sham-irradiated (top panel) and 1 day after 8.5 Gy TBI (bottom panel). Inserted images in the right bottom of each main image are enlargements of the regions inside the white squares in these main images. Green, γ-H2AX; red, DNA stained with PI. Errors bars represent standard deviations of the mean (n ≥ 4 for 1 Gy; n ≥ 5 for 3.5 Gy; n≥ 3 for 6.5 Gy; n≥7 for 8.5 Gy; n≥2 for Sham-IR).
4. Conclusion
Radiation accidents are more commonly characterized by acute partial-body or inhomogeneous rather than homogeneous TBI radiation exposures. Dose inhomogeneity can be a major confounder in dose assessment using biodosimetry (Kolanko et al., 2000; Prasanna, et al.). Two chromosome aberrations assays, metaphase-spread dicentric and ring and premature chromosome condensation or PCC, along with analysis methods, Qdr (Sasaki and Miyata, 1968) and Qpcc (Blakely, et al., 1995) respectively, for partial-body exposure assessment have been proposed for this purpose. Sak and colleagues evaluated the yield of γ-H2AX foci formation to allow estimation of the applied integral body dose after local radiotherapy to different sites of the body (Sak, et al., 2007). In 2007, a study by Rothkamm et al. described the application of a related cytogenetic data analysis method, the contaminated Poisson method by Dolphin, to detect partial body exposure using γ-H2AX-based biodosimetry in patients undergoing CT scans (Rothkamm et al., 2007). The authors observed an overdispersion of the γ-H2AX focus distribution from the Poison distributions after CT examinations that revealed a partial body exposure to radiation. Here, we introduce a partial-body analysis methodology (i.e., Qγ-H2AX, Fγ-H2AX), similar to that done for dicentrics (Qdr) and PCC (QPCC) assays, utilizing the gamma-H2AX foci assay. Utilizing γ-H2AX foci levels for these calculations has the advantage over micronuclei and damaged chromosomes of not requiring cell culturing and stimulation, since the foci are formed in the quiescent lymphocytes. Representing an alternative and rapid approach both for the assessment of dose intensity and inhomogeneity following acute ionizing radiation exposures, this approach could be useful for several practical biodosimetry applications.
However, we are aware that this method is most useful when the partial irradiation time is much less than the rate of blood circulation so that the blood pool renewal in the irradiated part of the body is limited. In addition, further analysis will be necessary to evaluate the influence of potential confounders on our method (i.e., inter-individual variations in background foci levels, gradient dose distributions rather than just 'clean' partial body irradiation, number of cells scored, response of special populations, etc). Because of the fast signal lost, we believe that Qγ-H2AX could be used in the first hours but less than a day following low irradiation doses (< 1Gy). Thus, Qγ-H2AX could be limited to high PBI doses. PBI doses higher than 3.5 Gy would result in long lasting γ-H2AX foci in irradiated lymphocytes with PBI doses ≥ 6.5 Gy resulting in a population of lymphocytes with “stable” γ-H2AX foci (non-repairable of DSBs)(Redon, et al., 2010a). With such high PBI irradiation doses, we could expect to use Qγ-H2AX for a few days after exposure (time that would also depend on the volume of irradiated body). Therefore, future studies will need to be performed to validate this model. Finally, because tissues from other parts of the body such as nails and teeth may be useful for biodosimetry (Fattibene and Callens, 2010; Romanyukha et al., 2010), their analysis together with γ-H2AX detection in other parts of the body, i.e., hairs plucked from the scalp and elsewhere, may develop into a robust tool for PBI dose assessment. Since non-clinical personnel could pluck hairs, this tissue will be well suited for large scale sample collection.
Acknowledgments
This research was supported by the NIAID Radiation/Nuclear Countermeasures Program, the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH and the Armed Forces Radiobiology Research Institute under research work unit number BD-13 (RBB4AR). Views presented in this paper are those of the authors; no endorsement by the Department of Defense has been give or should be inferred. The authors gratefully acknowledge Dr. Terry C. Pellmar (Silver Spring, MD) and Dr. Andrea L. DiCarlo-Cohen (NIAID) for their efforts to enable these studies, the assistance of the AFRRI Veterinarian, Dr. Jennifer Mitchell, and her colleagues in AFRRI’s Veterinary Science Department, radiation exposure and dosimetry support from AFRRI’s dosimetrist, Dr. Vitaly Nagy, and his colleagues in AFRRI’s Radiation Science Department, professional assistance in the rhesus macaque radiation model from Drs. Natalia I. Ossetrova and Gregory L. King, and technical support by David J. Sandgren, HM2 Sergio Gallego, Katya Kranopolsky, and Yvonne Eudy. We also thank Dr. O. Sedelnikova, NCI, for critical reading of the manuscript.
Abbreviations
- TBI
total-body irradiation
- PBI
partial-body irradiation
- NHP
non-human primate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare that they have no competing interests.
References
- Blakely WF, Prasanna PG, Kolanko CJ, Pyle MD, Mosbrook DM, Loats AS, Rippeon TL, Loats H. Application of the premature chromosome condensation assay in simulated partial-body radiation exposures: evaluation of the use of an automated metaphase-finder. Stem Cells. 1995;13 Suppl 1:223–230. [PubMed] [Google Scholar]
- Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattibene P, Callens F. EPR dosimetry with tooth enamel: A review. Appl Radiat Isot. 2010;68:2033–2116. doi: 10.1016/j.apradiso.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Kolanko CJ, Pyle MD, Nath J, Prasanna PG, Loats H, Blakely WF. In situ detection of a PCR-synthesized human pancentromeric DNA hybridization probe by color pigment immunostaining: application for dicentric assay automation. Biotech Histochem. 2000;75:91–98. doi: 10.3109/10520290009064153. [DOI] [PubMed] [Google Scholar]
- Kuefner MA, Grudzenski S, Schwab SA, Wiederseiner M, Heckmann M, Bautz W, Lobrich M, Uder M. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Invest Radiol. 2009;44:440–446. doi: 10.1097/RLI.0b013e3181a654a5. [DOI] [PubMed] [Google Scholar]
- Kyoizumi S, Suzuki T, Teraoka S, Seyama T. Radiation sensitivity of human hair follicles in SCID-hu mice. Radiat Res. 1998;149:11–18. [PubMed] [Google Scholar]
- Lobrich M, Rief N, Kuhne M, Heckmann M, Fleckenstein J, Rube C, Uder M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A. 2005;102:8984–8989. doi: 10.1073/pnas.0501895102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Takahashi A, Nagamatsu A, Omori K, Suzuki H, Shimazu T, Ishioka N. Detection of space radiation-induced double strand breaks as a track in cell nucleus. Biochem Biophys Res Commun. 2009;390:485–488. doi: 10.1016/j.bbrc.2009.09.114. [DOI] [PubMed] [Google Scholar]
- Porcedda P, Turinetto V, Lantelme E, Fontanella E, Chrzanowska K, Ragona R, De Marchi M, Delia D, Giachino C. Impaired elimination of DNA double-strand break-containing lymphocytes in ataxia telangiectasia and Nijmegen breakage syndrome. DNA Repair (Amst) 2006;5:904–913. doi: 10.1016/j.dnarep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Prasanna PG, Blakely WF, Bertho JM, Chute JP, Cohen EP, Goans RE, Grace MB, Lillis-Hearne PK, Lloyd DC, Lutgens LC, Meineke V, Ossetrova NI, Romanyukha A, Saba JD, Weisdorf DJ, Wojcik A, Yukihara EG, Pellmar TC. Synopsis of partial-body radiation diagnostic biomarkers and medical management of radiation injury workshop. Radiat Res. 2010;173:245–253. doi: 10.1667/RR1993.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redon CE, Nakamura AJ, Gouliaeva K, Rahman A, Blakely WF, Bonner WM. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS One. 2010a;5:e15544. doi: 10.1371/journal.pone.0015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redon CE, Nakamura AJ, Zhang YW, Ji JJ, Bonner WM, Kinders RJ, Parchment RE, Doroshow JH, Pommier Y. Histone {gamma}H2AX and Poly(ADP-Ribose) as Clinical Pharmacodynamic Biomarkers. Clin Cancer Res. 2010b;16:4532–4542. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Romanyukha A, Reyes RA, Trompier F, Benevides LA. Fingernail dosimetry: current status and perspectives. Health Phys. 2010;98:296–300. doi: 10.1097/01.HP.0000347999.01948.74. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Balroop S, Shekhdar J, Fernie P, Goh V. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology. 2007;242:244–251. doi: 10.1148/radiol.2421060171. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Horn S. Ggamma-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita. 2009;45:265–271. [PubMed] [Google Scholar]
- Sak A, Grehl S, Erichsen P, Engelhard M, Grannass A, Levegrun S, Pottgen C, Groneberg M, Stuschke M. gamma-H2AX foci formation in peripheral blood lymphocytes of tumor patients after local radiotherapy to different sites of the body: dependence on the dose-distribution, irradiated site and time from start of treatment. Int J Radiat Biol. 2007;83:639–652. doi: 10.1080/09553000701596118. [DOI] [PubMed] [Google Scholar]
- Sasaki MS, Miyata H. Biological dosimetry in atomic bomb survivors. Nature. 1968;220:1189–1193. doi: 10.1038/2201189a0. [DOI] [PubMed] [Google Scholar]
- Sieber VK, Sugden EM, Alcock CJ, Belton RR. Reduction in the diameter of human hairs following irradiation. Br J Radiol. 1992;65:148–151. doi: 10.1259/0007-1285-65-770-148. [DOI] [PubMed] [Google Scholar]