Abstract
Survival functions from smoking cessation interventions are described by a three-state Markov model. On quitting, smokers transit through a state of withdrawal characterized by a high rate of relapse, and then into a more secure state of long-term abstinence. The Markov model embodies the dynamic nature of the cessation/relapse process; it permits stronger inference to long-term abstinence rates, provides measures of treatment efficacy, describes the outcomes of new quit attempts, and suggests mechanisms for the survival process.
Keywords: quitting tobacco, survival model, nicotine, monoamine oxidase
Scientists construct and validate maps between events or processes and their descriptions with words, graphs, or equations. Those maps are most secure when close to the object of study, such as mechanics is to the movement of massive bodies and chemistry is to its constituents. However, sometimes there is an advantage to working at more molar or molecular levels—statistical mechanics are one level up, and quantum chemistry is one level down. The most promising level of attack is typically where the greatest order is found, which can serve as a stable fulcrum for leverage above or below. In this paper, an epidemiological model is applied to tobacco addiction, which allows inferences both to behavioral processes and underlying physiological dysfunction.
Tobacco causes ∼440,000 premature deaths and $157 billion in health-related economic losses annually in the United States (1). The habit is tenacious, with around 90% of unassisted quitters smoking again within 1 y and the best interventions seldom decreasing that by more than 15% (2). Most attempts fail within a few weeks, leaving most trials underpowered for evaluating interventions beyond 3 mo. Characterization of the relapse process would permit information from the early epochs to improve inferences concerning long-term abstinence. Noting that many survival functions decrease at a similar rate in the right tail, Piasecki et al. (3) called for a dynamic model of abstinence that would explain such uniformity. The present paper answers that call with a simple Markov model of the transitions between stages of the quitting process.
Three-State Markov Model
It only takes a few cigarettes for some individuals to initiate the addiction process (4), with the most susceptible losing autonomy over smoking soon thereafter (5). Conversely, the first days of smoking cessation are also critical. Behavior (6) and adrenocortical perturbations (7) on the first day predict long-term abstinence, the first week is critical for counseling interventions (8), and after 1 mo, attrition occurs at much slower rates. To respect this distinction between early and late relapse processes, a three-state model was developed, with states corresponding to regular use, the critical early period of withdrawal, and the long right tail of guarded abstinence.
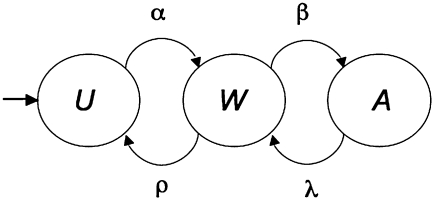
Almost one-half of current smokers attempt to quit at least one time per year (9), with some making multiple attempts. On any particular day, there is some probability α that they will quit, making the transition to a state of withdrawal (Fig. 1, W). All participants in cessation studies start in this second state, and with regrettably high probability ρ, they soon relapse to regular use. The minority of smokers who persevere will move into a state of long-term abstention (A) with probability β. They are never completely safe, because there remains a small probability λ that they will lapse and one cigarette will carry them back to renewed attempts to abstain, now with the higher probability (ρ) of relapse to habitual use. A single lapse does not guarantee relapse to regular smoking, but it is a good predictor of it (10), plays a causal role in relapse (11), and entrains a consequent relapse rate of 60–80% (12, 13). Individuals remain in the states with a complementary probability not shown in Fig. 1. The probability of continuing to smoke is 1 − α for the users. The probability of remaining in the transitional state W is 1 − ρ − β, and the probability of remaining abstinent for the longer term is 1 − λ.
Fig. 1.
A state diagram of the smoking cessation processes depicting the stages of habitual use (U), active struggle in the context of withdrawal symptoms (W), and long-term abstention (A). Greek symbols indicate the probability of transition on any given day. Cessation moves the individual through the states from left to right, with lapses from A, occurring with probability λ, returning the individual to W, with its higher probability ρ of relapse to habitual use.
Operational definitions of the key states and processes shown in Fig. 1, based on recommendations of the Measures Workgroup of the Society for Research on Nicotine and Tobacco (14), are particularized for the specific studies analyzed here. Regular use, U, is the state of participants before beginning cessation trials, typically smoking a pack a day for an extended time. It is the state to which they return after relapse. Lapse is the use of a few cigarettes by individuals attempting to abstain. Relapse is a return to regular smoking after a period of abstinence. Depending on the study, abstinence ranges from not even a single puff to passing a threshold of chemical validation to more than one cigarette per d for 1 wk. Therefore, it comprises both the state of withdrawal (W) and that of long-term abstention (A). The distinction between these is not independently measured in this analysis but inferred from data; W includes new abstainers and long-term abstainers who have recently lapsed but remain below detection threshold or use criteria. (Versions of the model that stipulate distinct states for these conditions are not supported by the data, given their burden of additional parameters.)
Fig. 1 is a classic Markov chain (15) corresponding to the transition matrix P and specifying how the behavioral and clinical outcome probabilities change from one observation to the next (Eq. 1):
 |
The rows of Eq. 1 represent the current states of regular use, withdrawal and renormalization of brain processes, and long-term abstention; the columns represent the status on the next day. Thus, the entries in the first row give the probabilities that a smoker will continue to smoke (1 − α) or quit (α) and thereby move into the second state, W. The second row gives the probability that an individual in W will, by the next observation, have relapsed (ρ), remained in W (1 − ρ − β), or made the transition to long-term abstention (β). The third row gives the probability of lapsing from abstention (λ), a lapse that, by reinstating the process of withdrawal, increases the likelihood of additional consumption, carrying him or her back to regular smoking with probability ρ. The current analysis uses 1 d as the epoch over which observations are made and probabilities are calculated.
Like any model, Fig. 1 simplifies a complex and idiosyncratic process. In particular, Markov models are history-independent; the probability of transition into any state depends only on the current state and not how long the individual resided in it or the number of prior visits to it. This may seem counterintuitive, but in one of the few studies reporting such data, the distribution of times to first lapse by abstainers was approximately exponential (13)—in particular, a geometric progression with transition probability λ = 0.035. Such exponential processes are memoryless, consistent with the Markov property. Whereas more frequent lapses do predict a greater probability of relapse, this is also predicted by the Markov model; with each visit to the W state, the individual reexposes him- or herself to increased probability of relapse ρ, with the odds of relapse (ρ/β) substantially greater after a lapse than those from the third state (λρ/β). The constancy of the transition probabilities over time does not entail that the hazard of relapse is constant over time: the interplay of the states causes the hazard function to decrease quickly at first and then more slowly as the process approaches its asymptote.
The structure of the model does not differentiate smokers by age, sex, or severity of dependence, and it does not take into account the crucial variables of social context and genetic predispositions (16–19). However, although the grammar of Eq. 1 is deaf to the particulars of individual case histories, its semantics—its parameters—distill the information in scores of data points into more robust estimates of underlying processes, providing clues as to the reasons for differential success in different groups. Knowing the state of the subject on the first day, one can calculate the probability that she will be in any one of the states on the nth d by raising Eq. 1 to the nth power, Pn. Because all subjects in intervention studies start out as new attempts, the second row of P, giving the probabilities for a population that starts in W, is key. In the second row, the first cell 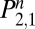 gives the probability of relapsing to habitual use by the end of the nth d. On the first day,
gives the probability of relapsing to habitual use by the end of the nth d. On the first day, 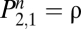 . Because the rows of Eq. 1 sum to 1,
. Because the rows of Eq. 1 sum to 1, 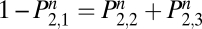 . The probability of not being back in the state of regular use, U, on the nth day (
. The probability of not being back in the state of regular use, U, on the nth day (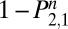 ) equals the probability of being in the state of withdrawal (
) equals the probability of being in the state of withdrawal (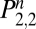 ) or the state of abstention (
) or the state of abstention (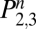 ). The primary data reported here are
). The primary data reported here are 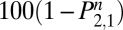 , the percentage of individuals who quit on day 0 that are not regularly smoking on day n. For more information on stochastic matrices, see a standard probability text such as ref. 20.
, the percentage of individuals who quit on day 0 that are not regularly smoking on day n. For more information on stochastic matrices, see a standard probability text such as ref. 20.
Asymptotics
Wherever the population starts—whether all in W, as in these studies or distributed across all states, as in the population at large—eventually all of the rows of Pn converge to the same array of values, with the population settling to an equilibrium distribution over the three states. If the probability of either of the bad transitions (the regressive arrows at the bottom of Fig. 1) is 0, in the long run no one will habitually smoke (Eq. 2). When only λ = 0, abstention will be an absorbing state, and when only ρ = 0, individuals will cycle between abstention and occasional cigarettes. Such chipping characterizes a well-known subset (∼4%) of smokers. If the probability of either of the two good transitions (the progressive arrows in the top of Fig. 1) is 0, in the long run no one would abstain (Eq. 4). If only α = 0, regular use would be the absorbing state, and if only β = 0, individuals would cycle between habitual use and doomed attempts to quit. If all parameters are positive, all states are transient, with asymptotic probabilities of residing in them (section 4.4 in ref. 20) being (Eqs. 2–4)
This model does not depict a state of full recovery. Most abstainers are but one lapse away from the active struggle of the second state, with its high relapse probability (21, 22). No relapse prevention interventions have shown efficacy over the long run (23)—that is, efficacy in reducing λ. Even after 1 y abstention, there remains a 10% probability of relapse every 1 y thereafter (24). For the populations studied here, smoking seems to be a chronic, relapsing disorder (25) (that is, λ > 0). Nonetheless, many smokers eventually manage to quit (26), with spontaneous and unassisted attempts actually faring better than outcomes from treatment programs (27). The latter, analyzed here, are, therefore, not fully representative of smokers at large (28, 29); they may be biased to more dependent smokers who have failed independent attempts to quit, leading them to participate in these clinical trials. Genetic predispositions play a role in determining who falls into and successfully struggles out of addiction (19). As we shall see, different subpopulations of smokers may be characterized by distinctive values of the parameters β and ρ.
Application to Survival Curves
Prediction requires that we know the transition probabilities a priori. The goal here is not prediction but understanding how effective an intervention is, what process it affects, or what ways two populations differ. To such ends, the Markov model can be reverse-engineered to infer the transition probabilities. This requires sufficiently detailed data to narrow confidence intervals on the parameters; unfortunately, most studies report only a handful of time points (30). A search of the Cochrane Report (31) for studies with at least 100 subjects per group and full survival functions returned the four studies displayed in Fig. 2. Each will serve to illustrate different aspects of the model.
Fig. 2.
The probability of not smoking regularly (U) given by the Markov model (smooth black lines) underscores various survival functions. The parameters are listed in Table 1.
For each function, the parameters (Table 1) were adjusted to maximize the likelihood of the data given the model, with the probability of not habitually using on day n given by 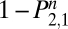 . Formally, this is a hidden Markov model (because the second and third states, W and A, are not directly distinguished from one another by observation). The Aikaike Information Criterion (AICc) (32) was used to determine when holding parameters invariant over conditions improved the performance of the model, measured as a combination of accuracy and parsimony. This criterion tells when additional parameters or parameters left free to vary over groups are likely to be fitting noise rather than reflecting reliable differences in datasets. A binomial error function was employed. The following paragraphs step through analyses of the studies to show use and interpretation of the model.
. Formally, this is a hidden Markov model (because the second and third states, W and A, are not directly distinguished from one another by observation). The Aikaike Information Criterion (AICc) (32) was used to determine when holding parameters invariant over conditions improved the performance of the model, measured as a combination of accuracy and parsimony. This criterion tells when additional parameters or parameters left free to vary over groups are likely to be fitting noise rather than reflecting reliable differences in datasets. A binomial error function was employed. The following paragraphs step through analyses of the studies to show use and interpretation of the model.
Table 1.
| Transition probabilities × 100 |
|||||
| Study | α | β | λ | ρ | Asymptotic P (not U) |
| Killen et al. (33) | |||||
| TNP | 0.2 | 9.3 | 5.5 | 2.6 | 0.20 |
| Placebo | 0.2 | 2.2 | 1.0 | 4.5 | 0.15 |
| Blondal et al. (40) | |||||
| NNS | 0.3 | 11.8 | 2.1 | 7.6 | 0.22 |
| Placebo | 0.9 | 11.8 | 5.7 | 15.6 | 0.17 |
| Zhu et al. (8) | |||||
| Multicounseling | 0.3 | 19.0 | 1.5 | 14.2 | 0.23 |
| Single counseling | 0.5 | 14.1 | 1.5 | 24.3 | 0.16 |
| Self-help | 0.5 | 9.3 | 1.5 | 24.3 | 0.13 |
| Swan et al. (42) | |||||
| Male | 0.4 | 4.7 | 1.2 | 4.3 | 0.24 |
| Female | 0.4 | 3.7 | 1.2 | 5.8 | 0.16 |
| CEASE | |||||
| 25 mg | 0.2 | 1.8 | 1.2 | 3.4 | 0.14 |
| 15 mg | 0.2 | 1.8 | 1.2 | 4.4 | 0.11 |
| 0 mg | 0.2 | 1.8 | 1.2 | 7.0 | 0.08 |
| Treatment |
Median |
||||
| Full* | 0.31 | 7.00 | 1.39 | 5.06 | 0.21 |
| Minimal | 0.37 | 5.75 | 1.35 | 11.31 | 0.14 |
Fig. 2 Upper Left comes from a comparison of 16 wk of transdermal nicotine patches (TNP) with placebo patches (33). Table 1 shows that the major benefits of this classic treatment were speeding the transition from second to third state (β) and reducing the probability of early relapse (ρ) by 50%. The reduction of relapse was probably caused by the patch's lessening of withdrawal symptoms associated with W (34, 35). The higher rate of lapsing (λ) in the treatment group was more than compensated by their higher probability of recommitting to abstention (β; this study's lenient criterion for relapse—7 d of smoking—kept brief lapses from being counted as failures). Table 1 shows that the long-run probability of abstinence (comprising W and A and predicted by the complement of Eq. 2) was substantially larger for the group with active patches. A null hypothesis of no long-term differential effectiveness may be tested by constraining this asymptotic probability to be equal for both groups, leaving other parameters free to optimize; the data are e−5 < 1/100 times as likely under that null hypothesis. The long-term differential effectiveness of TNP has recently been validated (36), although the relative benefit at 10 y was a slight five points—consistent with the asymptotic difference between groups seen in Table 1.
Measuring Health Benefits.
The long-term probability of not returning to regular use (Eq. 4) underestimates the benefits of treatment. Relapse and recommitment are normal parts of the cessation process, and any reduction of exposure to the toxins in tobacco may be cost-effective, even if the individual eventually returns to regular use. The reduction of exposure to tobacco is proportional to the area under the survival curves (AUC); the difference in areas between control and treatment curves is, therefore, a better metric of health benefit, one consistent with the recommendations of the Measures Workgroup (14) to favor comparative measures over absolute ones, such as percent abstinent. The AUC for treatment and control groups is easily computed by cumulating the values of 1 − 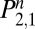 over the number of days of interest. In the case of 1 y, that equals
over the number of days of interest. In the case of 1 y, that equals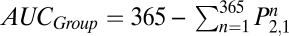 . The differential effectiveness is AUCTreatment − AUCControl. For the data in Fig. 2
Upper Left (33), this yields 41 d of not smoking per person during the first year above that achieved by the placebo group. Most survival curves are parallel beyond 1-y postcessation (37), and therefore, longer-term benefits may be estimated for those additional days by the differences in the asymptotic survival probabilities. The 5-y benefit of TNP in this study was 41 + 1,460 d/4 y × (0.196 − 0.147) = 112 fewer days smoking than controls over this epoch.
. The differential effectiveness is AUCTreatment − AUCControl. For the data in Fig. 2
Upper Left (33), this yields 41 d of not smoking per person during the first year above that achieved by the placebo group. Most survival curves are parallel beyond 1-y postcessation (37), and therefore, longer-term benefits may be estimated for those additional days by the differences in the asymptotic survival probabilities. The 5-y benefit of TNP in this study was 41 + 1,460 d/4 y × (0.196 − 0.147) = 112 fewer days smoking than controls over this epoch.
A much more sophisticated model of the health benefits of smoking cessation uses a two-stage Markov model for tobacco abstention coupled with an elaborate set of exposure risks to various illnesses in those states (38). This permits a coherent public health decision on the economics of various interventions. Nordstrom et al. (38) estimated the transition probabilities among states from published data using different probabilities for different intervals postquitting. The present three-stage model may provide a more sensitive and consistent front end to such pharmacoeconomic models.
Empirical leverage on the right tail of the survival function is provided by the data in Fig. 2 Upper Right comparing patch plus nicotine nasal spray (NNS) to patch plus placebo nasal spray (PNS) to 2 y (39). NNS substantially reduced the probability of lapse (λ of 2% vs. 6%) and relapse (ρ of 8% vs. 16%), leaving 22% of the treatment group vs. only 17% of the placebo group not users over the long term, a difference 50 times more likely than the null. The lower rate of lapsing (λ) and relapsing may have been because of the availability of the NNS as needed for up to 1 y. The values of β might have been not significantly different, because an active patch was used in both groups. The 1 and 5 y health benefits were 50 and 119 fewer d smoking than controls. The probability of being in A over the long term (Eq. 4) was projected to be 18% and 11%, which is consistent with the results of a 6-y follow-up on NNS plus patch by the same group (40) that showed a 16% probability of finding the treatment group in abstention vs. 9% for control.
Fig. 2 Lower Right compares the efficacy of zero [self-help alone (SH)], one (SC), or multiple (MC) sessions of phone counseling in 1,700 subjects that were able to quit for at least 1 d (8). Because of this eligibility criterion, these data are left-truncated (41), entering the analysis after the most difficult day in the quitting process. In analysis, therefore, 1 d was added to each reported time interval, and the model predictions were renormalized by dividing by the computed probability of having survived the end of the first day. Multiple sessions of strategically timed counseling (MC) doubled the rate of transition to long-term abstinence (β), while almost halving the relapse rate (ρ) over self-help. There was a decrease in α for the MC group, suggesting a less frequent cycling into and out of episodes of smoking. There was a monotonic increase in the asymptotic probability of abstention from 13% to 23% as a function of amount of intervention, with differences e100 more likely than no differences among groups. The median length of abstinence reported for year 1 was 58 d greater for the MC than the self-help group compared with the mean advantage computed from the AUC of 64 d (and 212 d benefit at 5 y).
Individual Differences.
Women have more difficulty quitting than do men, as attested by the survival curves for male and female smokers in smoking cessation counseling programs (42) in Fig. 2 Lower Left. The parameters of the fast processes favored the males, who had speedier transition to abstention (β ∼ 5% vs. 4%) and lower probability of relapse (ρ ≈ 4% vs. 6%) than females. None of the other parameters met AICc criteria for being different between groups, and there was no real evidence for differences in asymptotic probability of abstention, with unconstrained asymptotes increasing the likelihood of the model only threefold.
These investigators presented their survival curves using the Kaplan–Meier transformation. When an individual drops out of the study, the data are renormalized by subtracting a count from both the numerator and denominator to calculate percent surviving thereafter (43). This missing at random model is common in the creation of survival curves. However, most participants quit smoking cessation trials because they have returned to regular use; by ignoring them after they have dropped out rather than counting them as failures, Kaplan–Meier tests overestimate the efficacy of interventions (44, 45). There is no way to adjust for this bias posthoc. Kaplan–Meier reports are valuable for their estimates of relapse on a daily basis, but their characteristic renormalization for missing data undermines that utility.
Whether the intervention was the use of a transdermal nicotine patch, a nicotine nasal spray, or counseling, its primary contribution was to decrease the odds of relapse from W (ρ/β) on the average by two-thirds. The major benefit of a patch is to reduce the progression from lapse to relapse (46). The positive probability of lapse from the third state (λ) indicates that ex-smokers are never safe from the potential for relapse. Other studies of long-term abstinence confirm their continued vulnerability (47), even decades into the future (24, 48).
Alternate Measures of Relapse
Because the Markov model is memoryless, it is intrinsically a point prevalence measure. It was successful with prolonged abstinence data from Fig. 2, because relapse moved the individuals back to the first state of regular use, which is almost absorbing (α ≈ 0). An alternate measure of relapse is the more lenient prevalence (of abstinence) over the period of 1 wk, a standardized epoch that allows chemical validation (14). Such period prevalence regimens keep smokers who have been clean during the period in the subject pool, with earlier lapses ignored (49). Eq. 1 can track period prevalence data, because it is not necessarily monotonic. If transition into and out of A is slow (λ and β are small) but recommitment is allowed (α > 0) as intrinsic to period prevalence measures, the function can rise to a higher asymptote after an initial dip. Another way to be more realistic about the natural process of quitting is to allow slips (50). The nominal rate of relapse ρ then decreases and is commensurate with the more lenient relapse criteria, elevating the survival curves.
The asymptotic probability of finding an individual not smoking (the complement of Eq. 2) or abstinent for the long term (Eq. 4) allows lapses followed by recommitment, thus refreshing the population of nonsmokers. However, a lapse has serious implications. The odds of staying in A from one day to the next are the substantial (1 − λ)/λ; however, a single lapse carries the individual back to W, with the odds on returning to A being the much smaller β/ρ. More serious relapses to regular use require escape from the first state, which occurs with the relatively low probability α. The values of α shown in Table 1 are within the range reported for spontaneous quit attempts. It might seem that α should fall to 0 in studies with strict standards for abstinence, but those are difficult to enforce. The rate of false negatives depends on the criteria for abstinence (which must balance false positives against false negatives), the honesty of participants (potential lie rates are estimated to be 20% in some studies) (42), and the sensitivity of the assays (51). One study found that, despite a stringent criterion for abstinence (carbon monoxide ≤ 8 ppm), in groups with no onus for admitting slips, such admissions were critical for a realistic estimate of indulgence (52). Slips happen, but interventions can still benefit those who have lapsed.
The probability of not returning to habitual use, given by the complement of Eq. 2 and listed in Table 1, includes individuals who have relapsed but are again abstaining. To analytically exclude those who had returned to heavier smoking but crept back into the study, set α = 0 after parameter evaluation; this permits calculation of the mean residence time in the two states of abstinence as T = (I − P)−1 (that is, as the inverse of the complement of Eq. 1) (20). For the parameters in the bottom rows of Table 1, the typical intervention gives 17 d withdrawal (in W) and 103 d long-term abstention (in A) for a total of 120 d not smoking vs. 6 d withdrawal and 38 d abstention for a total of 45 d not smoking for the controls.
The benefit of Eq. 1 is its treatment of cessation as a dynamic recurrent process, with occasional lapses (λ) and recovery (β) and more serious relapses (ρ) into regular use but with a continuing process of new quit attempts (α). Because most people who achieve long-term abstention have failed in numerous previous attempts and may yet again relapse, only a dynamic model allowing flow through the various stages can provide an accurate picture of addiction and its remission as well as the attendant health implications. Despite the high asymptotic relapse rate in these studies, many smokers are eventually able to quit (38). Given the numerous times that most smokers make the attempt before they succeed, the small probabilities of success in each attempt eventually add up.
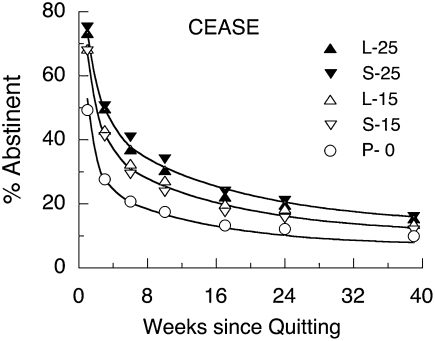
A disadvantage of Eq. 1 is that it overpowers data from interval-censored studies, which often report data from only a handful of time points (53). Unfortunately, these are the majority of the cases. The multisite Collaborative European Anti-Smoking Evaluation (CEASE) trial of nicotine patches (54), for instance, enrolled 3,500 smokers and found that higher dosage patches (25 vs. 15 vs. 0 mg) were most effective, but it also found that continuing their use beyond 8 wk (when most of the smokers would be out of W) made no difference. Because of the small number of points on the curve, the AICc was content with the same α, β, and λ for all conditions (Table 1), with ρ decreasing monotonically with nicotine dose level. The asymptotic probability of not being a regular user increased with dose level from 8% to 14%. Fig. 3 displays a good fit to the data; but without full survivor curves, or at least ones having dense coverage over the first month, we cannot be certain of the values or the invariance of the fast parameters, β and ρ.
Fig. 3.
Survival curves for the multisite Collaborative European Anti-Smoking Evaluation (CEASE) trial of nicotine patches (54) drawn by Eq. 1. Only ρ changed with dose, decreasing monotonically with its increase. Duration (L = 22 wk; S = 8 wk) had no effect on survival.
Alternate Models of Relapse
All models approximate more complicated states of nature. They can be more or less precise depending on the demand for clarity and generality, which are typically complementary to precision. The Pareto distribution, f(t) = (c/t)ρ (t ≥ c), is a simple function that characterizes some survival curves but is trumped by Eq. 1 in all of the data analyzed here, even with AIC allowing for its fewer parameters. Kirshenbaum et al. (55) showed that both tobacco and alcohol survival curves had similar forms: 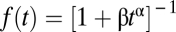 . This hyperboloid is a truncated series approximation to the Weibull, which is commonly used to describe survival processes. It provides a better account of these data than did the power function, but the AIC criterion preferred the Markov model to the above hyperboloid in each case of Fig. 2, with average log odds of 20 to 1. The hyperboloid and Markov provided comparable fits to the CEASE data. Either of these alternate models, thus, can provide a decent description of survival curves for data reported at sparse periods. The demonstration of the generality of the hyperboloid by Kirshenbaum et al. (55) holds hope for the potential use of the Markov model in analyses of withdrawal from other drugs of abuse.
. This hyperboloid is a truncated series approximation to the Weibull, which is commonly used to describe survival processes. It provides a better account of these data than did the power function, but the AIC criterion preferred the Markov model to the above hyperboloid in each case of Fig. 2, with average log odds of 20 to 1. The hyperboloid and Markov provided comparable fits to the CEASE data. Either of these alternate models, thus, can provide a decent description of survival curves for data reported at sparse periods. The demonstration of the generality of the hyperboloid by Kirshenbaum et al. (55) holds hope for the potential use of the Markov model in analyses of withdrawal from other drugs of abuse.
Alternate Starting Points
The initial distribution of a population over the three states of Fig. 1 may be represented by the row vector s(0) = [N1 N2 N3], giving the number in each of the states. To predict the subsequent distribution s(n), multiply this vector by Pn: s(n) = s(0)Pn. In the present analyses, all started as new quitters, and therefore, s(0) = [0 100 0]: zero in U and A and 100% in U. To predict the future states of a population of current smokers (56), set s(0) = [100 0 0]. To predict the future states of a population of long-term abstainers (24, 48), set s(0) = [0 0 100]. To predict the impact of a public health cessation intervention, increase in tobacco tax, sales restrictions, or other intervention (57, 58) on a population's status at any point into the future, set the initial values of s(0) according to the current distribution of the population over the states, adding an additional initial state for those who have never used tobacco.
Peters and Hughes (25) studied individuals who spontaneously decided to quit abruptly, decided to taper off, or had no a priori intention of quitting, and therefore, s(0) = [100 0 0]. The probability of being abstinent n days after that decision was geometrically increasing and well-described by  using the parameters in the bottom row of Table 1 but with unique values of α for each group.
using the parameters in the bottom row of Table 1 but with unique values of α for each group.
What Are the Correlates of the States?
Psychological.
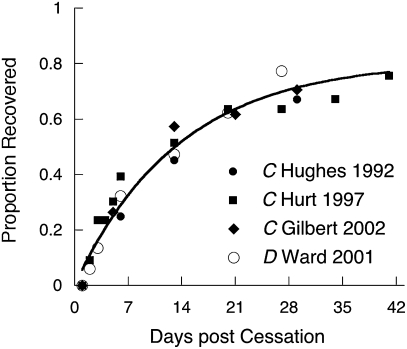
Why is it so difficult to remain abstinent immediately after quitting? Severe dysphoria, at levels comparable with those found in psychiatric outpatients (59), accompanies the early days of cessation, with irritability and anxiety as the predominant symptoms (60). Fig. 4 displays the time course of the malaise and desire to smoke in untreated individuals normalized to range from 0 (initial levels) to 1 (baseline; either prequit or nonsmoker levels). Also shown is the probability of entering state A over time for those who do not relapse (ρ set to 0) using the values of β and λ from the bottom row of Table 1. The approximate correspondence suggests that state W is associated with the symptoms of withdrawal, which decrease with entry into state A. Eq. 1 does not posit that withdrawal symptoms associated with W precipitate relapse, but Fig. 4 suggests that they may be an important causal factor for it.
Fig. 4.
Amelioration of withdrawal symptoms in untreated or placebo groups measured on composite scales (C) (106–108) or as the simple desire to smoke (D) (109). The curve traces the predicted entry into state A for subjects who do not relapse (ρ = 0) setting β and λ at their median values from the bottom row of Table 1.
Physiological.
Nicotine has traditionally been viewed as the prime culprit in tobacco addiction (61, 62). Nicotine potentiates the ability of stimuli to act as reinforcers for rats (63–65) and humans (66). The α7 nicotinic acetylcholinesterase receptors (nAChRs) enhance neurotransmitter release from glutamatergic terminals, including those originating in the prefrontal cortex that signal reinforcement (67, 68). Nicotine first activates and then deactivates nAChRs, with the deactivation lasting for 24 h and depressing the release of glutamate. Exogenous nicotine is an important adjuvant in supporting the ability of stimuli to function as reinforcers during this time, despite their longer-term negative role.
Nicotine is not the only villain, because nicotine replacement therapies make only a small dent in the probability of return to habitual use (69, 70). Alternate sources of nicotine, such as gum, patches, and inhalers, are seldom addictive (71, 72); abusers of pure nicotine are unknown (73). Smoking denicotinized cigarettes is as likely to cause relapse as smoking regular cigarettes (21). “Cigarette smoking can no longer be viewed in simplistic terms as an addiction to nicotine without regard to nonnicotine factors” (74); some of the other chemicals in tobacco must complement nicotine to make cigarettes so addictive.
Tobacco contains monoamine oxidase inhibitors (MAOIs) (75, 76), reviewed in ref. 77, which drive MAO activity 40% below normal levels. MAOs catalyze the oxidation of monoamines such as dopamine (78). By inhibiting MAOs, tobacco increases the base levels of neurotransmitters that play important roles in attention, motivation, and reinforcement. The MAOIs in tobacco enhance the reinforcing strength of nicotine (79, 80); sensitization to nicotine, which typically decreases to baseline over 3 mo, may be elevated much longer after treatment with MAOIs (81). The half-life for the washout of the motor effects of selegiline, an MAOI used to treat Parkinson's disease, is 8 d (82), on the same order as the effects shown in Fig. 4. In terms of direct effects and interactions with nicotinic effects and serotonergic effects (83), MAOIs have thus become a prime suspect in tobacco addiction (77, 84).
Therapy with MAOIs is no better than other therapies that ameliorate withdrawal symptoms (85, 86); indeed, nicotine withdrawal in the presence of MAOIs generates long-lasting aversion to associated stimuli (87). This is because MAOIs surfeit the brain with dopamine and other monoamine neurotransmitters, causing a down-regulation of D2 receptors (88, 89). Nicotine can boost neurotransmission at glutamatergic synapses over those elevated thresholds. Both are conveniently available in a cigarette, which affords both the instant relief of nicotine and conditioned relaxation/reinforcement, plus replenishment of MAOIs to maintain the need [a fact well-understood by the industry (90)]. During smoking cessation and the return of MAOs, the level of ambient dopamine decreases, lowering the level from which the elevated glutamatergic thresholds must be surmounted. Regrowth of dopamine receptors awaits the slow return of MAOs, and they may never fully reach their original density (88), which might explain why ex-smokers have a lifelong hazard for relapse. Treatment with MAOIs in conjunction with nicotine (86) may provide breathing space to change smoking habits, but it only delays the repopulation of D2 receptors. Other sources of MAOIs, such as alcohol, can also reset the clock on attempts to quit tobacco.
Survival functions from a number of addictions have a similar form (55); down-regulation of D2 receptors is also a universal feature of addictions (89, 91, 92), which may underlie that similarity of form. Addictive substances cause “a combination of chronic elevation of reward set point fueled by decreased function of reward circuits and recruitment of anti-reward systems” and that, inter alia, “acute withdrawal from all major drugs of abuse produces increases in reward thresholds, and increases in anxiety-like responses” (93). Fig. 1 may, therefore, provide a general model of recovery, because similar brain processes may—with differences in detail—underlie all addictions (94–96).
Discussion
Eq. 1 distills an extended process into four key parameters, each of psychological interest. The rate at which users attempt to quit (α) reflects personal and social forces, including public health or legislative interventions. Like all of the parameters, it is directly affected by the criteria for inclusion in the study or category. The severity of withdrawal symptoms affects the rate of return to regular use (ρ), which is reduced by nicotine replacement therapies. The speed of physiological renormalization may be captured by β. Friends who smoke, the use of alcohol, and other circumstantial events modulate the probability of lapsing after successful abstention, which is captured by λ. In the handful of studies analyzed here, interventions primarily reduced the odds of relapse to smoking from the initial state of withdrawal (ρ/β). The model permits more powerful inference concerning the probability of long-term recidivism, which is shown with a model comparison approach using AICc as a criterion for asymptotic efficacy of treatment relative to baseline. That index can, in turn, be used to predict the replicability of the intervention's effect (97).
Eq. 1 is a dynamic recurrent model that characterizes the process of quitting tobacco (25). Most smokers attempt to quit multiple times; their reentry into the flow is governed by α. Conversely, most individuals who have quit remain at risk, which is reflected in finite values of λ. Rather than attempting to suppress reentry or lament recidivism, this model permits mensuration of those natural flows. It permits new measures of the success of intervention, such as the excess number of cigarette-free days, and average residence time in the states (T).
A model such as Fig. 1 can abet the search for processes at other levels (98). Analogous compartment models may describe the renormalization of monoamine receptors after their destabilization by the MAOIs in tobacco. Ability to support nAChR functioning during this phase, which ameliorates the symptoms of withdrawal while speeding up the repopulation of dopamine receptors, is likely to be a key component of successful therapies. Because receptor recovery may never be complete, the last phase of abstention is seldom secure (λ > 0). Availability of and encouragement to use sources of nicotine that are free from MAOIs, such as lozenges, nasal sprays, and e-cigarettes (99), or deliver fewer MAOIs and carcinogens (100, 101) may be useful prostheses for the long haul (102). Conversely, cigarettes go famously well with alcohol and coffee, which contain substantial MAOIs (73, 103), and commensurately increase the risk of relapse (48, 104, 105). Giving up so many of life's pleasures when attempting to quit tobacco may seem to compound the impossible, but giving up cigarettes will prove impossible for many without such austerity.
Acknowledgments
I thank Elena Koustova, Paul Schnur, and David Shurtleff at the National Institute on Drug Abuse (NIDA) for introduction to the field, Jed Rose for encouragement to publish, Alan Leshner and Donald Pfaff for the opportunity to develop the model for this audience, and Federico Sanabria, Natalie Peartree, and anonymous reviewers for helpful comments on the manuscript.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Quantification of Behavior” held June 11-13, 2010, at the AAAS Building in Washington, DC. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/quantification.
This article is a PNAS Direct Submission.
References
- 1.Centers for Disease Control and Prevention From the Centers for Disease Control and Prevention. Annual smoking attributable mortality, years of potential life lost and economic costs—United States, 1995–1999. JAMA. 2002;287:2355–2356. [PubMed] [Google Scholar]
- 2.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- 4.Salber EJ, Abelin T. Smoking behavior of Newton school children—5-year follow-up. Pediatrics. 1967;40:363–372. [PubMed] [Google Scholar]
- 5.DiFranza JR, et al. Symptoms of tobacco dependence after brief intermittent use: The Development and Assessment of Nicotine Dependence in Youth-2 study. Arch Pediatr Adolesc Med. 2007;161:704–710. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- 6.Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Arch Intern Med. 1997;157:335–340. [PubMed] [Google Scholar]
- 7.al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhu SH, et al. Telephone counseling for smoking cessation: Effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996;64:202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 9.Gilpin E, Pierce JP. Measuring smoking cessation: Problems with recall in the 1990 California Tobacco Survey. Cancer Epidemiol Biomarkers Prev. 1994;3:613–617. [PubMed] [Google Scholar]
- 10.Nides MA, et al. Predictors of initial smoking cessation and relapse through the first 2 years of the Lung Health Study. J Consult Clin Psychol. 1995;63:60–69. doi: 10.1037//0022-006x.63.1.60. [DOI] [PubMed] [Google Scholar]
- 11.Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology (Berl) 1992;108:495–500. doi: 10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- 12.Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- 13.Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 14.Hughes JR, et al. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 15.Sonnenberg FA, Beck JR. Markov models in medical decision making: A practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 16.Frenk H, Dar R. A Critique of Nicotine Addiction. Boston: Kluwer; 2000. [Google Scholar]
- 17.Heyman GM. Addiction: A Disorder of Choice. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 18.Littrell J. Perspectives emerging from neuroscience on how people become addicted and what to do about it. J Soc Work Pract Addict. 2010;10:229–256. [Google Scholar]
- 19.Fowler JS, et al. Reversible inhibitors of monoamine oxidase-A (RIMAs): Robust, reversible inhibition of human brain MAO-A by CX157. Neuropsychopharmacology. 2010;35:623–631. doi: 10.1038/npp.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross SR. Introduction to Probability Models. 6th Ed. San Diego: Academic; 1997. [Google Scholar]
- 21.Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychol. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- 22.Hughes JR. Craving among long-abstinent smokers: An Internet survey. Nicotine Tob Res. 2010;12:459–462. doi: 10.1093/ntr/ntq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: A systematic review of trials. Arch Intern Med. 2006;166:828–835. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- 24.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: A meta-analysis. Addict Behav. 2008;33:1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters EN, Hughes JR. The day-to-day process of stopping or reducing smoking: A prospective study of self-changers. Nicotine Tob Res. 2009;11:1083–1092. doi: 10.1093/ntr/ntp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 27.West R, Sohal T. “Catastrophic” pathways to smoking cessation: Findings from national survey. BMJ. 2006;332:458–460. doi: 10.1136/bmj.38723.573866.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes JR, Callas PW. Data to assess the generalizability of samples from studies of adult smokers. Nicotine Tob Res. 2010;12:73–76. doi: 10.1093/ntr/ntp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiffman S, Di Marino ME, Sweeney CT. Characteristics of selectors of nicotine replacement therapy. Tob Control. 2005;14:346–355. doi: 10.1136/tc.2004.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 31.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation (Cochrane Review) The Cochrane Library. 2003. (John Wiley & Sons, Chichester, UK), Issue 4. Available at http://personales.unican.es/ayestaf/esh/Nicotine%20replacement%20therapy%20for%20smoking%20cessation.htm.
- 32.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd Ed. New York: Springer; 2002. [Google Scholar]
- 33.Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self-help video for cigarette smoking cessation. J Consult Clin Psychol. 1997;65:663–672. doi: 10.1037//0022-006x.65.4.663. [DOI] [PubMed] [Google Scholar]
- 34.Hughes JR. Effects of abstinence from tobacco: Etiology, animal models, epidemiology, and significance. A subjective review. Nicotine Tob Res. 2007;9:329–339. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- 35.Stapleton JA, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction. 1995;90:31–42. doi: 10.1046/j.1360-0443.1995.901316.x. [DOI] [PubMed] [Google Scholar]
- 36.Richmond RL, Kehoe L. Ten-year survival outcome of the nicotine transdermal patch with cognitive behavioural therapy. Aust N Z J Public Health. 2007;31:282–285. doi: 10.1111/j.1467-842x.2007.00062.x. [DOI] [PubMed] [Google Scholar]
- 37.Jamerson BD, et al. Late-term smoking cessation despite initial failure: An evaluation of bupropion sustained release, nicotine patch, combination therapy, and placebo. Clin Ther. 2001;23:744–752. doi: 10.1016/s0149-2918(01)80023-0. [DOI] [PubMed] [Google Scholar]
- 38.Nordstrom BL, et al. Predictors of continued smoking over 25 years of follow-up in the normative aging study. Am J Public Health. 2000;90:404–406. doi: 10.2105/ajph.90.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blöndal T, Franzon M, Westin A. A double-blind randomized trial of nicotine nasal spray as an aid in smoking cessation. Eur Respir J. 1997;10:1585–1590. doi: 10.1183/09031936.97.10071585. [DOI] [PubMed] [Google Scholar]
- 40.Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: Randomised trial with six year follow up. BMJ. 1999;318:285–288. doi: 10.1136/bmj.318.7179.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 42.Swan GE, Ward MM, Jack LM, Javitz HS. Cardiovascular reactivity as a predictor of relapse in male and female smokers. Health Psychol. 1993;12:451–458. doi: 10.1037//0278-6133.12.6.451. [DOI] [PubMed] [Google Scholar]
- 43.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method) BMJ. 1998;317:1572–1580. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satagopan JM, et al. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiffman S, West RJ, Gilbert DG, SRNT Work Group on the Assessment of Craving and Withdrawal in Clinical Trials Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res. 2004;6:599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- 46.Shiffman S, et al. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- 47.Stapleton JA, Sutherland G, Russell MAH. How much does relapse after one year erode effectiveness of smoking cessation treatments? Long-term follow up of randomised trial of nicotine nasal spray. BMJ. 1998;316:830–831. doi: 10.1136/bmj.316.7134.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: Findings from the VA Normative Aging Study. Nicotine Tob Res. 2002;4:95–100. doi: 10.1080/14622200110098428. [DOI] [PubMed] [Google Scholar]
- 49.Hughes JR, Carpenter MJ, Naud S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine Tob Res. 2010;12:756–762. doi: 10.1093/ntr/ntq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider NG, et al. Efficacy of a nicotine nasal spray in smoking cessation: A placebo-controlled, double-blind trial. Addiction. 1995;90:1671–1682. doi: 10.1046/j.1360-0443.1995.901216719.x. [DOI] [PubMed] [Google Scholar]
- 51.Cummings SR, Richard RJ. Optimum cutoff points for biochemical validation of smoking status. Am J Public Health. 1988;78:574–575. doi: 10.2105/ajph.78.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider NG, et al. Efficacy of a nicotine inhaler in smoking cessation: A double-blind, placebo-controlled trial. Addiction. 1996;91:1293–1306. [PubMed] [Google Scholar]
- 53.Lindsey JC, Ryan LM. Tutorial in biostatistics methods for interval-censored data. Stat Med. 1998;17:219–238. doi: 10.1002/(sici)1097-0258(19980130)17:2<219::aid-sim735>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 54.Tønnesen P, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: Results from the European CEASE trial. Eur Respir J. 1999;13:238–246. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- 55.Kirshenbaum AP, Olsen DM, Bickel WK. A quantitative review of the ubiquitous relapse curve. J Subst Abuse Treat. 2009;36:8–17. doi: 10.1016/j.jsat.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: Results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98:1395–1402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- 57.Hatziandreu EJ, et al. Quitting smoking in the United States in 1986. J Natl Cancer Inst. 1990;82:1402–1406. doi: 10.1093/jnci/82.17.1402. [DOI] [PubMed] [Google Scholar]
- 58.Roethig HJ, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob Res. 2009;11:1216–1225. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 59.Hughes JR. Clinical significance of tobacco withdrawal. Nicotine Tob Res. 2006;8:153–156. doi: 10.1080/14622200500494856. [DOI] [PubMed] [Google Scholar]
- 60.Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 61.Bevins RA, Caggiula AR. The Motivational Impact of Nicotine and Its Role in Tobacco Use. New York: Springer; 2009. [Google Scholar]
- 62.Windom RE. 20th Report of the Surgeon General. Washington, DC: Surgeon General, Superintendent of Documents; 1988. The health consequences of smoking: Nicotine addiction. [Google Scholar]
- 63.Donny EC, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- 64.Palmatier MI, et al. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rose JE. Nicotine addiction and treatment. Annu Rev Med. 1996;47:493–507. doi: 10.1146/annurev.med.47.1.493. [DOI] [PubMed] [Google Scholar]
- 67.Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- 68.Markou A. Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bała MM, Leśniak W, Strzeszyński Ł. Efficacy of pharmacological methods used for treating tobacco dependence: Meta-analysis. Pol Arch Med Wewn. 2008;118:20–28. [PubMed] [Google Scholar]
- 70.Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: A systematic review and meta-analysis. BMC Public Health. 2006;6:300. doi: 10.1186/1471-2458-6-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dar R, Frenk H. Do smokers self-administer pure nicotine? A review of the evidence. Psychopharmacology (Berl) 2004;173:18–26. doi: 10.1007/s00213-004-1781-2. [DOI] [PubMed] [Google Scholar]
- 72.West R, et al. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 2000;149:198–202. doi: 10.1007/s002130000382. [DOI] [PubMed] [Google Scholar]
- 73.Talhout R, Opperhuizen A, van Amsterdam JGC. Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacol. 2007;17:627–636. doi: 10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 74.Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 75.Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: β-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun. 2005;326:378–386. doi: 10.1016/j.bbrc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 76.Fowler JS, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- 77.Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 78.Youdim MBH, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 79.Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 80.Guillem K, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Villégier AS, Blanc G, Glowinski J, Tassin JP. Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol Biochem Behav. 2003;76:267–274. doi: 10.1016/s0091-3057(03)00223-5. [DOI] [PubMed] [Google Scholar]
- 82.Hauser RA, Holford NHG. Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson's disease. Mov Disord. 2002;17:961–968. doi: 10.1002/mds.10226. [DOI] [PubMed] [Google Scholar]
- 83.Lanteri C, et al. Inhibition of monoamine oxidases desensitizes 5-HT1A autoreceptors and allows nicotine to induce a neurochemical and behavioral sensitization. J Neurosci. 2009;29:987–997. doi: 10.1523/JNEUROSCI.3315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 85.George TP, Weinberger AH. Monoamine oxidase inhibition for tobacco pharmacotherapy. Clin Pharmacol Ther. 2008;83:619–621. doi: 10.1038/sj.clpt.6100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berlin I. Monoamine oxidase inhibitors for smoking cessation. In: George TP, editor. Medication Treatments for Nicotine Dependence. Boca Raton, FL: CRC; 2007. pp. 109–120. [Google Scholar]
- 87.Guillem K, Vouillac C, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically prolongs the duration of nicotine withdrawal-induced place aversion. Biol Psychiatry. 2008;63:158–163. doi: 10.1016/j.biopsych.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 88.Norman AB, Battaglia G, Creese I. Differential recovery rates of rat D2 dopamine receptors as a function of aging and chronic reserpine treatment following irreversible modification: A key to receptor regulatory mechanisms. J Neurosci. 1987;7:1484–1491. doi: 10.1523/JNEUROSCI.07-05-01484.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fehr C, et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- 90.Henningfield JE, et al. Reducing tobacco addiction through tobacco product regulation. Tob Control. 2004;13:132–135. doi: 10.1136/tc.2003.006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Amsterdam J, Talhout R, Vleeming W, Opperhuizen A. Contribution of monoamine oxidase (MAO) inhibition to tobacco and alcohol addiction. Life Sci. 2006;79:1969–1973. doi: 10.1016/j.lfs.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 95.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 96.Huang SY, et al. Monoamine oxidase-A polymorphisms might modify the association between the dopamine D2 receptor gene and alcohol dependence. J Psychiatry Neurosci. 2007;32:185–192. [PMC free article] [PubMed] [Google Scholar]
- 97.Ashby FG, O'Brien JB. The Prep statistic as a measure of confidence in model fitting. Psychon Bull Rev. 2008;15:16–27. doi: 10.3758/pbr.15.1.16. [DOI] [PubMed] [Google Scholar]
- 98.Miller R, et al. How modeling and simulation have enhanced decision making in new drug development. J Pharmacokinet Pharmacodyn. 2005;32:185–197. doi: 10.1007/s10928-005-0074-7. [DOI] [PubMed] [Google Scholar]
- 99.Bullen C, et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomised cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 100.Furberg H, et al. Is Swedish snus associated with smoking initiation or smoking cessation? Tob Control. 2005;14:422–424. doi: 10.1136/tc.2005.012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gartner CE, et al. Assessment of Swedish snus for tobacco harm reduction: An epidemiological modelling study. Lancet. 2007;369:2010–2014. doi: 10.1016/S0140-6736(07)60677-1. [DOI] [PubMed] [Google Scholar]
- 102.Caldwell B, Burgess C, Crane J. Randomized crossover trial of the acceptability of snus, nicotine gum, and Zonnic therapy for smoking reduction in heavy smokers. Nicotine Tob Res. 2010;12:179–183. doi: 10.1093/ntr/ntp189. [DOI] [PubMed] [Google Scholar]
- 103.Herraiz T, Chaparro C. Human monoamine oxidase enzyme inhibition by coffee and β-carbolines norharman and harman isolated from coffee. Life Sci. 2006;78:795–802. doi: 10.1016/j.lfs.2005.05.074. [DOI] [PubMed] [Google Scholar]
- 104.Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the Normative Aging Study. Addict Behav. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- 105.Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- 106.Gilbert DG, et al. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. J Consult Clin Psychol. 2002;70:142–152. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- 107.Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- 108.Hurt RD, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 109.Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addict Behav. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]