INTRODUCTION
It is estimated that between 350,000 – 540,000 children became infected with HIV in 2007, nearly 90% of them living in sub-Saharan Africa.1 In resource-limited settings it is estimated that 35% of HIV-infected infants die by one year of age and 53% by age two.2 In a natural history cohort study of HIV-infected infants from resource-limited settings, disease progression was rapid, with 85% of infants meeting WHO criteria for ART initiation within 6 months of age.3 In addition, a randomized strategy trial showed that among infants aged 6 to 12 weeks with CD4% ≥25, early ART reduced mortality by 76% over a median follow up period of 32 weeks.4 Nevirapine (NVP) for prevention of mother to child transmission has been the first line agent used in most resource-limited settings; however, limited data in HIV-infected infants who have failed single dose NVP suggest that there may be a higher rate of virologic failure when NVP is used for treatment within the first year of life.5 It is therefore important to study highly active agents other than NVP that could be used to treat very young infants.
Lopinavir/ritonavir (LPV/r) is safe and effective in infants and children older than 6 weeks of age, but its pharmacokinetic profile in younger infants has not been studied. Because infants in the first months of life generally have immature drug elimination processes and altered absorption, extrapolating doses from older children risks different drug exposure in young infants and incomplete virologic suppression and/or potential toxicity. The current study was initiated to evaluate the dose requirements as well as the safety, tolerability, and efficacy of LPV/r in infants <6 months of age; the 24 week results from the older cohort (≥ 6 weeks to < 6 months) have been reported elsewhere6 and this report includes 24 week results for the cohort of infants ages ≥14 days and <6 weeks.
MATERIALS AND METHODS
Study Design
Pediatric AIDS Clinical Trials Group (PACTG) protocol P1030 was a prospective multicenter, Phase I/II open label trial performed in HIV-infected infants aged ≥14 days and <6 months in the United States and Brazil; this report is limited to the 24 week follow-up for infants enrolled younger than 6 weeks of age. The study treatment consisted of the liquid formulation of LPV/r in combination with two nucleoside reverse transcriptase inhibitors (NRTI’s) for HIV-infected infants enrolled at ≥14 days and <6 weeks of age. Infants weighing ≥2.5 kg with confirmed HIV infection and HIV-1 RNA >10,000 copies/ml within 30 days of study entry were eligible for enrollment. Prior treatment with LPV/r was not allowed. Infants were not enrolled if they had any of the following: 1) laboratory values consistent with ≥ Grade 3 values as measured by the 1994 Division of AIDS (DAIDS) Toxicity Tables for Grading Severity of Pediatric Adverse Experiences7 (in which a score of 0 indicates no toxicity, 1 indicates mild toxicity, 2 indicates moderate toxicity, 3 indicates severe toxicity and 4 indicates potentially life-threatening events), 2) a newly diagnosed acute opportunistic infection or serious bacterial infection at the time of enrollment, 3) chemotherapy for active malignancy, or 4) gestational age <32 weeks at birth.
Due to the probability of higher oral clearance of protease inhibitors in young infants8,9, study subjects were treated with a starting dose of lopinavir 300 mg/m2 of body surface area and ritonavir 75 mg/m2 (300/75 mg/m2) by mouth twice daily in combination with two NRTI’s chosen by the principal investigator in consultation with the protocol team. NNRTIs and other antiretrovirals were not allowed. Adherence was checked by asking caregivers to report missed doses of medications in the 3 days prior to each scheduled visit. A 12 hour intensive pharmacokinetic (PK) study was performed at study week 2. If the trough LPV concentration was <1 mcg/mL, which is the value considered to be the minimum effective concentration in treatment-naïve adults10, in a subject whose adherence was assessed to be adequate, the LPV/r doses were increased to 450/112.5 mg/m2 twice daily. If the subject’s lopinavir area under the curve (AUC0–12) was >170 mcg *h/mL, which is approximately double the average exposure in adults, the LPV/r dosage was reduced to 230/57.5 mg/m2 twice daily. In subjects requiring dosage adjustments, a repeat PK study was performed 2 weeks following the dosage change.
Criteria for permanent discontinuation of study treatment included any of the following: 1) Grade 4 or recurrent/persistent Grade 3 toxicity, 2) confirmed deterioration of CD4 percentage to less than half of the study entry level, 3) confirmed HIV RNA increase to >50,000 copies/ml following initial suppression, 4) parent/guardian request, or 5) investigator discretion related to non-compliance with the protocol.
Subjects were evaluated at the screening visit and at study entry before receipt of any treatment, at 2 and 4 weeks, then every 4 weeks through week 24. Physical examination and non-fasting laboratory evaluations (including electrolytes, glucose, blood urea nitrogen, creatinine, total bilirubin, aspartate aminotransferase, alanine aminotransferase, calcium, phosphorus, triglycerides, cholesterol, total amylase, complete blood count with differential and platelets, and HIV-1 RNA level) were performed during each visit. Lymphocyte surface markers were evaluated every 12 weeks.
The study was approved by the Institutional Review Board for each participating site as well as by the Brazilian National Ethics Committee, and written informed consent was obtained from each child’s legal guardian before performance of any study-specific procedure. Guidelines of the United States Department of Health and Human Services governing experimentation in human subjects were followed.
Pharmacokinetic methods
The intensive pharmacokinetic study at week 2 of LPV/r therapy consisted of blood samples obtained before the morning dose and 2, 4, 8 and 12 hours following an observed dose. In subjects requiring dosage adjustment, a repeat PK study was done 2 weeks after the dosage change with blood samples obtained pre-dose and at 4 and 12 hours post dose. Predose concentration samples were also drawn at weeks 8, 12, 16 and 24, stored at –70°C and batched for analysis, as no further dosage adjustments were made after the intensive PK study. A multi-analyte HPLC assay using reverse phase HPLC separation was used to quantitate lopinavir and ritonavir and was performed at St Jude’s pharmacology laboratory.6 The assay was validated and peer-reviewed in accordance with the PACTG guidelines and the laboratory participated in the biannual PACTG external Quality Control program throughout the study.
Pharmacokinetic measures were calculated using standard non-compartmental methods to determine LPV area under curve (AUC0–12), maximum concentration (Cmax), and predose concentration (Cpre). The trough was defined as the lowest observed concentration (typically Cpre or C12) and used to determine if a dose increase was necessary for a specific subject. Pharmacokinetic data were also analyzed using compartmental methods to determine apparent clearance (CL/F) and apparent volume of distribution (V/F). The pre-dose concentration was set as the initial starting condition, and each subject’s data were fit to a one compartment model with a first order absorption (Ka) and lag time, if necessary using a maximum likelihood estimation and ADAPTII version 4.11
Laboratory methods
Chemistries, complete blood counts and urinalyses were performed in local clinical laboratories, and lymphocyte subset analysis was performed by standard flow cytometry according to standard procedures. The Amplicor HIV-1 Monitor test, version 1.5 (Roche Molecular Systems) was used to determine quantitative plasma HIV-1 RNA (lower limit of quantitation (LLOQ) = 400 RNA copies/mL). All assays from the US subjects were performed at a single core virology laboratory, which is certified by Division of AIDS (DAIDS) Virology Quality Assurance Program12, and samples from Brazilian infants were analyzed locally by labs approved by DAIDS and the Brazilian Society of Clinical Pathologists. Plasma samples were stored at −70°C until the assay was performed. Samples from the first 28 days of the study were assayed in batch format to avoid inter-assay variability, while subsequent samples were assayed in real time.
Endpoints
Toxicity was defined as the development of diagnoses or ≥ Grade 3 signs and symptoms and laboratory abnormalities that occurred while on study treatment as evaluated using the 1994 DAIDS Toxicity Table7 and considered related or possibly related to study treatment as determined by the Study Team. The protocol definition of a virologic endpoint included the following: a) fewer than one log10 copies/ml decrease in HIV-1 RNA from baseline to week 8, b) HIV-1 RNA >400 copies/ml at week 16 of treatment, or c) rebound of HIV RNA to >4000 copies/ml after week 16; all endpoints required confirmation on a repeat sample.
RESULTS
Human Subjects
Ten infants were enrolled from August, 2003 to September, 2006. The infants were enrolled at 8 clinical centers in the United States (n=9) and one center in Brazil (n=1). Three of the study participants were female, and the median age of infants on the date study treatment was dispensed was 5.7 weeks (range 3.6 to 5.9 weeks). Seven infants were black non-Hispanic and three were Hispanic. At entry, the median (range) HIV-1 RNA was 6.0 (4.7–7.2) log10 copies/ml, CD4% was 41 (16–59) % and CD8% was 21 (15–37) %.
Study Treatment
Ten infants initiated LPV/r as 300/75 mg/m2/dose with background nucleoside therapy that included lamivudine (n=9) in combination with either stavudine (n=5) or zidovudine (n=4); the other child received zidovudine plus didanosine. There was a protocol-directed dose increase in LPV/r to 450/112.5 mg/m2 for one child instituted at week four, based on a week 2 predose LPV concentration < 1 mcg/mL. At the repeat pharmacokinetic evaluation at week 6, the LPV AUC exceeded the protocol-specified limit of 170 ug*h/mL and the LPV dosage was reduced to 315 mg/m2. At week 8, LPV/r was held for four days because of Grade 3 neutropenia. At week 9 the dosage was lowered back to the starting LPV/r dose of 300/75 mg/m2 because the measured AUC again exceeded the study-defined limit. In a second child, LPV/r doses were erroneously administered only once a day instead of twice a day for six days after starting treatment before the deviation was discovered and corrected. No child permanently discontinued study treatment at or before week 24.
Pharmacokinetics
Pharmacokinetic measures from the intensive study at week 2 are available for 9 out of 10 enrolled subjects; one subject vomited after the observed dose, and the guardian refused to repeat the intensive PK study; therefore no results for this child were included in the intensive pharmacokinetic summary. One subject with poor adherence at the week 2 visit had the PK evaluation repeated; the PK data collected at the repeat evaluations while adherent were included in the data analysis.
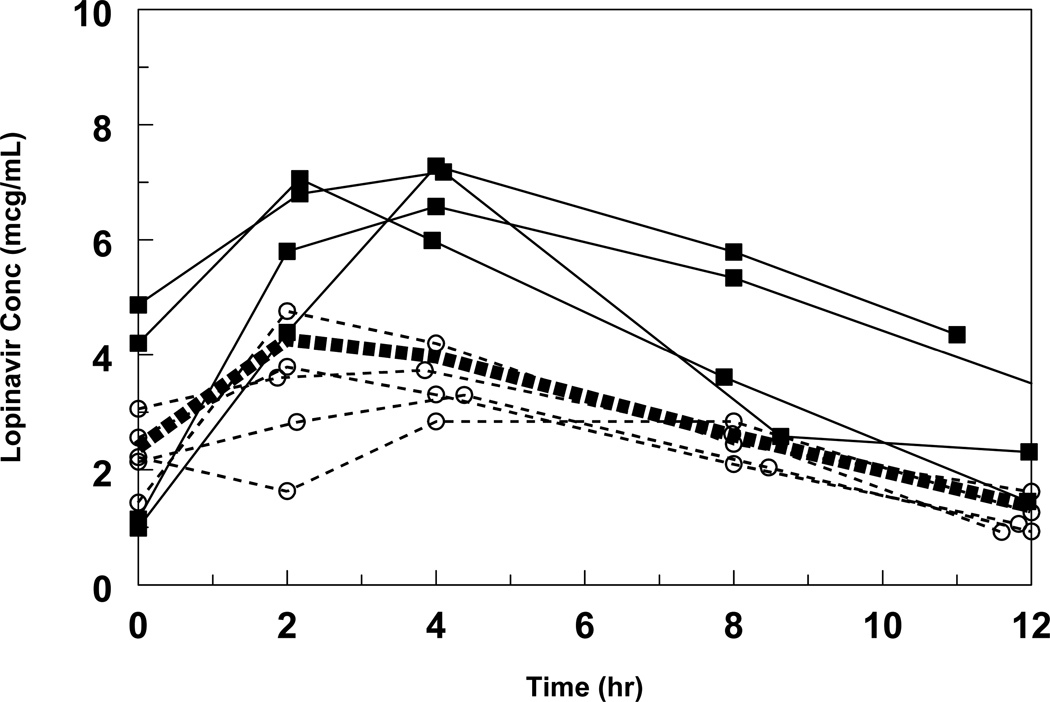
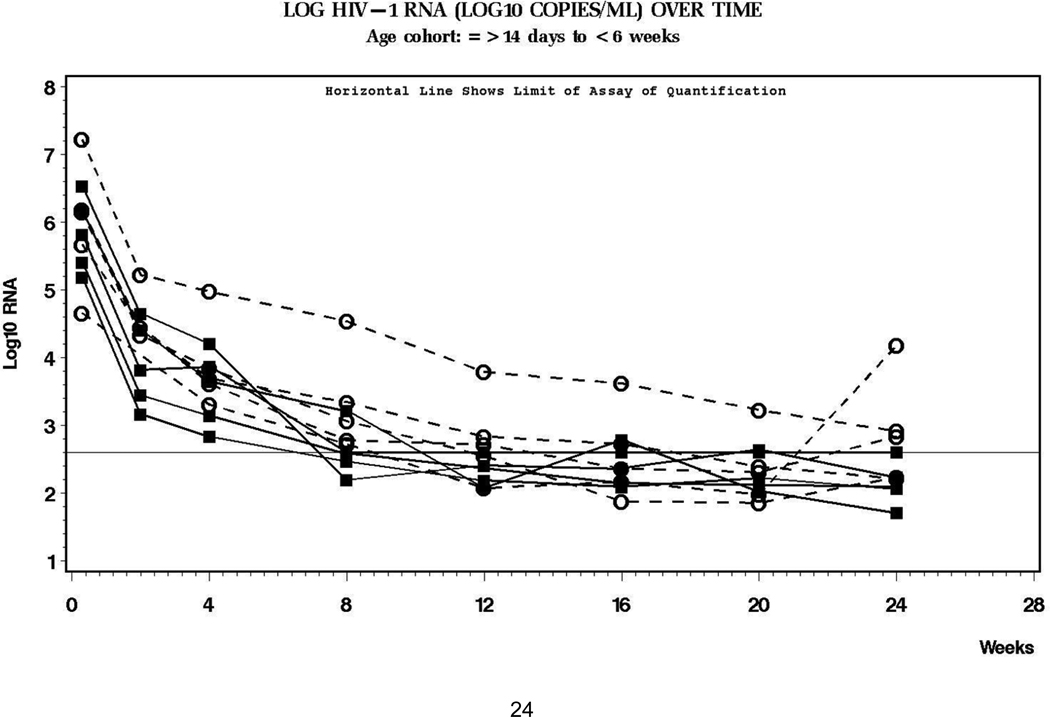
The ages and weights at study entry and median starting dose for the 9 patients are summarized in Table 1. The starting dose of LPV/r 300/75 mg/m2 every 12 hours initiated at study entry was not changed during the first 2 weeks regardless of changes in body surface area; therefore, the median (range) dose at the time of the pharmacokinetic study was 267 mg/m2 (246–305), which was equivalent to 15 mg/kg. The median concentration-time profile is shown in Figure 1, and pharmacokinetic measures of LPV and ritonavir are summarized in the Table. Infants with LPV exposure at or below the median had similar virologic responses to those with higher LPV exposure. (Figure 2)
Table 1.
Baseline data and Week 2* LPV/r and RTV pharmacokinetic results for 9 infants with evaluable pharmacokinetic analysis.
| Variable | Baseline Data and Pharmacokinetic Results at Week 2 | |||||
|---|---|---|---|---|---|---|
| mean | median | SD | CV% | min | max | |
| Age at entry (weeks) | 5.4 | 5.7 | 0.8 | 14% | 3.6 | 5.9 |
| Weight (Kg) | 4.81 | 4.7 | 0.88 | 18% | 3.6 | 6.1 |
| BSA (m2) | 0.27 | 0.27 | 0.04 | 14% | 0.21 | 0.33 |
| Dose (mg) | 71.9 | 64 | 9.6 | 13% | 64 | 87 |
| LPV Predose Conc (mcg/mL) | 2.51 | 2.22 | 1.34 | 53% | 0.99 | 4.87 |
| LPV Maximum Conc (mcg/mL) | 5.17 | 4.76 | 1.84 | 36% | 2.84 | 7.28 |
| LPV AUC (mcg*h/mL) | 43.4 | 36.6 | 14.8 | 34% | 27.9 | 62.6 |
| RTV AUC (mcg*h/mL) | 1.60 | 1.25 | 1.29 | 81% | <0.6 | 4.78 |
| LPV CL/F (L/h/kg) | 0.43 | 0.37 | 0.22 | 50% | 0.15 | 0.75 |
| LPV CL/F (L/h/m2) | 7.56 | 6.75 | 3.54 | 47% | 2.79 | 12.83 |
| LPV Ka (h-1) | 0.47 | 0.34 | 0.32 | 70% | 0.19 | 1.21 |
| LPV V/F (L/kg) | 2.03 | 1.86 | 0.88 | 43% | 0.80 | 3.79 |
| LPV T1/2 (h) | 3.67 | 3.51 | 1.46 | 40% | 2.06 | 5.80 |
One subject had PK determination at week 8, after good adherence was assured
BSA: body surface area
LPV: lopinavir
RTV: ritonavir
CV: coefficient of variation
Cl/F: apparent clearance (adjusted for weight [kg] or body surface area [m2])
Ka: absorption rate constant
V/F: apparent volume of distribution
T1/2 half-life
Figure 1. Individual LPV concentration time profiles.
All intensive PKs were performed at week 2 except for one performed at week 8, when adherence was assured. The bold dashed line represents the median concentration, the circles and squares represent subjects at or below and above the median, respectively.
Figure 2. Log HIV-1 RNA over time in study subjects.
The horizontal line is the lower limit of quantification of the assay (400 copies/ml). The circles and squares represent subjects whose intensive PK profiles were at or below (circles) and above (squares) the median.
Virology
Virologic responses to therapy are shown in Figure 2. All subjects had > 1 log10 copies/ml decline in HIV-1 RNA level at week 8. The median log10 decline in HIV-1 RNA from baseline to week 24 was 3.61 (range 4.3 to 2.83) (N = 10). One subject met a virologic endpoint at week 16 with HIV RNA >400 copies/ml; however, this child’s baseline value was in excess of 16,000,000 copies/ml and showed decreasing HIV-1 RNA at each successive measurement through week 24. A second subject reached a virologic endpoint at week 24 because of rebound to >4,000 copies/ml; this child had documented poor adherence to study treatment.
Immunology
Because of blood volume constraints, lymphocytes subsets were measured in only six of ten infants at all time points. The median entry CD4 percentage was 41% (range 16–59%, n=10) with a median change of −1% (range −10% to +18%, n=6) from baseline to week 24. The median entry CD8 percentage was 21% (range 15–37%, n=10) and at week 24, the median CD8 percentage change was −7% (range −16% to +11%, n=6).
Adverse Events
No Grade 4 events were recorded. A total of five Grade 3 adverse events occurred in three patients; all events were neutropenia and three were considered to be possibly treatment related. One patient had neutropenia temporally related to high blood concentrations of LPV/r and stabilized after re-starting LPV/r at lower dose. In the second patient neutropenia resolved after substituting stavudine for zidovudine. In the third patient neutropenia resolved after four days of treatment interruption. Non-fasting lipid samples were collected from all ten participants. When the 2004 Division of AIDS (DAIDS) Toxicity Tables for Grading Severity of Pediatric Adverse Experiences toxicity table11 (which utilizes fasting blood concentrations) is used as a reference, there were no Grade 3 lipid toxicities; all infants had triglycerides < Grade 2 (<500 mg/dL) through 24 weeks, four infants had cholesterol measurements ≤ Grade 1 and six had Grade 2 cholesterol measurements (200–300 mg/dL) during the observation period.
DISCUSSION
In this study, we found that doses of 300/75 LPV/r mg/m2 twice daily were safe and effective in HIV-infected infants initiating ART before 6 weeks of age. It is particularly important to understand antiretroviral treatment options for this age group given accumulating evidence that early therapy is associated with superior outcomes. Violari and colleagues4 showed that initiation of ART by 12 weeks of age is associated with significantly improved survival when compared with deferred therapy. The European Collaborative Study found that both initiation of therapy at <5 months of age and use of ART as a first regimen were strongly associated with a more rapid improvement in CD4 count, independent of immunologic and clinical status at initiation.14
Although early testing of newborns has not been readily available in many resource-limited settings, the urgency to start treatment will drive the demand for early diagnosis, increasing the proportion of infants who are initiating ART at a very young age. Furthermore, as late gestational prevention ART regimens increase in potency, the majority of infants who fail perinatal prevention will have been infected in utero, making their diagnosis possible within the first month of life.3 Thus, if the WHO objective of universal access to ART by 2010 is achieved in the setting of widespread early infant diagnostic capability, thousands of infants in this age group will soon be initiating treatment.15 Due to concerns about NNRTI resistance, protease inhibitors increasingly will be part of the preferred initial regimen in countries where single dose NVP is used for prevention of HIV transmission.5 LPV/r will be one of the first line agent choices in these settings, as it is one of the few protease inhibitors with a liquid formulation currently available.
Young infants in this trial exhibited lower LPV exposure than has been reported in older infants and children. (Table 2) Several mechanisms may be responsible for this finding including low ritonavir serum concentrations, altered protein binding and poor absorption in young infants. The RTV component of LPV/r is essential for inhibiting LPV metabolism and increasing LPV exposure. The RTV AUC was about half that of older infants and may have led to less inhibition of LPV metabolism.6 However, the half-life of LPV was similar in both age groups, suggesting that enhanced metabolism was not the primary cause for the lower concentrations.
Table 2.
Comparative Pharmacokinetic Data for Lopinavir
Reduced LPV bioavailability (F) is likely the primary cause for the lower LPV exposure in this population. While lower RTV concentrations may have had some impact on LPV metabolism, they could also be a marker for reduced absorption of protease inhibitors rather than the primary cause. Similar increases in CL/F and V/F (80–102%) and the strong correlation between these two measures (Spearman Correlation r=0.72, P=0.02) is consistent with reduced LPV absorption and suggests that bioavailibility is the primary source for intersubject variability. The later LPV peak time in the younger cohort and larger age-related differences in Cmax (4.8 vs. 8.1mcg/mL) compared with Cpre (2.2 vs. 2.4 mcg/mL) also suggests altered absorption processes.6 Bioavailability of many protease inhibitors, including LPV, is increased with food. This characteristic may lead to reduced absorption in infants as has been observed with nelfinavir, which also requires food for optimal absorption.8 During the intensive PK evaluation, the infants in this study were receiving formula as their sole form of nutrition, which may not be optimal for LPV absorption. There was no correlation between volume or timing of formula administration and LPV/r exposure (data not shown). It is likely that the older cohort in this study received cereal and other foods in addition to formula which may have enhanced LPV bioavailability for that age group.
The LPV pharmacokinetic measures in this study were determined from total (bound and unbound) concentrations. Lopinavir is 98–99% protein bound, thus its free fraction is sensitive to changes in plasma binding protein concentrations. Low albumin and alpha-1 acid glycoprotein levels present during the first few weeks of life may have maintained normal, or near-normal, unbound active LPV concentrations despite reduced total LPV concentrations, Reduced LPV plasma binding would increase both CL/F and V/F in parallel as was observed in this study, but it would not have had a differential effect between Cmax and Cpre. While drug binding protein concentrations are expected to be lower in younger infants than older infants, this difference is small and cannot by itself explain the observed differences in the two age groups in LPV AUC.
Another factor contributing to variable drug exposure is suboptimal adherence. Some caregivers found it challenging to administer medications to infants and required time to learn the best technique. Although poor adherence was encountered at the initiation of therapy in a few subjects, the intensive pharmacokinetic evaluation was repeated in these subjects at a time when they exhibited good overall adherence. The LPV concentration profiles in Figure 2 indicate similar C0 and C12 values as would be expected with good adherence.
Despite the lower peak and average LPV exposure in these young infants, the LPV trough was similar to that of older infants and sub-therapeutic troughs (<1 mg/mL) were not frequently encountered. (Table 2) A plasma concentration of 1 µg/mL still affords a 15-fold margin above the estimated IC50 for LPV, which has been used as a correlate of efficacy in treatment-naïve adults.16 Indeed, virologic response to LPV-based therapy was excellent, with only one of ten infants reaching a virologic endpoint at week 16 and a second experiencing virologic rebound at 24 weeks secondary to poor adherence. This short term efficacy is higher than seen in the older cohort of infants in this study in which only half of the infants had virologic suppression to <400 copies/ml at 24 weeks.6 In addition, entry CD4 percentages were high and remained stable throughout the study, although a normal decline would be expected in the first 6 months of life.17
It is difficult to determine whether the dosage studied in this trial is optimal for all infants less than 6 weeks of age. The 24-week virologic responses with LPV/r 300/75 mg/m2 were promising even in the subjects with lowest LPV concentrations. However, the number of subjects in this study was small, and the long term consequences of low LPV exposure (AUC) are unknown. There was great variability in LPV exposure, with approximately half of the infants in the range found in older infants. A higher LPV/r dose might put these young infants at risk for added toxicity. The one subject in this trial who had the dosage increased based on PK results subsequently required dosage reduction for excessive drug concentrations in serum and possible drug related toxicity. While it may be reasonable to consider initiating therapy with a dosage greater than 300 mg/m2, it would require careful monitoring of serum drug concentrations and toxicity in the setting of a study. Because dosage adjustments were delayed until after PK evaluations were completed in this study, the median doses at the time of the PK studies were >10% below the intended 300/75 mg/m2. One important way to optimize the likelihood of therapeutic LPV dosing is to adjust the dosage for incremental growth at more frequent intervals in the first months of life, during which time the infant experiences rapid weight gain. Long-term follow-up will be important to determine changes in pharmacokinetic profiles over time and longer-term efficacy in this vulnerable population.
ACKNOWLEDGEMENT
The P1030 Team thanks the patients and families who participated in this trial, and dedicates this manuscript to the memory of John H. Rodman, PharmD who passed away before its completion. The team also greatly appreciates the assistance and expertise of Marisol Martinez, MD, Mary Elizabeth Smith, MD, Quianna Douglas MHSA and Jennifer Gardella, MPH.
Support: This project was supported by Grant Number U01AI068632 and 1 U01 AI068616 from the National Institute of Allergy and Infectious Diseases. This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200800014C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. This study was also supported by Abbott Laboratories.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) [Accessed 5/21/08];AIDS epidemic update: December 2007. Available at: http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 2.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: A pooled analysis. Lancet. 2004;364(9441):1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Mphatswe W, Blanckenberg N, Tudor-Williams G, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;21(10):1253–1261. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- 4.Violari A, Cotton M, Gibb D, et al. Antiretroviral therapy initiated before 12 weeks of age reduces early mortality in young HIV-infected infants: Evidence from the Children with Early Antiretroviral Therapy (CHER) study [Abstract WESS103]. 4th International AIDS Society Conference on HIV Treatment and Pathogenesis; July 22 to 25, 2007; Sydney, Australia. [Google Scholar]
- 5.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. New Engl J Med. 2007;356(2):135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 6.Chadwick EG, Capparelli EV, Yogev R, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS. 2008;22(2):249–255. doi: 10.1097/QAD.0b013e3282f2be1d. [DOI] [PubMed] [Google Scholar]
- 7.Division of AIDS (DAIDS) [Accessed 5/21/08];Table for grading severity of pediatric (≤ 3 months of age) adverse experiences: April 1994. Available at: http://rcc.tech-res.com/DAIDS%20RCC%20Forms/ToxicityTables_Pediatric_Under3MonthsAge_v02.pdf.
- 8.Capparelli EV, Sullivan JL, Mofenson L, Smith E, Graham B, Britto P, et al. Pharmacokinetics of nelfinavir in human immunodeficiency virus-infected infants. Pediatr Infect Dis J. 2001;20(8):746–751. doi: 10.1097/00006454-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Chadwick EG, Rodman JH, Britto P, Powell C, Palumbo P, Luzuriaga K, et al. Ritonavir-based highly active antiretroviral therapy in human immunodeficiency virus type 1-infected infants younger than 24 months of age. Pediatr Infect Dis J. 2005;24(9):793–800. doi: 10.1097/01.inf.0000177281.93658.df. [DOI] [PubMed] [Google Scholar]
- 10.Kashuba ADM, Shapiro J, Burger DM. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:4–14. [Google Scholar]
- 11.D’Argenio DZ, Schumitzky A. ADAPT II user’s guide: Pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles, CA: Biomedical Simulations Resource; 1997. [Google Scholar]
- 12.Yen-Lieberman B, Brambilla D, Jackson B, et al. Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J Clin Microbiol. 1996;34(11):2695–2701. doi: 10.1128/jcm.34.11.2695-2701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Division of AIDS. [Accessed 5/21/08];Table for grading the severity of adult and pediatric adverse events. 2004 December; Available at: http://rcc.tech-res.com/DAIDS%20RCC%20Forms/ToxicityTables_DAIDS_AE_GradingTable_FinalDec2004.pdf.
- 14.Newell ML, Patel D, Goetghebuer T, Thorne C. European Collaborative Study. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: Is it associated with age at initiation? J Infect Dis. 2006;193(7):954–962. doi: 10.1086/500842. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) 2006. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: Towards universal access by 2010: How WHO is working with countries to scale-up HIV prevention, treatment, care and support. [Accessed 5/21/08]; Available at: http://www.who.int/hiv/toronto2006/towardsuniversalaccess.pdf.
- 16.Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS. 2001;15(1):F1–F9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]
- 17.HIV Pediatric Prognostic Markers Collaborative Study. Use of total lymphocyte count for informing when to start antiretroviral therapy in HIV-infected children: A meta-analysis of longitudinal data. Lancet. 2005;366(9500):1868–1874. doi: 10.1016/S0140-6736(05)67757-4. AIDS. 2006;20:1289. [DOI] [PubMed] [Google Scholar]
- 18.Sáez-Llorens X, Violari A, Deetz CO, et al. Forty-eight week evaluation of lopinavir/ritonavir, a new protease inhibitor, in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22:216–223. doi: 10.1097/01.inf.0000055061.97567.34. [DOI] [PubMed] [Google Scholar]