Abstract
Two hallmarks of the Firmicute phylum, which includes the Bacilli and Clostridia classes, are their ability to form endospores and their "Gram-positive" single-membraned, thick-cell-wall envelope structure. Acetonema longum is part of a lesser-known family (the Veillonellaceae) of Clostridia which form endospores, but which are surprisingly "Gram-negative", possessing both an inner and outer membrane and a thin cell wall. Here we present macromolecular resolution, 3-D electron cryo-tomographic images of vegetative, sporulating, and germinating A. longum cells showing that during the sporulation process, the inner membrane of the mother cell is inverted and transformed to become the outer membrane of the germinating cell. Peptidoglycan persists throughout, leading to a new, "continuous" model of its role in the process. Coupled with genomic analyses, these results point to sporulation as a mechanism by which the bacterial outer membrane may have arisen, and A. longum as an exciting "missing link" between single- and double-membraned bacteria.
INTRODUCTION
For decades bacteria have been classified into two main groups by whether or not they retain crystal violet, the so-called "Gram" stain. Gram-positive cells have a single membrane and a thick peptidoglycan (PG) cell wall, which retains the stain where as Gram-negative cells are enclosed by two membranes separated by a thin layer of PG, which does not retain the stain. While more recently the terms Gram "-positive" and "-negative" have fallen out of favor in the face of richer phylogenetic distinctions, the presence of either one or two enclosing membranes remains a fundamentally intriguing difference between bacterial species. Transport across the inner membrane (IM) of double-membraned bacteria and the single membrane of single-membraned bacteria is tightly regulated, as these membranes sustain proton gradients essential for metabolism. Outer membranes (OM)s of double-membraned bacteria are structurally and functionally quite different, containing large amounts of the immunologically important macromolecule lipopolysacharide (LPS, or "endotoxin") and numerous beta-barrel protein porins that allow passive diffusion of small molecules. Assuming the first cells were enclosed by a single membrane, it is unclear how and why second membranes evolved (Bos et al., 2007; Lake, 2009).
In the original bacterial classifications, Gram-positives were assigned to the phylum Firmicutes. Many species of the bacterial phylum Firmicutes respond to adverse growth conditions by forming endospores (Piggot and Hilbert, 2004). Sporulation begins with DNA replication, chromosome segregation and packing, asymmetric positioning of the Z-ring, and septation (reviewed in (Margolin, 2002)). This yields a mother cell and a daughter cell, or “prespore”, that are separated by a double-membraned septum. After septum formation the mother cell engulfs the prespore in a process morphologically similar to phagocytosis. Inside the mother cell the forespore matures, adding several layers of a protein coat and in some species an exosporium. Finally, when the mother cell lyses, the mature spore is released. These resting forms can remain viable for thousands of years without water or nutrients and can resist, among other environmental insults, UV irradiation, heat, pH extremes and oxidative damage (Setlow, 2007). When favorable conditions return, the spores germinate and new progeny emerge via outgrowth.
For decades, the model organism for studying both sporulation and the "Gram-positive" cell type has been the bacterium Bacillus subtilis. B. subtilis was the first sporulating bacterium to have its genome sequenced and in many ways is an excellent model organism. Its natural competency has facilitated genetic and biochemical characterization and its large size has benefited traditional electron microscopy (EM) and light microscopy (LM) investigations. Largely because in EM images of sporulating Gram-positive cells, the septum was clearly thinner than the thick, vegetative cell wall (Bechtel and Bulla, 1976), it has long been thought that any PG present in the septum is degraded before engulfment begins. Furthermore, little attention was paid to the fate of the OsM, since it was not part of the future germinating cell.
Acetonema longum is part of a lesser-known family of the Firmicutes (the Veillonellaceae), which form endospores, but which are surprisingly "Gram-negative": they stain Gram-negative, they are enveloped by two membranes and a thin cell wall, and their OMs contain LPS (Hofstad, 1978; Hofstad and Kristoffersen, 1970; Kane and Breznak, 1991; Mergenhagen, 1965; Rainey, 2009). Like B. subtilis, A. longum forms endospores that are both pasteurization-resistant and calcium dipicolinate-containing (Kane and Breznak, 1991). Germination results, however, in a double-membraned, Gram-negative cell, calling attention to the origin of the OM and the periplasmic PG.
Also unlike B. subtilis, A. longum cells are slender enough to image intact in a near-native state by electron cryo-tomography (ECT). Previous images of B. subtilis and other sporulating cells were obtained by chemically fixing, dehydrating, plastic embedding, sectioning, and staining the samples. Such approaches sometimes fail to preserve important details or even introduce misleading artifacts (Pilhofer et al., 2010). ECT involves neither plastic embedding nor staining, yielding "macromolecular" resolution, three-dimensional (3-D) images of biological samples in near-native, frozen-hydrated states (Ben-Harush et al.; Li and Jensen, 2009). ECT has been used for example to identify the architectures of the bacterial flagellar motor and chemoreceptor arrays (Briegel et al., 2009; Chen et al., 2011; Liu et al., 2009).
In this study, we imaged vegetative, sporulating and germinating A. longum cells and endospores with ECT. ECT analyses were supplemented with LM, immunofluorescent labeling, Western blotting, traditional EM, mass spectrometry, genome sequencing, and phylogenetic profiling. The images and analyses show that a thin PG layer persists in the septum throughout sporulation and germination, that a protein coat (likely SpoIVA) forms densely packed concentric rings on the mother side of the engulfing septum, and that a region of the IM of the mother cell is inverted and transformed to become the OM of the outgrowing cell. A. longum's peculiar characteristics as an endospore-forming Gram-negative cell suggest interesting new explanations for the evolution of all Firmicutes and the bacterial OM.
RESULTS
Vegetative cell ultrastructure
The life cycle of A. longum cells was studied first by LM. Two vegetative morphologies were observed: flexible rods between 50 – 70 µm in length and rigid rods less than 10 µm in length (Figure S1). The shorter form was observed as cultures entered stationary phase and was the form that produced endospores. Crystal violet was not retained at any time during the life cycle, establishing at least by the simple definition that A. longum cells are Gram-negative (data not shown).
Ultrastructural ECT studies (Figures 1–3A–E, Movies S1 and S2 available on line) revealed that the cell envelope of vegetative cells comprised an IM, periplasm, and an OM typical of other Gram-negative bacteria (Figure 1A) (Briegel et al., 2006; Komeili et al., 2006). Closer inspection of the periplasmic space (28 nm wide) revealed the presence of 2–3 PG-like periplasmic layers ~2-nm thick equally spaced 6 nm apart (peak-to-peak, as all other measurements reported below)(Figure 3A and Figure S3A). These multiple layers appeared similar to one another and were consistently present in the ~200 vegetative cells imaged. In areas where the OM was distended, the periplasmic layers remained associated with the IM, suggesting that the layers were more closely associated with the inner rather than the outer membrane.
Figure 1. Stages of sporulation in A. longum.
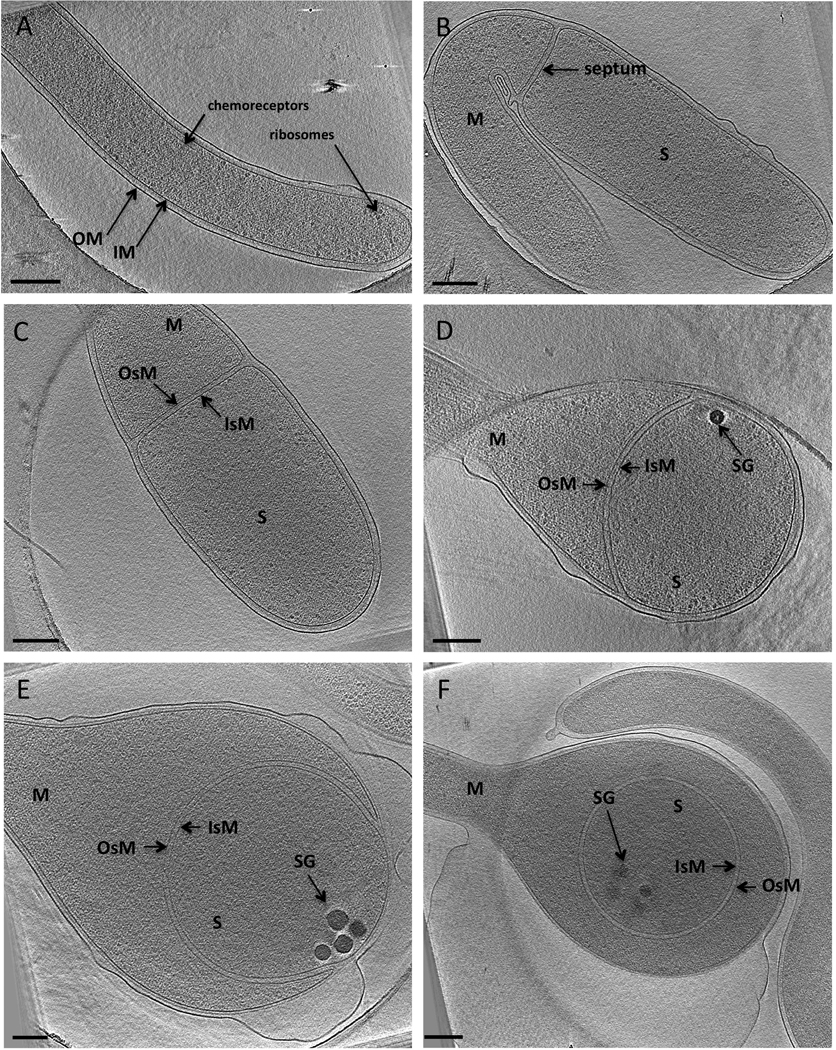
(A) A vegetative cell showing typical Gram-negative cell wall architecture.
(B) A sporulative septum separates the mother cell (M) from the prespore (S). The septum is formed from the inner membrane (IM) of the mother cell.
(C) The diameter of the prespore enlarges before engulfment, and the septum eventually turns into the inner spore membrane (IsM) and the outer spore membrane (OsM) of the prespore.
(D) Engulfment begins as the IsM and OsM curve and move along the mother cell wall. Storage granules appear at the leading edges of the engulfing membranes (SG, black bodies).
(E) The prespore continues to enlarge and eventually becomes spherical. The number and size of SGs increases as engulfment proceeds.
(F) Engulfment is completed and a forespore surrounded by an IsM and an OsM is formed in the middle of the mother cell. Each panel is a 20-nm thick tomographic slice through a 3-D reconstruction of an intact cell. Scale bar 200 nm (note E and F are slightly smaller scale to show the entire cell pole).
See also Figure S1 and Figure S2, Table S1 and Movie S1.
Figure 3. Structural details.
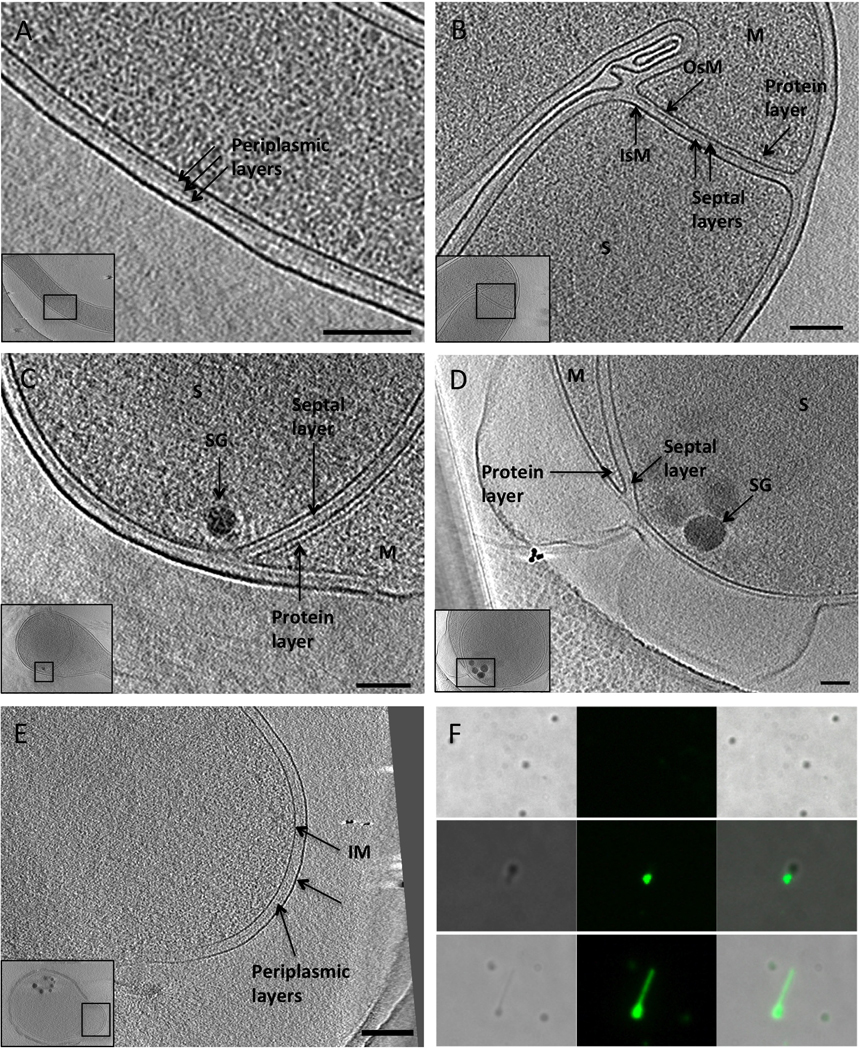
(A) 2 to 3 periplasmic layers are observed between the IM and OM of vegetative cells.
(B) Septa at early stages of sporulation exhibit two septal layers of density between the IsM and OsM. The layers are continuous with the innermost periplasmic layer. A layer of protein density is observed on the mother side of the septum.
(C) Leading edge of an engulfing membrane. A layer of septal material is present between the IsM and OsM, a protein layer is connected to the mother side of a septum and appears as regularly spaced densities connected to the OsM, and storage granules appear at the leading edge of the engulfing membranes.
(D) Advanced stage of engulfment. The number and size of the SG increases, the septal layer between the IsM and OsM is still observed, a protein layer is also present on the mother side of OsM.
(E) Presence of periplasmic layers between the IM and the OM of an outgrowing cell. Insets show the area of a cell that has been magnified. Scale bars 200 nm.
(F) Immunofluorescence images of quiescent (top) and outgrowing spores (middle and bottom) showing that LPS appears on the surface A. longum cells immediately upon outgrowth.
See also Figure S5 and Figure S6.
Vegetative septa
Two different types of septa were observed by ECT (Figure S2). While vegetative septa occurred at ~10 µm intervals along the long, flexible cells, sporulation septa were observed at the tips (~1.5 µm away from the poles) of the short (10 µm), straight cells. Vegetative septa were smaller than sporulation septa, and while the OMs surrounding vegetative septa ingressed, those surrounding sporulative septa remained flat (Figure 1B and Figure S2B).
Sporulation
Sporulation begins as the IM invaginates in a ring and constricts until a closed septum is formed across the cell, separating the prespore from the mother cell. Despite repeated attempts, we were unable to find cells exhibiting partial septa, suggesting that septum formation in A. longum is rapid. Figure 1B shows a complete sporulative septum accompanied by a minor enlargement in the diameter of the prespore (prespore calculated volume of 0.22 ± 0.07 µm3, Table S1). PG-like layers were also seen in the septum (Figure S3B). Closer inspection of the junction between the septum and the mother cell wall revealed that the innermost periplasmic layer of the mother cell was continuous with the septal layers (Figure 3B–D). As sporulation proceeded, the diameter of the spore continued to increase until it was 2–3 times the diameter of a vegetative cell. In addition, small dense bodies (likely storage granules) were formed at the leading edges of the engulfing membranes (Figure 1D and Figure 3C). Interestingly, the number and size of the storage granules (SGs) in the prespore increased as engulfment proceeded, reaching a final number of 8–12 per spore, with diameters ranging from 40 to 120 nm and together accounting for ~7% of the forespore volume (Figure 1E and Figure 3D).
As engulfment proceeded, the edge of the septum moved towards the spore pole along the sides of the mother cell wall. Our tomograms and volume calculations revealed that even though the shape of the prespore changed from cylindrical to spherical, the volume increase was slight (Table S1; Movie S2). Fusion of the leading edges of the engulfing membranes resulted in the formation of a forespore. While both membranes surrounding the forespore originated from the IM of the mother cell, we refer to these as the inner spore membrane (IsM) and the outer spore membrane (OsM) to distinguish them from the inner and outer membranes of the mother cell (Figure 1F). PG-like layers were observed between the IsM and OsM throughout engulfment.
Spore maturation
After engulfment and before the release of the mature spore via lysis of the mother cell, two layers of cortex between the IsM and OsM as well as coat layers outside the OsM of the forespore became apparent (Figure 2A–B). Due to the larger size of the sporulating cell and increased sensitivity to the electron beam, the reconstructions of the maturation process within the mother (5 total) were lower quality. In Figure 2A the OsM nevertheless appears thicker and less regular, indicating the beginning of the coat formation. Figure 2B shows the beginning of cortex synthesis. In this cell, the space between the IsM and OsM had increased to ~40 nm and separate PG-like layers were no longer visible, suggesting the accumulation of cortex.
Figure 2. Spore maturation, germination and outgrowth.

(A) A forespore (S) in the middle of a mother cell (M) is surrounded by a double membrane (IsM and OsM) and a PG layer between them. Multiple layers of coat are deposited on the outside of the OsM.
(B) The IsM and OsM are separated apart (40 nm) as cortex is synthesized.
(C) The main features of a mature spore: core, storage granules (SG), IsM, inner and outer cortex (ICx, OCx, respectively), OsM, coat, exosporium (Ex, also see D).
(D) A germinating spore shows that cortex (Cx) hydrolysis is uneven. The IsM and OsM come closer together as the cortex gets degraded.
(E) Cortex degradation nears completion before outgrowth begins.
(F) Outgrowth of a bacterium shows the new IM and OM that are derived from an IsM and the OsM. Again each panel is a 20-nm thick tomographic slice through a 3-D reconstruction. Scale bars 200 nm.
See also Figure S3 and Figure S4.
Mature spores
Due to the size and beam-sensitivity of mature spores, we augmented our studies of mature spores with traditional, room-temperature EM of plastic embedded thin-sections (Figure S4). Two layers of cortex, labeled ICx and OCx, for "inner" and "outer" cortex, respectively, were apparent based on their differential staining with osmium tetroxide and uranyl acetate. The same two layers could also be seen in cryo-tomograms, though with smaller overall difference in contrast (Figure 2C). ECT of plunge-frozen A. longum spores revealed the continuing presence of the IsM and OsM as well as an exosporium (Ex) composed of tightly packed, hair-like structures ~200 nm in length and 2–4 nm in diameter (Figure 2D). From the inside out, the following distinct features were therefore discernable in a mature spore: core, SG, IsM, ICx, OCx, OsM, spore coat and Ex. Notably, membranes, SGs and the Ex were not preserved by traditional EM methods, and may therefore have been less apparent in previous studies.
Germination and outgrowth
Pure spores (either heat-shocked or not) were placed in fresh 4YACo medium to induce germination and then plunge-frozen and imaged by ECT. No difference in the morphology between the heat-treated and untreated spores was observed, but since heat-shocked spores yielded faster-growing cultures, heat-shock likely promoted germination. Spores at different stages of germination and outgrowth were present at each time point after induction, making it difficult to synchronize the cells or determine the duration of germination. Vegetative cells were observed 12 hours after inoculation, however, indicating that the germination process can be completed within this amount of time. During germination, the cortex degraded non-uniformly (Figure 2D) until the distance between the IsM and OsM was again typical of a vegetative cell (~28 nm, Figure 2E and Figure S3C). Notably, where the OsM separated from the coat, the densities of the IsM and OsM appeared similar, suggesting that both membranes still had similar compositions (Figure S3C). Finally, the rupture of the protein coat of the spore accompanied outgrowth (Figure 2F). The area of the coat rupture where the new vegetative cell emerges has been termed ‘the cap’ (Steichen et al., 2007). Multiple periplasmic layers were again discernable during outgrowth (Figure 3E and Figure S3D).
The OM of A. longum possesses LPS
Following the progression of the septa in the cryo-tomograms revealed that the OM of germinating cells was derived from an inverted section of the mother cell's IM. Because the bacterial inner and OMs are so different in structure and function, the composition of A. longum's OM was checked by several methods. First, whole cells of A. longum (and B. subtilis as negative control) were subjected to the Limulus Amebocyte Lysate test, a highly sensitive, FDA-approved test for LPS. While the A. longum sample contained LPS (126200 EU/ml), the B. subtilis sample did not (less than 0.5 EU/ml). Next, LPS was purified from A. longum (with E. coli BL21 and B. subtilis serving as positive and negative controls, respectively). An antibody raised against the lipid A portion of LPS from E. coli O157 cells and shown to recognize the LPS of other typical Gram-negative bacteria was used for Western blots, which exhibited the hallmark ladder-like appearance of LPS (Chart et al., 1992)(Figure S6A). Pro-Q® Emerald 300 stains confirmed the presence of the expected carbohydrate components of LPS (Figure S6B), controlling against the possibility of false positives from the antibody. Finally, the composition of A. longum’s LPS was investigated by GC-MS: following water:phenol extraction, in the water layer various glycosyl residues were found as well as several beta-hydroxy fatty acids, Kdo, and GlcN, all well-established hallmarks of LPS (Figure S6C and S6D).
With the presence of LPS in A. longum cultures firmly established, its location and time of appearance on the surface of outgrowing cells was investigated by immunofluorescence. While pure spores showed no immunofluorescence, immediately after outgrowth, the tip of the emerging cell fluoresced strongly (Figure 3F). Later stages of outgrowth also showed fluorescent signal from the outgrowing vegetative cell. Positive and negative E. coli BL21 and B. subtilis control cells behaved as expected (Figure S5).
Protein layer coating the OsMs
A layer of ordered protein density was present on the outside of the engulfing OsM of sporulating cells (Figure 3B–D). The densities appeared equally spaced, were ~2.4 nm wide, and showed clear connectivity to the OsM. Since this layer appeared right after septum formation and was present throughout engulfment, we believe that one of the components of the protein layer is likely SpoIVA (Ramamurthi et al., 2006). This protein layer was clearly visible in a density profile of the septum (Figure S3B) and was located ~12 nm (peak to peak) from the OsM. By projecting this layer of density onto the OsM, the layer was seen to be organized as a series of parallel, concentric cables (Figure 4A). Accounting for the well-understood effects of the "missing wedge" in tomography, which elongates all densities along the direction of the electron beam and makes lines perpendicular to the beam difficult to discern, this pattern means that the cables form either concentric rings or a spiral (Figure 4B).
Figure 4. Higher order structure of a protein layer on the mother side of the septum.

(A) Density projections of a protein layer on the mother side of the OsM. Left column: tomographic slices through the cells showing the shape of the OsM and the protein density connected to it. Right column: projection of all the density within 5–15 nm on the mother cell's side of the OsM, revealing concentric, parallel cables. Control projections of density both further into the mother cell and on the spore side of the IsM appear random (data not shown).
(B) Schematic representation of the pattern generated by the observed protein layer (red rings) on the mother side of the OsM.
Negative-staining and ECT studies of purified sacculi
As described above, PG-like layers were present within the periplasm of vegetative cells, within septa throughout engulfment, and within the forespore between the IsM and OsM (Figure 3). To test whether the septal layers contained PG, sacculi of A. longum cells at different stages of engulfment were purified and imaged. While sacculi of vegetative cells were rod shaped, sacculi from sporulating cells were easily recognized by their enlarged poles. Negative staining of the purified sacculi showed round, approximately semi-circular dark patches near the tip of the enlarged pole consistent with the pattern that would be expected for collapsed prespores surrounded by the peptidoglycan of the mother cell (Figure 5A and Figure S7). Other sacculi exhibited very dark, spherical, spore-like shapes near the center of the enlarged pole suggesting that once the spore cortex matures, spore contents were trapped inside despite the harsh preparative procedure (Figure S7F). All imaged mother cell sacculi contained extra densities suggestive of prespore, forespore, or mature sacculus PG layers inside, but folds in the collapsed sacculi collected stain and complicated detailed interpretation.
Figure 5. Peptidoglycan is present between the IsM and OsM during engulfment.

(A) Images of negatively-stained, collapsed sacculi of engulfing cells.
(B) Tomographic slices through reconstructions of purified sacculi of engulfing cells. Black arrows mark the prespore PG and white arrows mark folds in the mother cell PG caused by the collapse of the sacculi on the EM grid. The inset shows a slice where the prespore PG can be seen merging with the mother cell PG at the tip.
(C) Two models for the structure and role of PG during sporulation. The key difference is the presence of PG between the IsM and OsM during engulfment, as highlighted in the red intermediate unique to the “continuous PG” model.
See also Figure S7 and Figure S8.
Purified sacculi were therefore also imaged with ECT (Figure 5B and Figure S8). In cryo-tomograms the cell wall surrounding sporulating poles was noticeably thicker than the wall surrounding vegetative cells. Numerous granules were observed within the sacculi of sporulating but not vegetative cells. These are likely glycogen, since energy storage is expected during sporulation and the purification procedure involved proteases, nucleases, harsh denaturants and detergents (boiling SDS) that would have destroyed other non-carbohydrate cellular macromolecules. While the granules were densely packed within the collapsed sacculi, they would not have been so in the inflated, living state. Prominent folds in the mother cell wall exhibited a characteristic pattern of two thick walls on either side of a cleft bereft of granules. Fully engulfed spores were easily identified due to their high contrast, size (known well from cryo-tomography of intact cells), and random placement within the enlarged pole of the mother cell. In addition to the folds, granules, and forespores, however, a thinner, vegetative-PG-like sack could be seen. The shape and size of the sacks matched those of engulfing prespores and the sacks were always found at the extreme tip of the mother cell. In some cases the boundary of the sack could be seen merging with the thicker mother cell wall around the tip, as would be expected of an engulfing septal PG. Numerous small, high contrast (black) spherical densities were also observed scattered over the region enclosed by and along the boundary of the sacks, as if they were specifically bound to the collapsed sack material, fortuitously marking its extent amidst the folds. We conclude that engulfing prespores are surrounded by PG.
Genome analysis
The genome of A. longum was sequenced using a 454 Flex Pyrosequencing platform. Based on SSU rRNA analysis A. longum belongs to the bacterial phylum Firmicutes (Kane and Breznak, 1991). Within this phylum, A. longum groups with the Veillonellaceae, a phylogenetically coherent family within the class Clostridia (Figure 6) (Rainey, 2009).
Figure 6. SSU rRNA phylogenetic tree.

The tree was constructed using the Fitch Distance method with Kimura 2-parameter correction and was based on 1117 unambiguously aligned nucleotide positions. The A. longum 16S gene sequence was aligned to the database, which incorporated the Silva SSU alignment (www.arb-silva.de), using parsimony methods. Values at nodes represent percent support for 1000 step bootstrap parsimony analyses of the dataset. Bar represents 10% sequence variation. Symbols: ●documented sporulation, Ø sporulation not observed. Where no symbol is given, spore formation was not classified. ✶ Certain members of the phylum Firmicutes are classified as Gram-negative or Gram-variable.
To investigate how the seldomly-combined sporulation and Gram-negative phenotypes could have arisen in A. longum, we compared A. longum's genome to those of other bacteria. First, using phylogenetic profiling (see Supplemental Experimental Procedures), we ranked the Pfam domains most strongly associated with the classification "Gram-negative", and then separately, with the ability to form endospores (from 1080 and 769 completed genomes, respectively). As expected, the genes most strongly correlated with Gram-negativity coded for known OM proteins such as LpxB-D and K, TolC, and Omp85. Components of the flagellar motor that localize to the OM (FlgH and FlgI) also scored highly (in the top 45, data not shown). Again not surprisingly, the genes that correlated most strongly with endospore formation included SpoIVA, SpoIIIAE, SpoIIP, and YabG. Homologs of nearly all the genes near the top of both lists were found in A. longum (Table S2). Thus, A. longum possesses a typical OM with proteins homologous to those found in numerous other Gram-negatives and forms endospores with the same basic machinery as other Firmicutes. These facts argue against either phenotype arising from convergent evolution.
To investigate whether either phenotype was likely the result of a recent horizontal gene transfer across phyla, we calculated phylogenetic trees for Omp85, TolC, LpxB-D, SecY, FlgH and FlgI, which are associated with Gram-negativity, and SpoIVA, SpoIIP, SpoVB and SpoIIIAE, which are predictive of endospore formation. One example of each (Omp85 and SpoIVA) is shown in Figure S9. The Omp85 protein family tree grouped A. longum with other members of Veillonellaceae but showed no clustering to any particular class of Gram-negative bacteria (Figure S9A). In contrast, the evolutionary relationship of chloroplasts to cyanobacteria and mitochondria to α-proteobacteria was clear. The SpoIVA family tree revealed A. longum and Thermosinus carboxydivorans, another member of the family Veillonellaceae, as a deeply branching and robustly-supported clade within the Clostridia class, separate from the Bacilli (Figure S9B), as expected from SSU rRNA-based species phylogeny. The trees for the other genes analyzed gave similar results, arguing against recent horizontal gene transfers as the source of either phenotype.
DISCUSSION
This study reports a comprehensive imaging survey of the processes of sporulation and germination in A. longum. Compared to previous work, our observations are novel in at least three ways: (1) we imaged a Gram-negative sporulating bacterium; (2) the cells were preserved in a near-native, "frozen-hydrated" state free from fixation, dehydration, and staining artifacts; and (3) the cells were imaged tomographically, resulting in full 3-D reconstructions. As a result, new details about periplasmic and septal layers, storage granules, protein coats, and membranes were obtained suggesting new models for the role of PG in sporulation and the evolution of the OM in bacteria.
Protein localization on the mother side of the engulfing membranes
We observed a layer of concentric rings (Figure 4A) on the mother side of the septum throughout engulfment (Figure 3B–D) that was clearly connected to the OsM. A candidate for the observed density is SpoIVA, an ATPase that is synthesized under the control of σE and appears immediately after septation (Roels et al., 1992; Stevens et al., 1992). In vitro, SpoIVA irreversibly self-assembles into bundles of cables ~10 nm in diameter (Ramamurthi and Losick, 2008). SpoIVA has been shown to be the base layer for attachment of more than 50 other coat proteins to the outer surface of the maturing spore after engulfment is completed, starting with CotE and CotJ (Driks, 2002). The predicted globular diameter (2.4 nm) (Erickson, 2009) and the width of the observed densities in our tomograms (~2.5 nm), supports the notion that the regularly spaced cables in A. longum correspond to SpoIVA, and we found a clear homolog of SpoIVA in the genomic sequence of A. longum (55% sequence identity to the protein in B. subtilis). Thus, we believe SpoIVA polymerizes into densely packed, concentric rings as engulfing membranes propagate around the prespore, exposing additional surface at the periphery (Figure 4B).
Ramamurthi et al. recently proposed a mechanism for how SpoIVA is localized to the septum in B. subtilis. A 26-amino-acid protein, SpoVM, forms an amphipathic helix that adheres to convex membranes via hydrophobic interactions (Ramamurthi and Losick, 2009). Affinity chromatography experiments showed that SpoIVA binds to SpoVM, suggesting that SpoVM targets SpoIVA to the curved engulfing membranes on the mother side of a septum. The literature has been ambiguous, however, about whether homologs of SpoVM exist in Clostridia (Onyenwoke et al., 2004; Prajapati et al., 2000). We searched for homologs in all sequenced Clostridia and many other sporulating species. Endospore-formers outside the family Veillonellaceae that possessed spoIVA also had a homologous sequence for spoVM, but surprisingly, the only two members of the family Veillonellaceae that have been sequenced and shown to sporulate (T. carboxydivorans and A. longum) lacked spoVM but had spoIVA. While it is possible that the sequencing coverage was incomplete, another possibility is that a different protein or mechanism serves the role of SpoVM in Veillonellaceae. We favor this idea because the putative SpoIVA protein layer we saw on the mother side of a septum localized there even before the septum began curving (Figure 3B), an observation quite different from B. subtilis (Ramamurthi et al., 2009).
Peptidoglycan and cortex
Because A. longum possesses both an inner and an OM throughout sporulation and germination, the data presented here call new attention to the continuity and development of the PG and cortex structures that fill the intermembrane space. PG is required for septation (Yanouri et al., 1993), and previous EM studies have shown PG is present in the septum right after septation (Hilbert and Piggot, 2004), but the fate of the septal PG thereafter is an active area of research. Thin-section EM images of sporulating Bacilli exhibit septa that are thinner and more irregular than the external cell wall, calling into question whether PG is retained (Gueiros-Filho, 2007). Numerous studies have reported that PG in the septum is completely degraded, starting at the center of the septum where closure occurs (Errington, 2003; Smith et al., 2000). PG hydrolase activity has been detected during engulfment and mutants that lack key PG hydrolases fail to complete engulfment (Errington, 1993; Illing and Errington, 1991). The formation of the SpoIIQ - SpoIIIAH channel between the mother and the forespore was suggested to depend on the lack of PG between the septum membranes (Blaylock et al., 2004), and SpoIIB was identified as the PG hydrolase necessary for degrading PG (Perez et al., 2000). PG hydrolysis was also shown to persist throughout membrane migration (Gutierrez et al., 2010). Following engulfment, two new layers of material were described between the IsM and OsM (Doi, 1989), and the synthesis of these new layers was defined as “stage IV” of sporulation (Buchanan et al., 1994). Based on the compositions of the two layers, it was suggested that one gives rise to the spore cortex, which is later discarded in germination, while the other persists to become the future vegetative cell wall (Henriques and Moran, 2007; Tipper and Linnett, 1976). Taken together, the model that emerges from the existing literature is therefore one of "complete degradation/re-synthesis," in which PG is used to advance the septum, but then is completely degraded, only to be re-synthesized later de novo as a double-layered foundation for the cortex and the future vegetative cell wall (Figure 5C).
PG-like densities were observed by cryo-tomography here, however, in the vegetative cell wall, within septa, between the IsM and OsM, in the cortex of mature spores, and during outgrowth. A key issue is whether these layers, and in particular the septal layers, are PG, or alternatively, if one or more are simply proteinaceous, consisting of for instance the periplasmic domains of integral membrane proteins or other proteins closely associated with a membrane. To test this, we purified sacculi from sporulating cells. The procedure involves treatments with detergents, nucleases, proteases, and boiling denaturants to remove all other cellular components besides large carbohydrate superstructures like glycogen granules and PG. If PG persists in septa throughout sporulation, engulfing sacculi should exhibit internal prespore compartments ranging from nascent septa to partially closed hemispheres (Figure 5C). With traditional EM and cryo-tomography methods, we observed many sacculi from engulfing cells that contained an additional internal "sack" fully consistent in size, shape, and position with prespores (Figure 5; Figure S7 and Figure S8). Thus, our results support a new, much simpler, "continuous PG" model in which the peptidoglycan required for septum formation is not completely hydrolyzed (Figure 5C). Instead, the PG produced during septation is remodeled during engulfment, then elaborated to form the two layers of cortex during spore maturation, and finally restored to its original (thin) state during germination to become the cell wall of the vegetative PG (Figure 3E). This model obviates the need to construct new PG layers de novo (either the cortex or later the outgrowing cell wall), a process without known precedent (Joseleau-Petit et al., 2007).
It seems reasonable that the cortex in pure spores could be derived from the septal PG because the structure of cortical PG is similar to vegetative PG, though less cross-linked (Atrih et al., 1996; Popham et al., 1996). The two septal PG layers likely serve as the foundation of the two cortices, which are simply expanded with modified building blocks. PG remodeling would require hydrolases such as SpoIIB and SpoIID, as described above, but also PG synthases to either drive or at least accommodate the morphological changes. Two promising candidate PG synthetases are MurG and MurAA, whose inhibition by fosfomycin blocked engulfing membrane migration (Meyer et al., 2010). Homologs of MurG and MurAA are present in A. longum.
Once the environment becomes favorable, spores germinate. For this to occur, nutrient molecules have to bind to receptors located on the IsM (Setlow, 2008). The first triggered event upon germination initiation is the release of dipicolinic acid and calcium ions from the spore coat and their replacement by water. Previous studies on Bacillus anthracis suggested that the release of these molecules also stimulated cortex hydrolases (such as YaaH (Dowd et al., 2008)), which in our model would be needed to degrade the thick layers of cortex between the inner and outer forespore membranes. A molecular determinant that governs the hydrolysis of the cortex material but not the vegetative PG during germination could be the difference in composition and cross-linking between the two. At the end of cortex degradation in A. longum, we observed the thinning of the two cortex layers into what became the multiple periplasmic layers in vegetative cells (Figure 2F and Figure S3D), supporting the notion that both are PG.
The continuous PG model has an intriguing implication. It suggests that A. longum can expand a thin, "Gram-negative" PG layer into a thick cortical layer that in other Firmicutes becomes the Gram-positive cell wall, and then reverse the process in germination. This suggests that Gram-negative and -positive cell walls share a common foundation and basic architecture. By direct imaging, we recently showed that the Gramnegative PG wall is "layered" (Gan et al., 2008), so Gram-positive walls are probably layered too (for other possibilities, see (Dmitriev et al., 2003; Hayhurst et al., 2008)).
Transformation of an IM into an OM
As with Bacillus and Clostridium spp., the sporulation septum in A. longum was generated by invagination of the IM (Figure 1B). This septum formed the two membranes (the IsM and the OsM) that surrounded the immature spore and that were visible throughout engulfment (Figure 1B–F). Even though the IsM and OsM were not observed in the plastic-embedded sections of pure spores (Figure S4) (Henriques and Moran, 2007), they were discernable in the cryo-tomograms (Figure 2B–E). The inner and outer cortex layers were deposited between the inner and OsMs. The OsM was clearly visible during coat hydrolysis and then outgrowth (Figure 2E–F), when it emerged as the OM of the germinating cell (Movies S2 and S3).
The presence or absence of an OsM during spore maturation and germination has not been discussed previously, since the other members of Bacilli and Clostridia that have been studied possess only a cytoplasmic membrane and therefore lose their OsM during outgrowth. As a simple result of the topology of engulfment, however, two membranes surround the mature endospores of all sporulating bacteria. Thus, a common morphological state exists between all double- and single-membraned sporulating bacteria. A. longum retains its second membrane during germination whereas other endospore-forming Bacilli and Clostridia lose theirs. Intriguingly, then, our data show that A. longum's OM is derived from an "inverted" IM of the mother cell.
OMs are typically very different, however, from IMs in protein composition, the presence of lipopolysaccharide, and permeability. Yet, by all indications, A. longum's second membrane is a bona fide, fully differentiated OM that is part of a typical Gram-negative bacterial cell envelope: (1) A. longum fails to retain the Gram stain just like all other typical Gram-negative cells (data not shown); (2) the OM of A. longum appears just slightly denser in cryo-tomograms than the IM (Figure 3A–C; Figure S3A), just as has been observed in other Gram-negative bacteria (Briegel et al., 2009), confirming its special composition and structure; (3) the entire envelope, including the width of the periplasm, the appearance of the peptidoglycan layers, and the presence of flagella spanning both membranes with accompanying rings are all typical of Gram-negative bacteria; (4) A. longum produces LPS, as confirmed here by Western blotting, Pro-Q® Emerald 300 staining, the LAL test, and gas chromatography-mass spectrometry (Figure S6, (Kane and Breznak, 1991); (5) the LPS appears on the surface of cells immediately upon outgrowth, as shown here through immunofluorescence (Figure 3F and Figure S5A); and finally (6) 22 of the top 25 Pfam domains that correlate most strongly with Gram-negativity are present in A. longum including OM proteins (Omp85, TolC), flagellar proteins (FlgH, FlgI), periplasmic chaperones (Skp), and LPS biosynthesis proteins (LpxA-C, MsbA) (Table S2A). (Ironically, the term "OM" was actually first applied to a member of the Veillonellaceae (Bladen and Mergenhagen, 1964), and only later was the term widely accepted and used to describe the structure in all other Gram-negatives!)
The IM of sporulating A. longum cells must therefore be transformed in composition and function during sporulation, germination, and outgrowth into a typical OM. We do not know exactly when this transformation occurs, but based on our immunofluorescent experiments (Figure 3F) and density profiles of membranes at different stages of sporulation (Figure S3), we hypothesize that LPS is synthesized when the outgrowing cell begins to elongate out of the spore. As the OM grows, new material including LPS and beta-barrel porins is likely added. This then simply dilutes out the initial IM material.
Evolutionary implications
How the OM first arose in Bacteria is an interesting question (Cavalier-Smith, 2004). It has been suggested that a symbiosis between an ancient actinobacterium and an ancient clostridium produced the last common ancestor of all double-membraned bacteria (Lake, 2009). Over long periods of time, some or all of the outer genome could have transferred into the inner organism. An alternative hypothesis is that a single-membraned cell autogenously evolved the machinery to synthesize a second membrane. One could imagine an internal vesicle being repurposed to serve as an IM, and the genome being moved to within the vesicle, or alternatively that external patches of membrane arose and then expanded over generations (proteins are known which could stabilize unclosed patches of membrane). If it were ever found that spheroplasts (Gram-negative bacterial cells stripped of their OM) could recover their OMs by extruding OM material, this would constitute strong support for this hypothesis.
Our work with A. longum reveals a third possible mechanism, however, and shows that it does in fact happen in nature frequently (every time A. longum germinates): second membranes are generated by sporulation and can then be transformed into typical LPS-containing OMs. Considering a primordial single-membraned cell with the capability to form a septum and divide, a small number of mutations might be required to cause the membrane of one daughter cell to stick to the other, which might eventually lead to some kind of engulfment and primitive sporulation. The single genome directing this process would already be found inside the spore. If upon escape from the engulfing mother cell (some kind of primitive “germination”), the second membrane were retained, as it is in A. longum, a double-membraned vegetative cell would emerge. Over generations the OM could have evolved to have very different properties than the IM. Whether or not this is the mechanism by which all OMs in Bacteria arose we may never know, but A. longum makes it clear that the basic membrane transformations can and do happen.
One test of this hypothesis (that a primitive sporulation-like process gave rise to the bacterial OM) is whether A. longum's abilities to elaborate an OM and sporulate are ancient and widely shared, or alternatively are the result of either recent horizontal gene transfers or convergent evolution. To explore these questions we used phylogenetic profiling to objectively rank genes that are most predictive of an organism's ability to either sporulate or form an OM, and then created phylogenetic trees of several top-scoring genes (Figure S9). In each of the proteins we analyzed (Omp85, TolC, LpxB-D, SecY, FlgH and FlgI, for OM), A. longum grouped with Thermosinus carboxydivorans, another member of the Veillonellaceae family, but not with any other Gram-negative bacteria outside the Veillonellaceae (Figure S9A). Thus, there is no evidence to suggest that recent lateral gene transfer played a role in its acquisition of an OM. The possibility of convergent evolution of the OM in the Veillonellaceae is ruled out by the presence of many unambiguous homologs of different subsystems associated with OM biogenesis and function in other Gram-negative phyla. Concerning sporulation, phylogenetic trees of Firmicute sporulation genes showed the same basic evolutionary relationships as their 16S ribosomal subunit (Figure S9B), again arguing against any recent horizontal gene transfer or convergent evolution. A. longum's machinery to form an OM and sporulate therefore derives from widely shared, ancient sources, supporting our hypothesis that the origin of the OM in all Bacteria could have been a primitive sporulation.
Finally, our studies of A. longum shed light on the evolution of the Firmicute phylum itself. Since all endospores pass through a common state with two membranes, we speculate that the last common Firmicute ancestor was also double-membraned. The diversity of the Firmicutes and the associated phylum Tenericutes could then be explained by losses of OM and/or sporulation properties rather than separate evolutionary gains: some members of the family Veillonellacea, including A. longum, retained both properties while others (such as Selenomonas ruminantium) lost the ability to sporulate but retained an OM. Conversely, many of the Clostridia and Bacilli retained their ability to form endospores but lost their OM, perhaps for reasons of increased sporulation and germination efficiency. Others (such as Ruminococcus productus) lost both the ability to sporulate and their OM, as did the Tenericutes, which further discarded PG.
Loss of entire gene suites is plausible, since it is now known, for instance, that there are very few pseudogenes in bacteria, as these are removed right away (Kuo and Ochman, 2010), and that mitochondria have lost nearly all of their genes. It is certainly easier to imagine unneeded genes being lost from bacterial lineages than gained, since acquisition requires that they be integrated into the existing networks in a fashion that immediately enhances (or at least preserves) cellular fitness. It is important to note, however, that our hypothesis does not require that modern Firmicutes be ancestral to any or all of the Gram-negatives. We are only proposing that a single-membraned last common ancestor of both Firmicutes and Gram-negatives possessed some primitive sporulation/phagocytotic-like ability, which gave rise to the OM. From this newly double-membraned sporulation ancestor many phyla could have diverged. Any one of these could have lost the OM and given rise to the Firmicutes. Other phyla could have lost the ability to sporulate at different times, giving rise to the current models of microbial phylogeny.
EXPERIMENTAL PROCEDURES
Sample preparation
Vegetative A. longum strain APO-1 (DSM 6540) and sporulating cells were grown anaerobically as described in (Leadbetter and Breznak, 1996). For ECT studies on sporulation, cells were harvested from cultures entering stationary phase. At this time, phase-bright (mature) spores were clearly visible and were still contained within mother cells. Pure spores were harvested by centrifugation and purified from mother cells as in (Nicholson and Setlow, 1990). For ECT studies on germination, purified spores were added to growth medium and either heat-shocked or not. Images of cells harvested 6, 7, 8 and 9 days after inoculation and spores harvested 0h, 4h, 8h, 12h and 24h after inoculation were collected. Sacculi were prepared as in (Poindexter and Hagenzieker, 1981). Samples were plunge-frozen across EM grids as described (Iancu et al., 2006).
EM data collection and processing
Cryo-tomograms of vegetative (~200), sporulating (~150) and germinating (~50) A. longum cells and mature spores (~50) were collected using an FEI Polara™ (FEI Company, Hillsboro, OR, USA) 300 kV FEG transmission electron microscope equipped with a Gatan energy filter and a lens-coupled 4k × 4k UltraCam (Gatan, Pleasanton, CA). Three-dimensional reconstructions were calculated using IMOD (Kremer et al., 1996) and analyzed with Amira (Mercury Computer systems) and in-house software. Purified sacculi were dried on glow-discharged EM grids, stained with 1% uranyl acetate, and imaged with a Tecnai T12 EM. Chemical fixation of pure spores was based on protocols developed by Sabatini et al (Sabatini et al., 1963).
Immunofluorescence
Germinating spores were fixed, washed, deposited onto glass slides, air-dried, blocked, and incubated with primary and then secondary antibodies. The primary antibody used in this study was a polyclonal goat Ab raised against the lipid A portion of LPS from E. coli O157. See Supplemental Experimental Procedures for more details.
Phylogenetic analysis
Genomic DNA was sequenced using a 454 Flex Pyrosequencing platform. Protein phylogenies were constructed using the MUSCLE algorithm (Edgar, 2004). Trees were constructed using PHYLIP. Values given above the nodes of trees represent percent support (when 70% or greater) calculated using 100 bootstrap maximum likelihood analyses of the datasets. Nodes indicated with dots are confirmed and supported by 100 bootstrap protein parsimony analyses (when 70% or greater).
Supplementary Material
Figure 7. Schematic highlighting key findings.

Frames 1–3 were taken from Movie S2 and illustrate the transformation of an IM (IM, green) to an OsM (green) and finally the OM (black) of an outgrowing cell. A yellow star is used to show that the IM also gets inverted in the process. PG is shown in red and is present in the septum throughout engulfment, elaborating to form the cortex of the mature spore and then degrading to restore the thin cell wall of the outgrowing cell.
Acknowledgments
We thank Dr. Alasdair McDowall and Mark Ladinsky for their help with the preparation of mature spores by traditional EM methods and Dr. Martin Pilhofer for helping with the immunofluorescence and Western blotting experiments. The GC-MS analysis of LPS was performed at the Complex Carbohydrate Research Center and was supported by DOE grant DE-FG02-09ER20097. We thank Everett Kane for the generation of Movie S2 and Jane H. Ding for helping with the generation of Movie S1. We thank Ivan Tochev for generating Figure 4B. We thank Dr. Stephen Quake and Richard White at Stanford University for performing the shotgun genome sequencing of A. longum. The authors gratefully acknowledge the IGS annotation engine. This work was supported by a Natural Sciences and Engineering Research Council of Canada Postdoctorate Fellowship (to E.I.T.), the Howard Hughes Medical Foundation, and gifts to Caltech from the Gordon and Betty Moore Foundation including support for the Caltech Center for Integrative Study of Cell Regulation. E.G.M was supported by the Department of Energy (award DE-FG02-07ER64484 to J.R.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atrih A, Zollner P, Allmaier G, Foster SJ. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel DB, Bulla LA., Jr Electron microscope study of sporulation and parasporal crystal formation in Bacillus thuringiensis. J Bacteriol. 1976;127:1472–1481. doi: 10.1128/jb.127.3.1472-1481.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Harush K, Maimon T, Patla I, Villa E, Medalia O. Visualizing cellular processes at the molecular level by cryo-electron tomography. J Cell Sci. 2010;123:7–12. doi: 10.1242/jcs.060111. [DOI] [PubMed] [Google Scholar]
- Bladen HA, Mergenhagen SE. Ultrastructure of Veillonella and Morphological Correlation of an OM with Particles Associated with Endotoxic Activity. J Bacteriol. 1964;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock B, Jiang X, Rubio A, Moran CP, Jr, Pogliano K. Zipper-like interaction between proteins in adjacent daughter cells mediates protein localization. Genes Dev. 2004;18:2916–2928. doi: 10.1101/gad.1252704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Robert V, Tommassen J. Biogenesis of the gram-negative bacterial OM. Annu Rev Microbiol. 2007;61:191–214. doi: 10.1146/annurev.micro.61.080706.093245. [DOI] [PubMed] [Google Scholar]
- Briegel A, Dias DP, Li Z, Jensen RB, Frangakis AS, Jensen GJ. Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography. Mol Microbiol. 2006;62:5–14. doi: 10.1111/j.1365-2958.2006.05355.x. [DOI] [PubMed] [Google Scholar]
- Briegel A, Ortega DR, Tocheva EI, Wuichet K, Li Z, Chen S, Muller A, Iancu CV, Murphy GE, Dobro MJ, et al. Universal architecture of bacterial chemoreceptor arrays. Proc Natl Acad Sci U S A. 2009;106:17181–17186. doi: 10.1073/pnas.0905181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CE, Henriques AO, Piggot PJ. Bacterial cell wall. 1 edn. Vol 1. Elsevier Science Pub Co; 1994. [Google Scholar]
- Cavalier-Smith T. Organelles, genomes, and eukaryote phylogeny: an evolutionary synthesis in the age of genomics. London: CRC Press; 2004. [Google Scholar]
- Chart H, Okubadejo OA, Rowe B. The serological relationship between Escherichia coli O157 and Yersinia enterocolitica O9 using sera from patients with brucellosis. Epidemiol Infect. 1992;108:77–85. doi: 10.1017/s0950268800049529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Beeby M, Murphy GE, Leadbetter JR, Hendrixson DR, Briegel A, Li Z, Shi J, Tocheva EI, Muller A, et al. Structural diversity of bacterial flagellar motors. EMBO J. 2011 doi: 10.1038/emboj.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev BA, Toukach FV, Schaper KJ, Holst O, Rietschel ET, Ehlers S. Tertiary structure of bacterial murein: the scaffold model. J Bacteriol. 2003;185:3458–3468. doi: 10.1128/JB.185.11.3458-3468.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi RH. Sporulation and germination. In: Harwood CR, editor. Bacillus. London: Plenum; 1989. pp. 169–215. [Google Scholar]
- Dowd MM, Orsburn B, Popham DL. Cortex peptidoglycan lytic activity in germinating Bacillus anthracis spores. J Bacteriol. 2008;190:4541–4548. doi: 10.1128/JB.00249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driks A. Maximum shields: the assembly and function of the bacterial spore coat. Trends Microbiol. 2002;10:251–254. doi: 10.1016/s0966-842x(02)02373-9. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online. 2009 doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol. 2003;1:117–126. doi: 10.1038/nrmicro750. [DOI] [PubMed] [Google Scholar]
- Gan L, Chen S, Jensen GJ. Molecular organization of Gram-negative peptidoglycan. Proc Natl Acad Sci USA. 2008;105:18953–18957. doi: 10.1073/pnas.0808035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueiros-Filho F. Bacillus Celular and Molecular Biology. 1 edn. Vol 1. Caister Academic Press; 2007. [Google Scholar]
- Gutierrez J, Smith R, Pogliano K. SpoIID peptidoglycan hydrolase activity is required throughout engulfment during Bacillus subtilis sporulation. J Bacteriol. 2010;192:3174–3186. doi: 10.1128/JB.00127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhurst EJ, Kailas L, Hobbs JK, Foster SJ. Cell wall peptidoglycan architecture in Bacillus subtilis. Proc Natl Acad Sci U S A. 2008;105:14603–14608. doi: 10.1073/pnas.0804138105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques AO, Moran CP., Jr Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol. 2007;61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- Hilbert DW, Piggot PJ. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol Mol Biol Rev. 2004;68:234–262. doi: 10.1128/MMBR.68.2.234-262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstad T. Chemotypes of Veillonella lipopolysaccharides. Acta Pathol Microbiol Scand B. 1978;86:47–50. doi: 10.1111/j.1699-0463.1978.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Hofstad T, Kristoffersen T. Chemical composition of endotoxin from oral Veillonella. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78:760–764. doi: 10.1111/j.1699-0463.1970.tb04367.x. [DOI] [PubMed] [Google Scholar]
- Iancu CV, Tivol WF, Schooler JB, Dias DP, Henderson GP, Murphy GE, Wright ER, Li Z, Yu Z, Briegel A, et al. Electron cryotomography sample preparation using the Vitrobot. Nat Protocols. 2006;1:2813–2819. doi: 10.1038/nprot.2006.432. [DOI] [PubMed] [Google Scholar]
- Illing N, Errington J. Genetic regulation of morphogenesis in Bacillus subtilis: roles of sigma E and sigma F in prespore engulfment. J Bacteriol. 1991;173:3159–3169. doi: 10.1128/jb.173.10.3159-3169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau-Petit D, Liebart JC, Ayala JA, D'Ari R. Unstable Escherichia coli L forms revisited: growth requires peptidoglycan synthesis. J Bacteriol. 2007;189:6512–6520. doi: 10.1128/JB.00273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MD, Breznak JA. Acetonema longum gen. nov. sp. nov., an H2/CO2 acetogenic bacterium from the termite, Pterotermes occidentis. Arch Microbiol. 1991;156:91–98. doi: 10.1007/BF00290979. [DOI] [PubMed] [Google Scholar]
- Komeili A, Li Z, Newman DK, Jensen GJ. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science. 2006;311:242–245. doi: 10.1126/science.1123231. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kuo CH, Ochman H. The extinction dynamics of bacterial pseudogenes. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JA. Evidence for an early prokaryotic endosymbiosis. Nature. 2009;460:967–971. doi: 10.1038/nature08183. [DOI] [PubMed] [Google Scholar]
- Leadbetter JR, Breznak JA. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol. 1996;62:3620–3631. doi: 10.1128/aem.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jensen GJ. Electron cryotomography: a new view into microbial ultrastructure. Curr Opin Microbiol. 2009;12:333–340. doi: 10.1016/j.mib.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin T, Botkin DJ, McCrum E, Winkler H, Norris SJ. Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography: evidence for stator ring curvature and rotor/C-ring assembly flexion. J Bacteriol. 2009;191:5026–5036. doi: 10.1128/JB.00340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin W. Bacterial sporulation: FtsZ rings do the twist. Curr Biol. 2002;12:R391–R392. doi: 10.1016/s0960-9822(02)00882-5. [DOI] [PubMed] [Google Scholar]
- Mergenhagen SE. Polysaccharide-lipid complexes from Veillonella parvula. J Bacteriol. 1965;90:1730–1734. doi: 10.1128/jb.90.6.1730-1734.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P, Gutierrez J, Pogliano K, Dworkin J. Cell wall synthesis is necessary for membrane dynamics during sporulation of Bacillus subtilis. Mol Microbiol. 2010;76:956–970. doi: 10.1111/j.1365-2958.2010.07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, Setlow P. Dramatic increase in negative superhelicity of plasmid DNA in the forespore compartment of sporulating cells of Bacillus subtilis. J Bacteriol. 1990;172:7–14. doi: 10.1128/jb.172.1.7-14.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyenwoke RU, Brill JA, Farahi K, Wiegel J. Sporulation genes in members of the low G+C Gram-type-positive phylogenetic branch (Firmicutes) Arch Microbiol. 2004;182:182–192. doi: 10.1007/s00203-004-0696-y. [DOI] [PubMed] [Google Scholar]
- Perez AR, Abanes-De Mello A, Pogliano K. SpoIIB localizes to active sites of septal biogenesis and spatially regulates septal thinning during engulfment in Bacillus subtilis. J Bacteriol. 2000;182:1096–1108. doi: 10.1128/jb.182.4.1096-1108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis. Curr Opin Microbiol. 2004;7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Pilhofer M, Ladinsky MS, McDowall A, Jensen G. In: BacterialTEM: new insights from cryo-microscopy. In Electron Microscopy of Model Systems. Mueller-Reichert T, editor. Elsevier; 2010. [Google Scholar]
- Poindexter JS, Hagenzieker JG. Constriction and septation during cell division in caulobacters. Can J Microbiol. 1981;27:704–719. doi: 10.1139/m81-109. [DOI] [PubMed] [Google Scholar]
- Popham DL, Helin J, Costello CE, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati RS, Ogura T, Cutting SM. Structural and functional studies on an FtsH inhibitor from Bacillus subtilis. Biochim Biophys Acta. 2000;1475:353–359. doi: 10.1016/s0304-4165(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Rainey F. Bergey's manual of systematic bacteriology. 2nd edn. Vol 3. Springer; 2009. [Google Scholar]
- Ramamurthi KS, Clapham KR, Losick R. Peptide anchoring spore coat assembly to the outer forespore membrane in Bacillus subtilis. Mol Microbiol. 2006;62:1547–1557. doi: 10.1111/j.1365-2958.2006.05468.x. [DOI] [PubMed] [Google Scholar]
- Ramamurthi KS, Lecuyer S, Stone HA, Losick R. Geometric cue for protein localization in a bacterium. Science. 2009;323:1354–1357. doi: 10.1126/science.1169218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Losick R. ATP-driven self-assembly of a morphogenetic protein in Bacillus subtilis. Mol Cell. 2008;31:406–414. doi: 10.1016/j.molcel.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Losick R. Negative membrane curvature as a cue for subcellular localization of a bacterial protein. Proc Natl Acad Sci U S A. 2009;106:13541–13545. doi: 10.1073/pnas.0906851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels S, Driks A, Losick R. Characterization of spoIVA, a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:575–585. doi: 10.1128/jb.174.2.575-585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DD, Bensch K, Barrnett RJ. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007;15:172–180. doi: 10.1016/j.tim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Setlow P. Dormant spores receive an unexpected wake-up call. Cell. 2008;135:410–412. doi: 10.1016/j.cell.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Blackman SA, Foster SJ. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000;146(Pt 2):249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- Steichen CT, Kearney JF, Turnbough CL., Jr Non-uniform assembly of the Bacillus anthracis exosporium and a bottle cap model for spore germination and outgrowth. Mol Microbiol. 2007;64:359–367. doi: 10.1111/j.1365-2958.2007.05658.x. [DOI] [PubMed] [Google Scholar]
- Stevens CM, Daniel R, Illing N, Errington J. Characterization of a sporulation gene, spoIVA, involved in spore coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:586–594. doi: 10.1128/jb.174.2.586-594.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper DJ, Linnett PE. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976;126:213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanouri A, Daniel RA, Errington J, Buchanan CE. Cloning and sequencing of the cell division gene pbpB, which encodes penicillin-binding protein 2B in Bacillus subtilis. J Bacteriol. 1993;175:7604–7616. doi: 10.1128/jb.175.23.7604-7616.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


