Abstract
The circadian rhythms of melatonin and body temperature are set to an earlier hour in women than in men, even when the women and men maintain nearly identical and consistent bedtimes and wake times. Moreover, women tend to wake up earlier than men and exhibit a greater preference for morning activities than men. Although the neurobiological mechanism underlying this sex difference in circadian alignment is unknown, multiple studies in nonhuman animals have demonstrated a sex difference in circadian period that could account for such a difference in circadian alignment between women and men. Whether a sex difference in intrinsic circadian period in humans underlies the difference in circadian alignment between men and women is unknown. We analyzed precise estimates of intrinsic circadian period collected from 157 individuals (52 women, 105 men; aged 18–74 y) studied in a month-long inpatient protocol designed to minimize confounding influences on circadian period estimation. Overall, the average intrinsic period of the melatonin and temperature rhythms in this population was very close to 24 h [24.15 ± 0.2 h (24 h 9 min ± 12 min)]. We further found that the intrinsic circadian period was significantly shorter in women [24.09 ± 0.2 h (24 h 5 min ± 12 min)] than in men [24.19 ± 0.2 h (24 h 11 min ± 12 min); P < 0.01] and that a significantly greater proportion of women have intrinsic circadian periods shorter than 24.0 h (35% vs. 14%; P < 0.01). The shorter average intrinsic circadian period observed in women may have implications for understanding sex differences in habitual sleep duration and insomnia prevalence.
Keywords: biological rhythm, gender, phase angle
On average, women go to bed earlier and wake up earlier than men, and they are more likely to rate themselves as morning types than men on standardized questionnaires (1). We recently reported a substantial sex difference in the entrainment of human circadian rhythms, such that the circadian rhythms of melatonin and temperature were entrained to an earlier time relative to the nightly sleep/darkness episode in women compared with men (2). The neurobiological mechanism underlying this sex difference in entrained circadian phase, which may have important implications for sleep quality and daytime alertness in women, remains unknown. Animal studies suggest that such differences in entrainment, technically called a phase angle difference between an endogenous rhythm (e.g., nightly secretion of melatonin) and the 24-h environmental light-dark (and wake-sleep cycle) to which the rhythm is synchronized, may be attributable to underlying differences in either the resetting sensitivity to environmental synchronizers or the intrinsic period of the circadian pacemaker(s) driving circadian rhythmicity (3–5). Little is known about sex differences in the sensitivity to photic resetting in humans, and no sex difference in the sensitivity to melatonin suppression by light has been reported in most studies (6–8). Findings from multiple studies in nonhuman animals have demonstrated that circadian period is shorter in females than in males, however (9, 10).
In humans, Wever (11) reported in 1984 that in a self-selected light-dark/activity-rest/wake-sleep cycle (a “free-running” protocol), women had more sleep and shorter sleep-wakefulness and body temperature periods than men when the sleep-wake cycle remained synchronized with the body temperature rhythm. When the sleep-wake and temperature rhythms became desynchronized, he reported that the women and men had similar temperature periods, leading him to conclude that there was a sex difference in sleep-wake period but not in temperature period (11). At that time, the powerful resetting effect of indoor levels of retinal light exposure on the human circadian timing system was not known (12–16) and likely had a strong influence on the assessment of circadian temperature period in those studies. It was subsequently recognized that the timing of the self-selected free-running rest-activity rhythm, commonly used as a circadian phase marker in nocturnal rodents, is not a reliable marker of the status of the circadian timing system in adult humans (17, 18), whose behavioral choices are affected by many factors other than circadian rhythmicity, including homeostatic sleep drive. Unlike the self-selected rest-activity/sleep-wakefulness rhythm in humans, body temperature and pineal melatonin rhythms have been shown to be reliable markers of the hypothalamic circadian pacemaker that drives endogenous circadian rhythms of sleep propensity, rapid eye movement sleep propensity, urine production, cortisol, thyroid-stimulating hormone, and neurobehavioral performance (17, 19–24). Therefore, it is difficult to determine from that prior study whether the reported sex difference was attributable to a sex difference in circadian period, light sensitivity, or behavior.
In 1938, Nathaniel Kleitman (25) pioneered the use of an alternative protocol to assess the endogenous nature of circadian rhythms in humans by scheduling participants to a day length outside the range of entrainment [i.e., a forced desynchrony (FD) protocol] in an environment shielded from the Earth's 24-h light-dark cycle (i.e., deep within a cave). Conducting such a protocol in dim light minimizes the confounding effects of both behavioral choices and photic resetting on the assessment of period. Findings from mathematical modeling studies (15) have revealed that intrinsic circadian period can be estimated much more accurately from the Kleitman FD protocol than from the free-running paradigm, provided that: (i) external and internal factors that might influence the phase or period of the circadian pacemaker are minimized at the time of measurement (e.g., exposure should be limited to dim light during the waking day, and such dim light exposure should be distributed uniformly across the circadian cycle during the protocol) and (ii) the imposed period of the activity-rest, wake-sleep, light-dark and feeding-fasting cycles is sufficiently different from the near-24-h intrinsic circadian period, well outside the range of entrainment. Because the circadian timing system consists of multiple central and peripheral oscillators, as recognized from physiological studies in humans (26–28) and from molecular studies in animals (29–31), it is important to measure more than one circadian output (e.g., core body temperature, plasma melatonin) (15). Twenty-five years ago, we began using Kleitman's FD protocol to assess intrinsic circadian period in humans studied in an environment free of external time cues (23). Using that protocol, our research group reported that the intrinsic period of the human circadian pacemaker(s) driving the rhythms of body temperature, melatonin, and cortisol secretion was stable and averaged 24.18 ± 0.15 h, with a percent coefficient of variation (PCV) of 0.6% (16). We then functionally validated the FD method of assessing the intrinsic circadian period of this stable oscillatory system, which also drives the circadian rhythm of sleep propensity, by demonstrating that the range of entrainment to a weak photic/behavioral synchronizer included an imposed T-cycle period of 24.0 h but not 24.6 h (32), a result consistent with an average intrinsic circadian period closer to 24 h than to 25 h.
Therefore, to resolve whether a sex difference in intrinsic circadian period underlies the sex difference in entrained circadian phase, in the present study we analyzed precise estimates of intrinsic circadian period collected from a series of 157 men and women who were studied using the Kleitman FD protocol.
Results
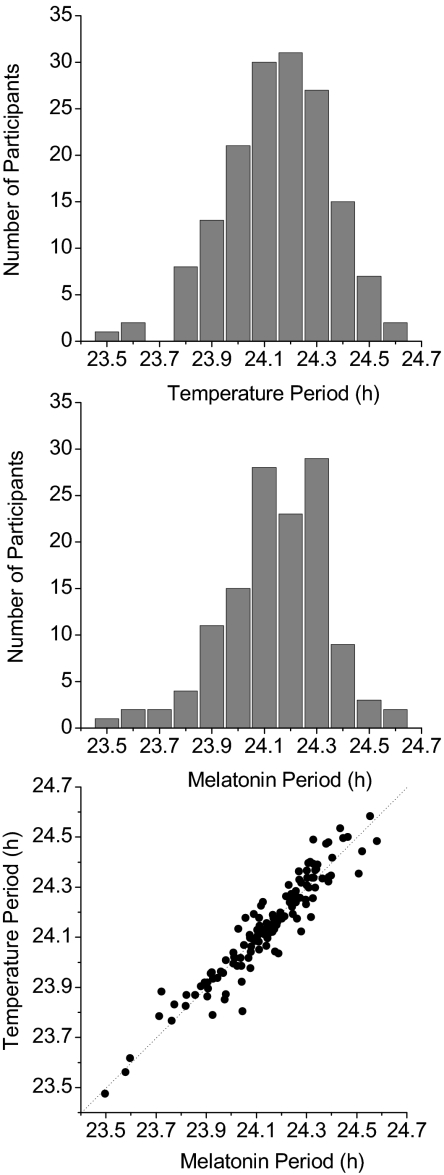
Data from 157 participants [52 women, 105 men; age (mean ± SD): 33.1 ± 17.4 y, range: 18–74 y] each studied for 2–6 wk in the laboratory, for a total of more than 5,000 d were included in our analysis. The average intrinsic circadian period was 24.15 h (SD = 0.2 h, SEM = 0.016 h, PCV = 0.8%) as assessed by core body temperature (n = 157; Fig. 1, Top) and was the same in the subset of subjects (n = 129) in whom we could also assess circadian period using melatonin data (Fig. 1, Middle). In these subjects, the period estimates derived from core body temperature and plasma melatonin data were highly correlated (n = 129; r = 0.95 ± 0.07; P < 0.0001; Fig. 1, Bottom). The circadian period was not significantly affected by the duration of the imposed rest-activity/light-dark cycle (T cycle; temperature period: F1,155 = 0.18, P = 0.67; Table 1).
Fig. 1.
Histogram of circadian periods as assessed using core body temperature (Top) or melatonin (Middle) in a group of adults studied in FD protocols. (Bottom) Relationship between temperature and melatonin period for each of the 129 subjects in whom both measures were determined. The slope of the dashed line, which represents a linear regression fit through these data with a forced intercept at 0, is 0.99988 ± 0.00024.
Table 1.
Period estimates (mean ± SD) for three of the FD T cycles
| T = 20 h | T = 28 h | T = 42.85 h | |
| Temperature | 24.12 ± 0.15 | 24.16 ± 0.21 | 24.15 ± 0.17 |
| (n = 26) | (n = 114) | (n = 16) | |
| Melatonin | 24.12 ± 0.14 | 24.15 ± 0.22 | 24.17 ± 0.15 |
| (n = 26) | (n = 89) | (n = 14) |
Average (±SD) intrinsic circadian period estimates derived from core body temperature and melatonin data of participants studied on three different FD T cycles. One participant studied on a T cycle of 11 h is not included in this table. Intrinsic circadian period data derived from core body temperature and melatonin were similar within and between each T-cycle group.
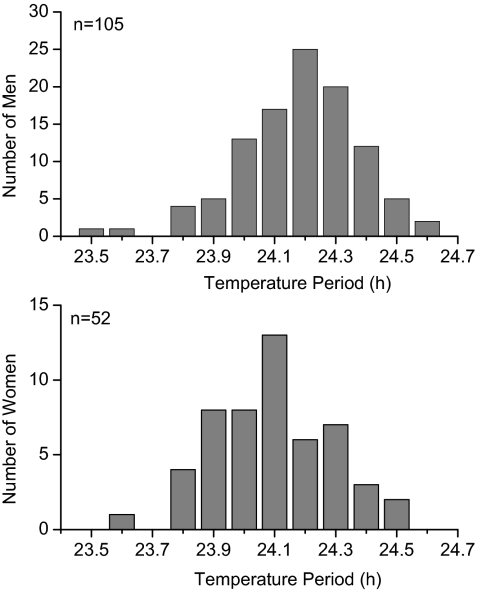
There was a significant influence of sex on circadian period as assessed using both temperature (F1,155 = 8.54, P < 0.01) and melatonin (F1,127 = 7.76, P < 0.01) data. The intrinsic period of the circadian pacemaker(s) driving those rhythms was significantly shorter in women than men as assessed by both temperature [women (n = 52): 24.09 ± 0.2 h, men (n = 105): 24.19 ± 0.19 h; P < 0.01; Fig. 2] and melatonin [women (n = 43): 24.08 ± 0.19 h, men (n = 86): 24.18 ± 0.19 h; P < 0.01]. Because of this finding of a significant relationship between sex and circadian period, we included sex in our statistical model for all subsequent analyses.
Fig. 2.
Histogram of circadian periods as assessed using core body temperature in men (Upper) and women (Lower).
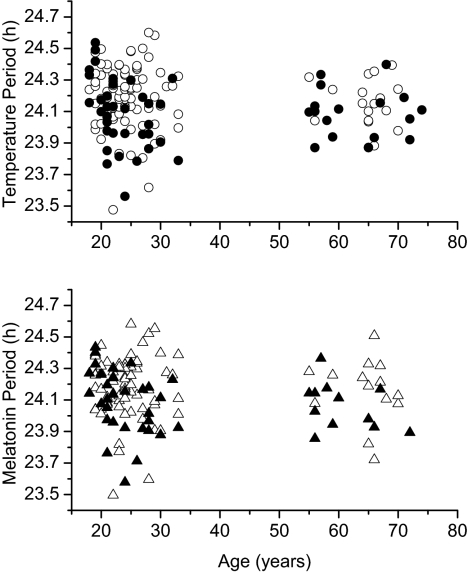
Consistent with a prior report based on results from a subset of these participants (16), in the group as a whole we found no significant influence of age on intrinsic period as estimated from temperature (F1,154 = 1.06, P = 0.3) or melatonin (F1,126 = 0.78, P = 0.38) data (Fig. 3). Comparison of the periods in young and older women by t test supported the latter [temperature period: young women (n = 35): 24.09 ± 0.22 h, older women (n = 17): 24.09 ± 0.15 h, P = 0.98; melatonin period: young women (n = 31): 24.09 ± 0.2 h, older women (n = 12): 24.06 ± 0.15 h, P = 0.72], as did comparisons between the young and older men [temperature period: young men (n = 86): 24.19 h ± 0.20 h, older men (n = 19): 24.15 ± 0.15 h, P = 0.36; melatonin period: young men (n = 72): 24.18 ± 0.19 h, older men (n = 14): 24.16 ± 0.20 h, P = 0.72].
Fig. 3.
Scatter plots of temperature and melatonin periods with respect to the age of the participants. (Upper) Age (in years) vs. temperature period (in hours) for all 157 participants, with data from women in filled circles (●) and data from men in open circles (○). (Lower) Age vs. melatonin period for the subset of 129 subjects in whom melatonin data were also available, with data from women in filled triangles (▲) and data from men in open triangles (△).
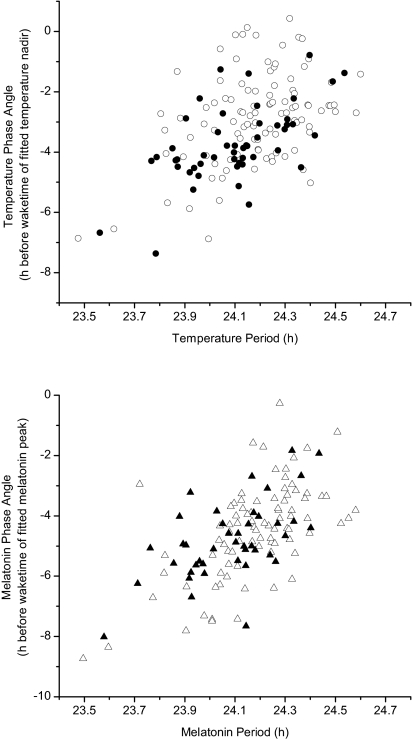
The nadir of the fitted core body temperature rhythm, as determined from nonorthogonal spectral analysis (NOSA) of the body temperature data collected throughout the FD protocol and projected back to the last baseline night of sleep, occurred, on average, 3.12 ± 1.52 h before habitual wake time (range: 7.37 h before to 1.18 h after), whereas the circadian phase of the fitted melatonin peak occurred, on average, 4.6 ± 1.50 h before habitual wake time (range: 8.74 h before to 0.27 h before). When we examined the latter by sex, the circadian phase of the core body temperature nadir occurred 3.75 ± 1.27 h before wake time in the women (range: 7.37–0.79 h before wake time, n = 50), which was significantly earlier with respect to wake time than in the men (2.81 ± 1.54 h before wake time, range: 6.88 h before to 1.18 h after wake time, n = 103; P = 0.0003). Although the circadian phase of the fitted melatonin peak was earlier in the smaller subset of women in whom we had melatonin data than in the men, this did not reach statistical significance (women: 4.82 ± 1.3 h before wake time, range: 8.02–1.84 h before wake time, n = 42 vs. men: 4.49 ± 1.58 h before wake time, range: 8.74–0.27 h before wake time, n = 86; P = 0.2). The phase angle of entrainment relative to the sleep-wake and associated light-dark cycles was significantly associated with intrinsic circadian period for both core body temperature (F1,150 = 36.47, P < 0.0001; Fig. 4, Upper) and melatonin (F1,125 = 71.4, P < 0.0001; Fig. 4, Lower) rhythms, with longer periods associated with a later temperature nadir and melatonin maximum relative to sleep/darkness. Linear regression analysis on the relationship between period and phase angle of entrainment showed that a given difference in period was associated with a 3.6 ± 0.5-fold larger difference in temperature phase angle and a 4.6 ± 0.5-fold larger difference in melatonin phase angle. Thus, the average 0.1-h (6-min) period difference between women and men was associated with an average temperature phase angle difference of 0.36 h (22 min) and an average melatonin phase angle difference of 0.46 h (28 min).
Fig. 4.
Scatter plot of relationship between circadian period and phase angle of entrainment in women and men studied in FD protocols. Phase angle of entrainment refers to the interval between circadian phase (fitted core body temperature minimum or melatonin maximum projected from the entire FD dataset to the beginning of the FD segment) and wake time/lights-on. (Upper) Relationship in all 157 subjects as assessed using circadian phase of the core body temperature minimum; linear regression analysis yields a slope of 3.6 ± 0.5 h. (Lower) Relationship as determined using circadian phase of the melatonin maximum in the subset of 129 subjects in whom melatonin data were available; linear regression analysis yields a slope of 4.6 ± 0.5 h. Filled symbols represent women, and open symbols represent men.
The range of observed circadian periods provides additional information about the process of entrainment in this group of individuals. The daily phase shift (adjustment) needed to maintain entrainment to the 24-h day ranges from a delay of 0.52 h (31 min) in the individual with the shortest period to an advance of 0.60 h (36 min) in the individual with the longest period, whereas individuals with average circadian periods require a daily phase advance of 0.15 h (9 min). As can also be seen in Fig. 1, ∼21% of the individuals had circadian periods that were shorter than 24 h. When we examined this by sex, a significantly greater proportion of the women had periods shorter than 24 h compared with the men (34.6% vs. 14.3%; Fisher's exact test, P = 0.0061).
In the subjects overall, the clock hour of wake time was significantly associated with temperature (F1,151 = 4.55, P < 0.05) and melatonin period (F1,126 = 4.68, P < 0.05), as was the clock hour of bedtime with temperature period (F1,151 = 4.52, P < 0.05), such that shorter periods were associated with earlier bedtimes and wake times and longer periods were associated with later bedtimes and wake times. Clock hour of bedtime tended to be associated with melatonin period, although this did not reach statistical significance (F1,126 = 3.80, P = 0.053). The association between period and the clock hour of bedtime and wake time was no longer significant when sex was included in our statistical model [bedtime-temperature period (F1,150 = 2.67, P = 0.1), bedtime-melatonin period (F1,125 = 2.02, P = 0.16), wake time-temperature period (F1,150 = 2.98, P = 0.09), and wake time-melatonin period (F1,125 = 3.18, P = 0.08)], indicating that the observed sex difference in period was sufficient to explain the relationship between sleep timing and period.
Discussion
Findings from the current study conclusively demonstrate that the intrinsic period of the human circadian pacemaker(s) driving the melatonin and temperature rhythms is significantly shorter in healthy adult women than in healthy adult men. This finding resolves the inconsistency from Wever's early report (11) that was derived from free-running studies, in which participants were allowed to self-select their sleep-wake (and thus light-dark) timing. In that report, when the sleep-wakefulness and body temperature rhythms remained synchronized, the women slept for a greater fraction of each cycle and exhibited shorter sleep-wake and body temperature rhythm periods than the men. After the sleep-wake and body temperature rhythms spontaneously desynchronized, the sleep-wake rhythms continued to show a sex difference but the body temperature rhythms were not different between the women and men. It is now known that the self-selected light exposure in those studies was of sufficient strength to influence the human circadian system (33, 34). Furthermore, as a result of the circadian rhythm in sleep propensity, subjects in free-running studies whose sleep-wakefulness and temperature rhythms remain synchronized typically choose sleep-wakefulness times that result in uneven light exposure across circadian phases, although when those rhythms become desynchronized, the light exposure becomes somewhat more evenly spread across circadian phases. Thus, the different sleep-wake and associated light-dark exposures between the sexes in the two conditions in that study very likely confounded the body temperature period estimates and led to the discrepancy (15). The advantage of the FD protocol used in the present study is that light levels were kept low and light exposure was spread evenly across circadian phases, minimizing the phase-dependent phase-shifting influence of light and allowing a more accurate estimate of period to be obtained. In doing so, we observed a significant sex difference in intrinsic circadian period. Using a different and much shorter protocol (35), researchers failed to detect this small (6-min) but statistically and biologically significant sex difference in intrinsic circadian period, likely because that protocol produces less precise period estimates and, together with the small sample size in that study, resulted in insufficient statistical power to detect a difference of this magnitude.
Consistent with our finding of a sex difference in intrinsic circadian period, findings from studies in a number of nonhuman animals have demonstrated that circadian period is shorter in females than in males (9, 10). Although circadian period is a genetically determined and stable property of the circadian timing system (16, 17, 29, 31), some environmental and chemical exposures have been shown to alter period (36–39). The shorter intrinsic circadian period we observed in women may be related to their higher estrogen levels, because it has been shown that continuous administration of estradiol benzoate results in a significant shortening of period in blind, ovariectomized, female hamsters (39). Our finding of a shorter intrinsic circadian period in women may therefore be attributable, in part, to the higher circulating levels of estrogen in women. Alternatively, it may be that exposure to high estrogen at some point during development alters the hypothalamic circadian pacemaker, leading to the sexual dimorphism that has been reported in suprachiasmatic nucleus structure (40, 41). We found that there was no significant interaction between age and sex and that the pre- and postmenopausal women in our study had similar periods, even though the postmenopausal women presumably had lower circulating estrogen levels. Further study with large numbers of participants throughout development will be required to characterize the sex difference in intrinsic circadian period and understand its physiological basis.
The finding that nearly four of five individuals have circadian periods longer than 24 h is consistent with the observation that most people report easier adaptation to westward travel, which requires that circadian rhythms be reset to a later hour, than to eastward travel, which requires that such rhythms be reset to an earlier hour. Our findings also suggest that more women than men may find it easier to travel eastward than westward, because women are more likely than men to have a circadian period shorter than 24 h.
The significantly shorter endogenous circadian period in women may partially account for our recent finding of an earlier timing of the endogenous melatonin and temperature rhythms relative to sleep/darkness in young women compared with young men (2), as well as the report of an earlier temperature nadir during sleep in older women compared with older men (42). Although the 6-min difference in intrinsic circadian period between men and women that we observed in the present study may appear small, our present data suggest that this period difference may account for a substantial fraction of the phase angle difference that we observed (2, 43, 44). Our population data indicate that a given period difference is associated with an approximately fourfold greater difference in entrained phase, consistent with our prior report (43). The 6-min average difference in intrinsic circadian period that we observed between women and men thus accounts for nearly a half-hour earlier alignment of the entrained melatonin rhythm relative to sleep. This intrinsic period difference could thus account for about 75% of the 36-min difference we previously observed in the timing of melatonin onset between women and men (2). Analysis of the temperature data yields a qualitatively similar conclusion, although less of the 1.5-h observed difference in entrained temperature phase from our prior study can be accounted for by the average sex difference in intrinsic circadian period observed in the present study.
Such a sex difference in entrained circadian phase, largely attributable to the sex difference in intrinsic circadian period reported here, results in women sleeping and waking at a later circadian time than men, a finding that has important implications for sleep quality in women. Studies in young men have found that the ability to have a consolidated ∼8-h sleep episode is greatest when sleep is initiated approximately 5 to 6 h before the circadian phase of the core body temperature nadir (20, 21). In our previous study, the men had habitual bedtimes and wake times that were nearly ideal for the maintenance of high sleep efficiency, whereas the women had a relatively delayed timing of sleep with respect to the timing of their circadian rhythms. That delayed biological time of sleep would be expected to result in greater sleep maintenance problems and may predispose women to develop sleep disorders, such as sleep maintenance insomnia and early morning awakening, which are more prevalent in women than in men (45, 46). This increased likelihood of sleep disruption in women attributable to the biological time of sleep may be exacerbated with age (47–49), as the sex difference in insomnia complaints increases further in people over 45 y of age (46, 50). Our findings of a sex difference in intrinsic circadian period and phase angle of entrainment suggest that light treatments for patients with circadian rhythm sleep disorders should be sex-specific and that additional studies of the phase angle of entrainment in middle-aged participants and in participants with complaints of sleep-maintenance insomnia, as well as studies in which sex differences in light sensitivity are explored, should be undertaken.
Our present analyses confirm that intrinsic circadian period in healthy humans is very close to 24 h and shows a low coefficient of variation, similar to observations obtained from other mammalian species. We also found that the Kleitman FD technique for assessing circadian period was robust, producing observed periods independent of the imposed rest-activity cycle (T cycle), consistent with our previous report (16). The near-24-h average intrinsic circadian periods reported here are consistent with a number of reports on smaller groups of sighted participants studied under similar conditions when observed circadian rhythmicity was dissociated from the timing of the light-dark and sleep-wake cycles (35, 51–54). They are also consistent with our demonstration that human circadian pacemakers can be entrained with a very weak (∼1.5 lux) light-dark/sleep-wake cycle to a 24-h day but not to a 24.6-h day (32). Our data also confirm our prior report from a subset of these participants (16) that intrinsic circadian period does not shorten with age in humans, although longitudinal studies in humans have not been performed. In several cross-sectional rodent studies, older animals were reported to exhibit shorter circadian activity rhythm periods than young animals (e.g., 55–57), although longer periods in older animals have also been reported (58). Findings from rigorously controlled longitudinal animal studies (59, 60) have revealed that, overall, there is no systematic shortening of intrinsic circadian period within individuals as they age.
Our finding of a significant association between intrinsic circadian period and phase angle of entrainment is in accordance with findings from both animals and humans (3, 5, 43), including findings from earlier reports on subsets of the participants included here (44, 61). We found that the interval between the melatonin peak or temperature nadir and wake time (time of lights-on) was longer in participants with the shortest periods and shortest in participants with the longest periods. Thus, individuals with longer periods, who need to receive large phase advance shifts to remain entrained, were waking at an earlier biological time, closer to the peak sensitivity to the phase-advancing influence of light. In contrast, individuals with shorter periods were going to bed at a later biological time, such that they received more phase-delaying evening light exposure. This finding has considerable practical relevance for understanding and treating circadian rhythm sleep disorders. Individuals in our study with the shortest and longest periods required a phase shift of approximately 35 min/d to remain stably entrained. If they were not exposed to light of sufficient strength and/or duration or chose behaviors (e.g., staying up late, getting up early) that altered their light exposure timing, that behavior could result in phase shifts of insufficient magnitude to allow for stable entrainment, leading to a circadian rhythm sleep disorder. Notably, when the circadian period is less than 24 h, the pattern of light exposure required for stable entrainment is qualitatively different, with evening light exposure required to induce a daily circadian phase delay shift to a later hour rather than morning light exposure being required to induce a daily phase advance shift to an earlier hour. The consequence of an insufficient synchronizing stimulus is also qualitatively different in those with a less than 24-h intrinsic circadian period, resulting in progressively earlier rather than later circadian timing. The fact that one-third of women have an intrinsic circadian period shorter than 24 h suggests that such women may be more prone to the symptoms of advanced circadian phase, such as early morning awakening.
Our study had a number of limitations. First, we did not have a sufficiently diverse sample to evaluate the recent report of racial differences in circadian period (35), although we did test this and found no evidence for a racial difference in circadian period in our sample. Participants in that study were repeatedly exposed to ambient light intensities of up to ∼100 lux, which has considerable phase-shifting effects (14); therefore, the reported racial differences in the circadian rhythm of light sensitivity could account for all the apparent differences in circadian period observed on that protocol (35). It should also be noted that the ultrarapid (in two cycles) method for assessing period used in that report has not been validated. Further studies assessing intrinsic circadian period during FD in dim light in larger numbers of well-characterized individuals will be required to distinguish between those possibilities. Second, the several weeks of study required to assess intrinsic circadian period in the FD protocol precluded us from assessing in women whether intrinsic circadian period varied with the phase of the menstrual cycle, which was not assessed in this protocol. Third, we were unable to evaluate the impact of genetic polymorphisms on intrinsic circadian period in the entire group, because the participants studied decades ago were not asked to provide consent for genetic evaluations.
In summary, our results on a large cohort of individuals in whom intrinsic circadian period was precisely measured demonstrate that intrinsic period of the human circadian timing system is, on average, slightly longer than 24 h; shows similar individual variability to that from other mammalian species; and does not shorten with age in healthy older individuals. We found a sex difference in intrinsic circadian period, with women having a significantly shorter average intrinsic circadian period than men and a much greater percentage of women than men having periods shorter than 24 h. The shorter intrinsic circadian period observed in women is consistent with our recent observation in a different cohort of young subjects, who were carefully matched for age and sleep-wake timing, that young women have a later biological time of sleep (i.e., an earlier circadian phase relative to the timing of sleep). The range of periods observed in this sample and the shorter periods in women have implications for understanding the development of circadian rhythm sleep disorders and for the higher rates of insomnia among women.
Materials and Methods
Protocol and Data Collection.
The data reported here were collected in a series of FD studies conducted in our laboratory over a 25-y period from 1985 to 2010 (16, 44, 62–66). In the FD protocol, the imposed light-dark (and corresponding activity-rest) cycle (the T cycle) is scheduled to a duration much shorter or longer than the near-24-h intrinsic circadian period and the light level during scheduled wake episodes was kept low to reduce the ability of the circadian system to entrain (synchronize) to the period of the imposed rest-activity cycle (67). This results in both the photic and nonphotic influences on the circadian system being distributed across all phases of the circadian cycle. This schedule is maintained for at least 1 wk, although the precision of the period estimate is greater if data are obtained for 2 wk or longer. Data for the present analysis were drawn from several studies of differing durations and FD T-cycle (imposed rest-activity) lengths. T cycles in the studies included here were 11 (n = 1), 20 (n = 26), 28 (n = 114), or 42.85 (n = 16) h, and the FD segments were at least 2 wk (range: 14–43 d). Light levels throughout the FD segments were less than 20 lux anywhere in the study rooms.
During FD, physiological variables that have a circadian oscillation (those that are modulated, in part, by the circadian pacemaker) are collected and assessed for periodicity within the circadian range (see below). In the studies presented in this report, core body temperature was collected at 1-min intervals throughout each FD study using a rectal thermistor (Measurement Specialties). The temperature recordings were visually inspected for brief sensor removals (for showers or toilet use) and sensor slips, which were edited out before analysis. In many of the studies, we also collected blood (n = 127) or saliva (n = 2) samples approximately once per hour for all or part of the FD segment for analysis of melatonin. The plasma or saliva was frozen; after each study was completed, the samples were assayed for melatonin (by Pharmasan Labs, Inc., by the General Clinical Research Center Core Laboratory at Brigham and Women's Hospital, or by Solidphase, Inc.).
Subject Screening and Selection.
In all the studies, participants underwent a medical and psychological screening process to ensure they were healthy before they were included in the study. This consisted of a medical history and physical examination, a 12-lead electrocardiogram, clinical biochemical tests on their blood and urine, and completion of psychological screening questionnaires and a structured interview with a clinical psychologist or psychiatrist. All participants aged 55 y or older also had an all-night clinical polysomnographic screening evaluation to screen out those with clinically significant obstructive sleep apnea.
Participants in all the studies reported no recent (within 1 y) history of regular night work and no recent (within 3 mo) crossing of more than one time zone. All participants were instructed to maintain a regular sleep-wake schedule for at least 1 wk before their study, and their compliance with this instruction was verified by a wrist activity monitor they wore during that week.
Data from an additional 97 FD participants studied in our laboratory between 1985 and 2010 were not included in our analyses. The majority of these participants (n = 70) had taken part in an FD study in which a drug, treatment, or intervention that had the potential to alter circadian period was tested in a between-subjects design, and they were randomized to a treatment group. Data from another 13 participants were not included because those individuals took part in an FD study in which a treatment that had the potential to alter period was tested in a within-subjects design and they received the treatment first, 2 additional participants who had a protocol disruption during their study that could have had an impact on the period estimate were not included, and data from 12 participants whose complete study records were not available at the time we completed the present analysis were also not included. Six individuals took part in more than one FD study in our laboratory, and we only included data from their first FD study in our analysis.
Data Analysis.
Our group developed a method of NOSA to estimate circadian period precisely from FD data. This method takes into account the periodic influence of the imposed activity-rest cycle (the T cycle) and then searches for the unknown periodicity in the circadian range (16).
To be consistent in our cross-study analysis, the temperature and melatonin data segment submitted to analysis always began at the first FD wake time and included as much of the available data as possible for an integral number of FD cycles (where an FD cycle is defined as having the rest-activity periods cover the 24-h cycle one time). The minimum FD duration included in the present analysis was two beat cycles, whereas the maximum number was six beat cycles.
The NOSA analysis was also used to determine circadian phase using all the data from the FD segment and projecting back to the start of the segment. For core body temperature, we used the minimum of the waveform fit to the FD data as the circadian phase marker, whereas for melatonin, we used the maximum of the waveform fit to the FD data as the circadian phase marker. Phase angle of entrainment was calculated by comparing the duration between the timing of the circadian phase marker and wake time/light onset time (the average wake/lights-on time from the week before the start of the inpatient study).
Correlation analysis between core body temperature and melatonin periods was performed using a Pearson correlation. Tests for main effects on period were performed using mixed model analysis of variance. Comparisons between age groups and sexes were done using Student t tests, correcting for variance homogeneity where necessary. Proportions of men and women with periods longer or shorter than 24.0 h were compared using Fisher's exact test. Examination of the relationship between period and phase angle of entrainment was performed using linear regression. All statistical analyses were performed using SAS (SAS Institute).
Acknowledgments
The authors thank the participants and the participant recruitment staff of the Division of Sleep Medicine; S. Driscoll and the technical and nursing staff of the Brigham and Women's Hospital Center for Clinical Investigation; C. F. Dennison, P. Dempsey, and E. J. Silva for assistance with data processing; Dr. W. Wang for statistical advice; Prof. R. E. Kronauer for his important contributions to the development of the FD protocol and the NOSA analysis and to interpretation of the resulting period data; Prof. E. N. Brown and Dr. J. F. Mitchell for their important contributions to developing the NOSA program; and Drs. J. S. Allan, S. P. Grady, J. T. Hull, and K. D. Scheuermaier and Mr. D. W. Rimmer for their contributions to carrying out some of the FD studies. The studies were supported, in part, by National Institutes of Health (NIH) Grants P01 AG09975, R01 HL080978, R01 HL52992, R21 AT02571, U01 AG12642, and T32 HL07901; by National Aeronautics and Space Administration Grants NAS9-19435 and NAG 5-3952 and NASA Cooperative Agreement NCC9-58 with the National Space Biomedical Research Institute; and by Air Force Office of Scientific Research Grants F49620-94-1-0398, F49620-95-1-0388, and F49620-00-1-0266. S.W.C. was supported, in part, by a fellowship from the Natural Sciences and Engineering Research Council of Canada. M.Y.M. was supported, in part, by a fellowship from Jazz Pharmaceuticals. The studies reported here were carried out in the Brigham and Women's Hospital Center for Clinical Investigation (formerly a General Clinical Research Center supported by NIH Grant M01 RR02635), currently part of Harvard Catalyst (Harvard Clinical and Translational Science Center), supported by NIH Award UL1 RR025758 and financial contributions from the Brigham and Women's Hospital and from Harvard University and its affiliated academic health care centers.
Footnotes
Conflict of interest statement: The authors declare a conflict of interest. The following authors declare no conflicts of interest: J.F.D., S.W.C., A.-M.C., A.J.K.P., M.Y.M., C.G., and D.-J.D. K.P.W. reports that he is a consultant for Takeda, Cephalon, and Zeo; he is the Chair of the Scientific Advisory Board and a stockholder in Zeo. J.K.W. reports that he has received investigator-initiated research funding from Respironics on a topic unrelated to the present paper. C.A.C. reports that he has received consulting fees from or served as a paid member of scientific advisory boards for Actelion, Ltd.; Bombardier, Inc.; the Boston Celtics; Cephalon, Inc.; Delta Airlines; Eli Lilly and Co.; Garda Síochána Inspectorate; Global Ground Support; Johnson & Johnson; Koninklijke Philips Electronics, NV; the Minnesota Timberwolves; the Portland Trail Blazers; Philips Respironics, Inc.; Sanofi–Aventis, Inc.; Sepracor, Inc.; Sleep Multimedia, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc.; and Zeo, Inc. He also owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc.; and Zeo, Inc., and he has received royalties from McGraw–Hill, the Massachusetts Medical Society/New England Journal of Medicine, the New York Times, Penguin Press, and Philips Respironics. He has received lecture fees from the Alliance for Epilepsy Research; American Academy of Sleep Medicine; Duke University School of Medicine; Mount Sinai School of Medicine; National Academy of Sciences; North East Sleep Society; Sanofi–Aventis, Inc.; Society for Obstetric Anesthesia and Perinatology; St. Luke's Roosevelt Hospital; University of Virginia Medical Center; University of Washington Medical Center; and University of Wisconsin Medical School. He has also received research prizes with monetary awards from the American Academy of Sleep Medicine and the New England College of Occupational and Environmental Medicine; clinical trial research contracts from Cephalon, Inc.; and an investigator-initiated research grant from Cephalon, Inc. His research laboratory at the Brigham and Women's Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon, Inc.; Koninklijke Philips Electronics, NV; ResMed; and the Brigham and Women's Hospital. The Harvard Medical School Division of Sleep Medicine, which C.A.C. directs, has received unrestricted research and educational gifts and endowment funds from Boehringer Ingelheim Pharmaceuticals, Inc.; Cephalon, Inc.; George H. Kidder, Esq.; Gerald McGinnis; GlaxoSmithKline; Jazz Pharmaceuticals; Lilly USA; Merck & Co., Inc.; Peter C. Farrell, PhD; Pfizer; Praxair US Homecare; ResMed; Respironics, Inc.; Sanofi–Aventis, Inc.; Select Comfort Corporation; Sepracor, Inc.; Sleep Health Centers, LLC; Somaxon Pharmaceuticals; Takeda Pharmaceuticals; Tempur-Pedic; Watermark Medical; and Zeo, Inc. The Harvard Medical School Division of Sleep Medicine Sleep and Health Education Program has received educational grant funding from Cephalon, Inc.; Takeda Pharmaceuticals; Sanofi–Aventis, Inc.; and Sepracor, Inc. C.A.C. is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc., and holds a number of process patents in the field of sleep and/or circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, he has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Quantification of Behavior” held June 11-13, 2010, at the AAAS Building in Washington, DC. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/quantification.
This article is a PNAS Direct Submission.
References
- 1.Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19:709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- 2.Cain SW, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25:288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol A. 1976;106:291–331. [Google Scholar]
- 4.Aschoff J, Pohl H. Phase relations between a circadian rhythm and its zeitgeber within the range of entrainment. Naturwissenschaften. 1978;65:80–84. doi: 10.1007/BF00440545. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann K. Zur beziehung zwischen phasenlage und spontanfrequenz bei der endogenen tagesperiodik. Z Naturforsch. 1963;18b:154–157. [Google Scholar]
- 6.Boyce P, Kennaway DJ. Effects of light on melatonin production. Biol Psychiatry. 1987;22:473–478. doi: 10.1016/0006-3223(87)90169-7. [DOI] [PubMed] [Google Scholar]
- 7.Nathan PJ, Wyndham EL, Burrows GD, Norman TR. The effect of gender on the melatonin suppression by light: A dose response relationship. J Neural Transm. 2000;107:271–279. doi: 10.1007/s007020050022. [DOI] [PubMed] [Google Scholar]
- 8.Nathan PJ, Burrows GD, Norman TR. The effect of dim light on suppression of nocturnal melatonin in healthy women and men. J Neural Transm. 1997;104:643–648. doi: 10.1007/BF01291882. [DOI] [PubMed] [Google Scholar]
- 9.Schull J, et al. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav. 1989;46:341–346. doi: 10.1016/0031-9384(89)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244:R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- 11.Wever RA. Sex differences in human circadian rhythms: Intrinsic periods and sleep fractions. Experientia. 1984;40:1226–1234. doi: 10.1007/BF01946652. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 13.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 14.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klerman EB, Dijk DJ, Kronauer RE, Czeisler CA. Simulations of light effects on the human circadian pacemaker: Implications for assessment of intrinsic period. Am J Physiol. 1996;270:R271–R282. doi: 10.1152/ajpregu.1996.270.1.R271. [DOI] [PubMed] [Google Scholar]
- 16.Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 17.Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–597. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- 18.Czeisler CA. The effect of light on the human circadian pacemaker. Circadian Clocks and Their Adjustment (Ciba Foundation Symposium 183) In: JM Waterhouse., editor. Chichester, UK: John Wiley and Sons; 1995. pp. 254–302. [DOI] [PubMed] [Google Scholar]
- 19.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 20.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 21.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 23.Czeisler CA, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 24.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 25.Kleitman N. Sleep and Wakefulness. Chicago: Univ of Chicago Press; 1939. [Google Scholar]
- 26.Aschoff J, Wever R. Human circadian rhythms: A multioscillatory system. Fed Proc. 1976;35:2326–2332. [PubMed] [Google Scholar]
- 27.Wever R. The circadian multi-oscillator system of man. Int J Chronobiol. 1975;3:19–55. [PubMed] [Google Scholar]
- 28.Kronauer RE, Czeisler CA, Pilato SF, Moore-Ede MC, Weitzman ED. Mathematical model of the human circadian system with two interacting oscillators. Am J Physiol. 1982;242:R3–R17. doi: 10.1152/ajpregu.1982.242.1.R3. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 30.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 31.Yoo SH, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42:167–168. [Google Scholar]
- 35.Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS ONE. 2009;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents III. Heavy water and constant light: Homeostasis of frequency? J Comp Physiol A. 1976;106:267–290. [Google Scholar]
- 37.Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus) Proc Natl Acad Sci USA. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheer FA, Wright KP, Jr, Kronauer RE, Czeisler CA. Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE. 2007;2:e721. doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- 40.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 41.Swaab DF, Chung WC, Kruijver FP, Hofman MA, Hestiantoro A. Sex differences in the hypothalamus in the different stages of human life. Neurobiol Aging. 2003;24(Suppl 1):S1–S16. doi: 10.1016/s0197-4580(03)00059-9. discussion S17–S19. [DOI] [PubMed] [Google Scholar]
- 42.Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: Relationships to sleep quality. Sleep. 1989;12:529–536. [PubMed] [Google Scholar]
- 43.Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci USA. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger MH, Roth TR, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Ed. Philadelphia: Elsevier Saunders; 2005. pp. 626–647. [Google Scholar]
- 46.Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19(3 Suppl):S7–S15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 47.Duffy JF, et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 48.Sherman B, Wysham C, Pfohl B. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab. 1985;61:439–443. doi: 10.1210/jcem-61-3-439. [DOI] [PubMed] [Google Scholar]
- 49.Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: Temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3:299–309. doi: 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- 50.Voderholzer U, Al-Shajlawi A, Weske G, Feige B, Riemann D. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress Anxiety. 2003;17:162–172. doi: 10.1002/da.10101. [DOI] [PubMed] [Google Scholar]
- 51.Campbell SS, Dawson D, Zulley J. When the human circadian system is caught napping: Evidence for endogenous rhythms close to 24 hours. Sleep. 1993;16:638–640. [PubMed] [Google Scholar]
- 52.Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. 1996;5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- 53.Hiddinga AE, Beersma DGM, Van den Hoofdakker RH. Endogenous and exogenous components in the circadian variation of core body temperature in humans. J Sleep Res. 1997;6:156–163. doi: 10.1046/j.1365-2869.1997.00047.x. [DOI] [PubMed] [Google Scholar]
- 54.Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- 55.Pittendrigh CS, Daan S. Circadian oscillations in rodents: A systematic increase of their frequency with age. Science. 1974;186:548–550. doi: 10.1126/science.186.4163.548. [DOI] [PubMed] [Google Scholar]
- 56.Witting W, Mirmiran M, Bos NPA, Swaab DF. The effect of old age on the free-running period of circadian rhythms in rat. Chronobiol Int. 1994;11:103–112. doi: 10.3109/07420529409055896. [DOI] [PubMed] [Google Scholar]
- 57.Morin LP. Age, but not pineal status, modulates circadian periodicity of golden hamsters. J Biol Rhythms. 1993;8:189–197. doi: 10.1177/074873049300800302. [DOI] [PubMed] [Google Scholar]
- 58.Wax TM. Runwheel activity patterns of mature-young and senescent mice: The effect of constant lighting conditions. J Gerontol. 1975;30:22–27. doi: 10.1093/geronj/30.1.22. [DOI] [PubMed] [Google Scholar]
- 59.Davis FC, Viswanathan N. Stability of circadian timing with age in Syrian hamsters. Am J Physiol. 1998;275:R960–R968. doi: 10.1152/ajpregu.1998.275.4.R960. [DOI] [PubMed] [Google Scholar]
- 60.Duffy JF, Viswanathan N, Davis FC. Free-running circadian period does not shorten with age in female Syrian hamsters. Neurosci Lett. 1999;271:77–80. doi: 10.1016/s0304-3940(99)00519-4. [DOI] [PubMed] [Google Scholar]
- 61.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 62.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 63.Wyatt JK, Dijk DJ, Ritz-de Cecco A, Ronda JM, Czeisler CA. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–618. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 64.Grady S, Aeschbach D, Wright KP, Jr, Czeisler CA. Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacology. 2010;35:1910–1920. doi: 10.1038/npp.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva EJ, Wang W, Ronda JM, Wyatt JK, Duffy JF. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep. 2010;33:481–490. doi: 10.1093/sleep/33.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cain SW, Rimmer DW, Duffy JF, Czeisler CA. Exercise distributed across day and night does not alter circadian period in humans. J Biol Rhythms. 2007;22:534–541. doi: 10.1177/0748730407306884. [DOI] [PubMed] [Google Scholar]
- 67.Czeisler CA, Allan JS, Kronauer RE. A method for assaying the effects of therapeutic agents on the period of the endogenous circadian pacemaker in man. In: Montplaisir J, Godbout R, editors. Sleep and Biological Rhythms: Basic Mechanisms and Applications to Psychiatry. New York: Oxford Univ Press; 1990. pp. 87–98. [Google Scholar]