SUMMARY
Protein-tyrosine phosphatases (PTPs), along with protein-tyrosine kinases, play key roles in cellular signaling. All Class I PTPs contain an essential active site cysteinyl residue, which executes a nucleophilic attack on substrate phosphotyrosyl residues. The high reactivity of the catalytic cysteine also predisposes PTPs to oxidation by reactive oxygen species, such as H2O2. Reversible PTP oxidation is emerging as an important cellular regulatory mechanism and might contribute to diseases such as cancer. We exploited these unique features of PTP enzymology to develop proteomic methods, broadly applicable to cell and tissue samples, that enable the comprehensive identification and quantification of expressed classical PTPs (PTPome) and the oxidized subset of the PTPome (oxPTPome). We find that mouse and human cells and tissues, including cancer cells, display distinctive PTPomes and oxPTPomes, revealing additional levels of complexity in the regulation of protein-tyrosine phosphorylation in normal and malignant cells.
INTRODUCTION
The phosphorylation of proteins on tyrosyl residues is controlled by protein-tyrosine kinases (PTKs) (Lemmon and Schlessinger, 2010) and protein-tyrosine phosphatases (PTPs) (Alonso et al., 2004), which direct diverse cellular processes, such as cell growth, proliferation and migration (Tonks, 2006). Aberrations in PTKs or PTPs contribute to several human diseases, including cancer (Julien et al., 2011; Lahiry et al., 2010). A systems level understanding of cell signaling requires methods to identify PTKs and PTPs, determine their expression levels and monitor their regulation. Most activated PTKs are phosphorylated on tyrosine and can be identified by phosphotyrosine (pY)-proteomics (Rikova et al., 2007; Zhang et al., 2005). By contrast, proteomic approaches to assess PTP expression and regulation have lagged behind.
The PTP superfamily comprises 107 genes, divided into four families (Alonso et al., 2004). The largest, the class I cysteine-based PTPs, can be sub-divided into classical and dual-specificity PTPs (DUSPs). The classical PTP sub-family consists of 21 receptor and 17 non-receptor PTPs that exclusively hydrolyze pY residues. Classical PTPs contain a conserved “signature motif”, [I/V]HCSXGXGR[S/T]G, within their active sites, wherein the invariant cysteine is essential for catalysis (Andersen et al., 2001). Owing to its low pKa (4.5–5.5), the catalytic cysteine exists in the thiolate (S−) state at physiological pH (Zhang et al., 1994). This property facilitates nucleophilic attack on substrate phosphotyrosines (Denu and Dixon, 1998), but also renders PTPs highly susceptible to oxidation by reactive oxygen species (ROS) (Salmeen and Barford, 2005; Tanner et al., 2010).
Although high levels of ROS damage cellular components, ROS, particularly H2O2, also function as intracellular second messengers [reviewed in (den Hertog et al., 2005; Rhee et al., 2000; Tonks, 2005)]. Early work showed that receptor tyrosine kinase (RTK) activation leads to transient H2O2 production, which is required for full RTK phosphorylation and downstream signaling (Sundaresan et al., 1995). PTPs were proposed as important ROS targets (Finkel, 1998), and soon PTPN1 (PTP1B) was found to be reversibly oxidized and inactivated in response to EGFR activation (Lee et al., 1998). Subsequently, studies have reported that oxidation of specific PTPs, including PTPN11 (SHP2) in PDGF signaling (Meng et al., 2002), PTPN1 and PTPN2 (TC-PTP) in insulin signaling (Meng et al., 2004) and PTPN6 (SHP1) in B cell receptor signaling (Singh et al., 2005), is required for optimal responses to various stimuli.
Cancer cells often produce high levels of ROS [reviewed in (Cairns et al., 2011; Liou and Storz, 2010)], which could decrease basal PTP activity and enhance tyrosyl phosphorylation (Ostman et al., 2006). Indeed, total PTP activity is decreased in BCR/ABL (Sattler et al., 2000)- and Src (Gianni et al., 2008)-transformed cells and can be restored by antioxidants or PTK inhibitors. Furthermore, over-expression of the ROS-producing protein Nox1 can cause cellular transformation and tumor formation (Arnold et al., 2001), implicating excess ROS production in carcinogenesis.
The identification of ROS-inactivated PTPs might provide important clues to key PTPs that regulate normal and oncogenic signaling pathways. Yet while approaches to identify specific oxidized PTPs are available, it has been difficult to develop general methods that detect and quantify any reversibly oxidized PTP in different physiological and pathological contexts. A modified in-gel phosphatase assay is currently the best global assay for PTP oxidation (Meng et al., 2002), but this approach is biased toward non-receptor PTPs (Burridge and Nelson, 1995), is not quantitative, and requires the oxidized PTP(s) to be identified by immunodepletion. We exploited the biochemistry of PTP catalysis and oxidation and an available monoclonal antibody against “hyper-oxidized” PTP active sites to develop global quantitative proteomic approaches that monitor classical PTP expression and oxidation.
RESULTS
Design of qPTPome and q-oxPTPome assays
H2O2 promotes reversible oxidation of PTPs to the sulfenic acid (PTP-SOH) state (Rhee et al., 2000), which is labile and, in different family members, rapidly rearranges to form a sulfenylamide with the adjacent main chain nitrogen (Salmeen et al., 2003; van Montfort et al., 2003) or a disulfide bond to a nearby cysteine (Chen et al., 2009). The sulfenylamide and disulfide states help limit “hyper-oxidation” to the sulfinic (PTP-SO2H) and sulfonic (PTP-SO3H) acid states, which are biologically irreversible (Tonks, 2005).
We exploited these biochemical properties to develop methods for quantifying classical PTP expression (qPTPome) and oxidation (q-oxPTPome), respectively (Figure 1A). Both methods use a monoclonal antibody (oxPTP Ab) raised against the signature motif of PTPN1 oxidized to the sulfonic acid (VHCSO3HSAG) (Persson et al., 2005) to isolate “hyper-oxidized” PTPs (PTP-SO3H). This antibody has been used for immunoblotting of cell lysates and to monitor oxidation of specific PTPs (Groen et al., 2005; Persson et al., 2004; Weibrecht et al., 2007). Its precise reactivity had not been defined, but because the signature motif is highly conserved, we suspected (and found; see below) that the oxPTP Ab would react with most, if not all, classical PTPs.
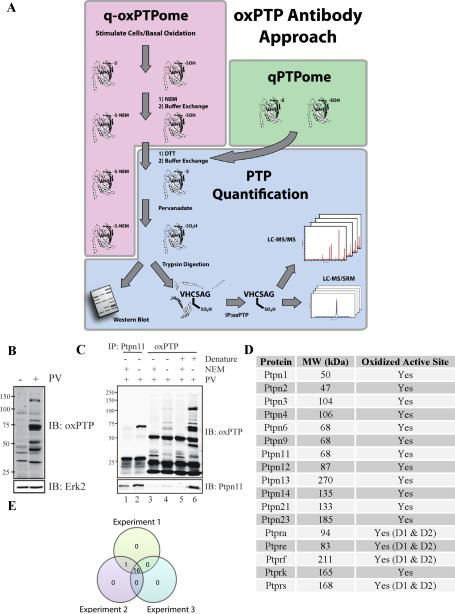
Figure 1. Development of qPTPome and q-oxPTPome.
(A) Scheme for each assay. (B) NIH3T3 cells were lysed in presence of DTT, applied to a gel filtration column, treated with PV or left untreated, and then analyzed by immunoblotting with the oxPTP Ab. (C) Native and denatured PV-treated lysates from (B) were immunoprecipitated with oxPTP or Ptpn11 antibodies, as indicated, and analyzed by immunoblotting. (D) PTPs in NIH3T3 cells, as identified by qPTPome. (E) Venn diagram demonstrating reproducibility of the qPTPome assay (n=3). See Figure S1 for additional variations of these assays.
Basal or ligand-induced PTP oxidation results in two pools of PTPs: oxidized (PTP-SOH; inactive) and reduced (PTP-S−; active). For q-oxPTPome, active PTPs are alkylated with N-ethylmaleimide (NEM; PTP-S-NEM), rendering them resistant to further modification, while oxidized PTPs remain unaffected (Figure 1A). Excess NEM is removed by gel filtration, and reversibly oxidized PTPs are reduced with dithiothreitol (DTT). Following a buffer exchange, the reduced PTPs (representing PTPs that initially were reversibly oxidized), are oxidized to the sulfonic acid (PTP-SO3H) using pervanadate (PV) (Huyer et al., 1997). For qPTPome, alkylation is omitted, and cells are lysed in the presence of DTT to reduce PTPs to the active (PTP-S−) state. Then, PTPs are “hyper-oxidized” to the SO3H state and detected by immunoblotting with the oxPTP Ab or processed for mass spectrometry (MS). For MS, PV-treated lysates are digested with trypsin, and PTP-SO3H active site peptides are purified with the oxPTP Ab and identified (“discovery” proteomics) by LC-MS/MS. Quantification can be achieved by various methods, including labelling with stable isotopes and/or chemical tags [reviewed in (Gstaiger and Aebersold, 2009; Walther and Mann, 2010; Yates et al., 2009)] or, as follows, by selected reaction monitoring (SRM, also known as multiple reaction monitoring or MRM) [reviewed in (Elschenbroich and Kislinger, 2010)].
qPTPome reliably identifies classical PTPs
For qPTPome, NIH3T3 cells were lysed in the presence of DTT, followed by a buffer exchange and treatment with PV. Multiple bands were detected in immunoblots with the oxPTP Ab in PV-treated, compared with untreated, lysates (Figure 1B). Next, native or denatured PV-treated lysates were immunoprecipitated and immunoblotted with the oxPTP Ab (Figure 1C). Again, new bands were detected in the PV-treated lysates (Figure 1C, compare lanes 5 and 6). Denaturing the lysate prior to immunoprecipitation increased recovery of oxidized PTPs (Figure 1C, compare lanes 4 and 6), suggesting that the epitope for the oxPTP Ab is not very accessible in the native PTP-SO3H conformation. As expected, NEM treatment prior to reduction markedly decreased oxPTP Ab-immunoreactive bands (Figure 1C, lanes 3 and 5). We also immunoblotted oxPTP and anti-Ptpn11 immunoprecipitates from denatured lysates with anti-Ptpn11 antibodies. Similar levels of Ptpn11 were recovered in each immunoprecipitate, suggesting that immunoprecipitation by the oxPTP Ab was quantitative (Figure 1C, compare lanes 2 and 6).
To identify the PV-inducible species, cell extracts were digested with trypsin, and peptides immunopurified with the oxPTP Ab were analyzed by LC-MS/MS. These experiments revealed active site PTP-SO3H peptides from 17 classical PTPs (Figure 1D). Importantly, unlike the in-gel PTPase approach (see Introduction), qPTPome detected the D1 and D2 domains from several RPTPs (Ptpra, Ptpre, Ptprf and Ptprs). Near-complete overlap in PTP expression was observed in biological replicates using NIH3T3 cells (Figure 1E) and other cell lines and tissues (Figure S2A).
To determine the range of classical PTPs that could be detected, we performed qPTPome on tissues from C57BL/6J mice (Figure 2A and Tables S1 and S2), several human cell lines (Figure 2B and Tables S1 and S2) and the rat basophilic leukaemia line RBL2H3 (Figure S2B and Tables S1 and S2). Nearly all classical PTPs (17/17 nonreceptor and 19/21 RPTPs) were identified, and PTPs accounted for ~70% of the Cys-SO3H-containing proteins recovered in these experiments (Table S1). Notably, members of other phosphatase families (e.g., DUSPs) were not detected, indicating that the oxPTP Ab is highly specific for the classical PTP signature motif. Other recovered peptides had sequence similarity to this motif (see below) or were from abundant proteins (Table S1).
Figure 2. qPTPome broadly identifies classical PTPs in cell lines and tissues.
The indicated mouse (A) and human (B) cell lines and tissues were profiled by qPTPome. Classical PTPs identified are shown as black boxes. (C) Comparison of qPTPome results (blue boxes) with published microarray data (black boxes). Correlations for each PTP were evaluated by Spearman r coefficient, as represented by the black-red scale. (D) PTPs in human platelets, as identified by qPTPome. (E) Multiple sequence alignment showing sequence of tryptic fragments containing the catalytic cysteines of each classical PTP. PTPs identified by qPTPome are bolded. Also, see Figure S2.
Each cell line and tissue had a unique PTP profile. For example, PTPN1 and PTPN2 were expressed ubiquitously, whereas PTPRC and PTPRD were present more selectively. There was near-complete overlap between PTPs identified by qPTPome and in published microarray analyses (Figure 2C and Table S1), providing further evidence of the reliability of our assay. This correlation was not absolute, though, indicating that post-transcriptional mechanisms contribute significantly to determining PTP protein levels.
We also applied our method to a cell type with an unknown PTP expression profile. Platelets are anuclear and express only small amounts of residual megakaryocyte mRNA, making it difficult to define their PTPome by RNA-based approaches. Using qPTPome, we identified 16 PTPs in platelets (Figure 2D), including PTPN2, PTPRA, and PTPRE, which had not been identified previously. When antibodies were available, these identifications were confirmed by immunoblotting (Figure S2C).
The above dataset allowed us to define the specificity of the oxPTP Ab. We aligned the sequences of the tryptic peptides from the active sites of all classical PTPs, highlighting those detected by qPTPome (Figures 2E and S2D). Although the D1 domains of Ptprc, Ptprm, Ptprk, Ptprt and Ptpru were identified, their D2 domains, which cluster at the top of the multiple sequence alignment, were not. The active site sequences of these D2 domains (VHCRDG and [I/V]HCLNG) diverge substantially from the sequence used to generate the oxPTP Ab. To ask if the oxPTP Ab could recognize these sequences, we performed immunoprecipitations with synthetic PTP-SO3H peptides. As expected, the oxPTP Ab isolated the Ptpn1 peptide, but not the D2 domains of Ptprk, Ptprm, Ptprt and Ptpru (data not shown). These experiments suggested that the oxPTP Ab recognizes [I/V]HCSO3HS[A/S]G, and that given their divergent active sites, Ptprn (VHCSDG) and Ptprn2 (VHCSDG) also would not be detectable. Notably, Ptprn and Ptprn2 are catalytically inactive (Gross et al., 2002); hence, qPTPome can identify all catalytically active classical PTPs in a single MS experiment.
Quantification of the PTPome
Selected reaction monitoring (SRM) is a label-free method that determines the relative abundance of peptide ions by measuring signals associated with specifically defined, distinctive, co-eluting parent ion-to-product ion transitions (Picotti et al., 2009). We developed a multiplexed SRM (mSRM) protocol to quantify the classical PTPome or oxPTPome from human, mouse or rat (Figure S3A). Following enrichment by the oxPTP Ab, PTP peptide ions and their product ion fragments first were observed in “discovery mode” LC-MS/MS, and then SRM transitions for each PTP were designed, optimized, and combined into a single mSRM protocol (Table S3). The optimized mSRM transitions were validated for several (~50%) mouse and human PTPs using synthetic peptides (Table S3). Furthermore, all active site peptides from classical PTPs in NIH3T3 (Figure S3B) and A431 (Figure S3C) cells were immunodepleted by the oxPTP Ab, demonstrating that the assay is quantitative.
Next, we assayed Ptpn11fl/KO fibroblasts (Figure 3A), in which 4-hydroxytamoxifen (4-OHT) addition activates a Cre-ER fusion protein and evokes deletion of a floxed Ptpn11 allele (Ptpn11KO/KO). Notably, the Ptpn11 signal from 4-OHT-treated cells was much lower than from controls, whereas their Ptpn1 signals were similar (Figure 3B). Moreover, the SRM signals paralleled the respective Ptpn11 and Ptpn1 levels detected by immunoblotting (Figure 3C). To display the mSRM signals for all PTPs simultaneously, we developed a heat map representation (Figure 3D and Table S3), in which the relative abundance of each PTP is shown in shades of red and the magnitude of the SRM signal for each PTP (from control cells) is indicated by shades of blue (black denotes no signal/undetectable expression). With the exception of Ptpn11, PTP expression was largely unaffected by 4-OHT treatment.
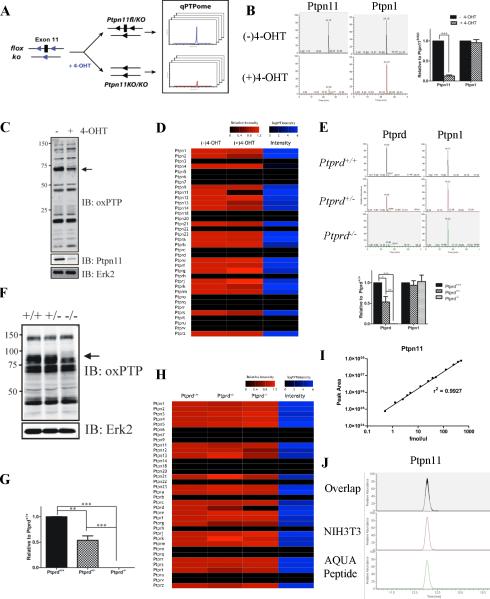
Figure 3. PTP quantification using qPTPome.
(A) Ptpn11fl/KO cells stably expressing CreER were treated with 4-OHT or left untreated, then assessed by qPTPome. (B) Representative SRM profiles for Ptpn11 and Ptpn1. Quantification of each PTP is displayed next to its elution profile (*** p<0.002, paired Student's t-test; 2 biological replicates, each with 2 technical replicates). (C) Immunoblot showing PTP expression levels. Arrow shows position of Ptpn11. (D) Heat map showing relative changes (compared with untreated cells) in PTP expression (measured by qPTPome) following 4-OHT treatment. The intensity value is the average signal for the SRM profiles for each PTP in untreated cells (E) Ptprd+/+, Ptprd+/− and Ptprd−/− brains were profiled by qPTPome (n=3; 3 technical replicates each). Representative SRM profiles are shown for Ptprd and Ptpn1, with quantification of each displayed next to the profiles. Data represent mean ± SEM (* p<0.05; ** p<0.01; *** p<0.001, ANOVA with Bonferroni post-test). (F) Immunoblot with oxPTP Ab showing PTP expression in Ptprd mutant brains. Arrow shows position of Ptprd. (G) Ptprd mRNA levels (assessed by qPCR; n=3). Data represent mean ± SEM (*** p<0.001, ANOVA with Bonferroni post-test). (H) Relative levels of PTP expression (measured by qPTPome) in Ptprd mutant (compared with Ptprd+/+) brains. (I) Standard curve of MS peak area for Ptpn11 AQUA peptide (n=3). (J) SRM peak of the AQUA peptide mixed with native Ptpn11 peptide from NIH3T3 cells. Also, see Figure S3.
To see if qPTPome could be used for tissues, we assayed brains from Ptprd+/+, Ptprd+/− and Ptprd−/− mice (Mizuno et al., 1993). As expected, Ptprd levels, obtained by SRM of the Ptprd D1 domain, were progressively lower in Ptprd+/+, Ptprd+/−, and Ptprd−/− brains (Figure 3E); by contrast, Ptpn1 expression remained similar. These findings agreed with a global immunoblot using the oxPTP Ab (Figure 3F) and with RT-PCR measurements of Ptprd mRNA (Figure 3G). Also consistent with the oxPTP immunoblot, there were no significant changes in classical PTP expression aside from Ptprd in these samples (Figure 3H and Table S3).
As implemented above, qPTPome displays the relative level of each classical PTP. We asked if, by combining qPTPome with absolute quantification (AQUA) (Gerber et al., 2003), the absolute amount of a PTP could be determined (Figure S1). We synthesized a Ptpn11-SO3H active site tryptic peptide containing a “heavy” arginine (six 13C and four 15N). Using a standard curve generated with this peptide (Figure 3I), we found that our SRM assay is linear over at least three orders of magnitude. By comparing the intensities of co-eluting “heavy” (AQUA) and “light” (endogenous) Ptpn11-SO3H peaks recovered by the oxPTP Ab (Figure 3J) and assuming complete digestion with trypsin, we determined that there are ~32,000 copies of Ptpn11 in each NIH3T3 cell. This measurement compares well (within 4-fold) with immunoblot determinations (Figure S3D).
Quantification of the PTP 'redoxome'
Next, we attempted to quantify PTP oxidation (Figure 4A). NIH3T3 cells were stimulated with increasing concentrations of H2O2 for 5 minutes and analyzed by q-oxPTPome. In parallel, qPTPome measurements were obtained to assess the stoichiometry of PTP oxidation. Under these conditions, the Ptpn6 and Ptpn11 SRM profiles showed dose-dependent, but differential increases in oxidation (Figure 4A), which were confirmed by immunoblotting (Figure 4B). We used a heat map to display the oxidized proportion of each PTP relative to its total amount (q-oxPTPome/qPTPome; Figure 4C and Table S4). As expected, under normal growth conditions, most PTPs had very low levels of basal oxidation to the PTP-SOH or SO3H states. By contrast, Ptpn23, Ptpra D2, Ptprf D2 and Ptprk D1 had high basal oxidation. With the exception of Ptprk D1, these PTP domains reportedly are catalytically inactive (Gingras et al., 2009; Persson et al., 2004). The D1 and D2 domains of specific RPTPs showed clear differences in oxidation sensitivity. For example, Ptpra D1 had a low level of oxidation, while its D2 domain was highly oxidized. By contrast, Ptprs D1 and D2 both showed low basal oxidation. As expected, increasing the H2O2 concentration increased the oxidation of multiple PTPs, including Ptpn1, Ptpn3, Ptpn9 and Ptpra D1 (Figure 4C). Surprisingly, though, Ptpn23 and Ptpra D2 oxidation appeared to decrease.
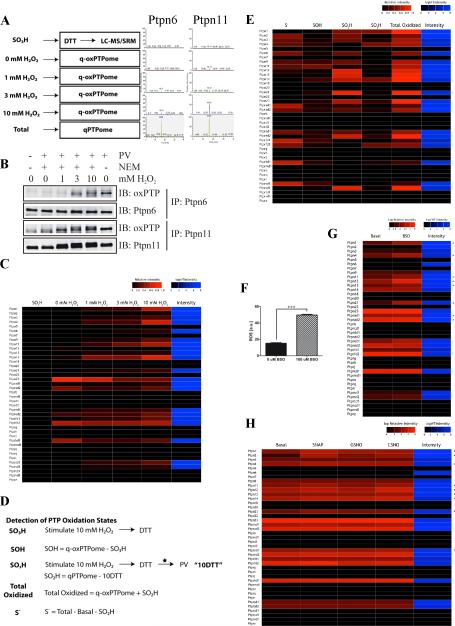
Figure 4. Monitoring multiple PTP oxidation states by q-oxPTPome.
(A) Experimental scheme (left). NIH3T3 cells were stimulated with the indicated concentrations of H2O2 for 5 min and analyzed by q-oxPTPome (n=2; 4 technical replicates each). Representative SRM profiles for Ptpn6 and Ptpn11 (right). (B) Detection of Ptpn6 and Ptpn11 oxidation. Following H2O2 stimulation, cells were processed by q-oxPTPome, immunoprecipitated with Ptpn6 or Ptpn11 antibodies and analyzed by immunoblotting, as indicated. (C) Heat map displaying levels of PTP oxidation following H2O2 stimulation, measured by q-oxPTPome. A black box indicates low levels of PTP oxidation; red boxes denote high levels. The intensity value is the average signal for the SRM profiles for each PTP measured by qPTPome. (D) Scheme showing modifications of qPTPome and q-oxPTPome to assess all potential PTP oxidation states (n=2; each with 3 technical replicates); * indicates a buffer exchange. (E) Fraction of each PTP in each potential oxidation state (i.e. S−, SOH, SO2H, SO3H) following 10 mM H2O2 stimulation. (F) ROS levels in NIH3T3 cells treated with 100 μM BSO (n=6 *** p<0.001, Student's t-test). Data represent mean ± SEM. (G) Levels of PTP oxidation in cells treated with 100 μM BSO or left untreated (n=2; 2 technical replicates each). * indicates PTPs with increased oxidation following BSO treatment. (H) Levels of nitrosylation measured by q-oxPTPome following stimulation with S-nitroso-N-penicillamine (SNAP), S-nitrosoglutathione (GSNO) or S-nitrosocysteine (CSNO). * indicates PTPs that were nitrosylated.
We suspected that this might reflect oxidation to the SO2H state, which should not be converted to the SO3H state by PV (Huyer et al., 1997) and hence would not be recognized by SRM. To test this possibility, we modified the q-oxPTPome protocol (Figure 4D; see Experimental Procedures for details) to enable identification and quantification of all PTP oxidation states (S−, SOH, SO2H, SO3H). Not surprisingly, most PTPs were oxidized by 10 mM H2O2 (Figure 4E and Table S4). Yet while several PTPs were converted to the SO2H state, including Ptpn23 and Ptpra D2, very few were converted to the SO3H state. Hence, extremely high levels of oxidative stress are required to completely oxidize PTPs inside cells. Most other PTPs, including Ptpn11 and Ptprs D1, showed high levels of reversible oxidation (PTP-SOH), whereas a few remained reduced.
We next assessed the changes in PTP oxidation caused by exposure of NIH3T3 cells to buthionine sulphoximine (BSO), which inhibits glutathione synthesis. As expected, intracellular ROS levels increased (Figure 4F), and concomitantly, several PTPs showed increased oxidation (Figure 4G and Table S4). Interestingly, although PTP oxidation levels were similar in BSO-treated and H2O2 (1 mM)-stimulated cells, the profile of oxidized PTPs differed significantly (compare Figures 4C and G).
Quantifying PTP nitrosylation
PTPs also can be regulated by reversible nitrosylation of their active site cysteines. Targeted indirect approaches have been used to detect nitrosylation of a few PTPs, including PTPN1 and PTPN11 (Li and Whorton, 2003). To see if our method could monitor PTP nitrosylation, NIH3T3 cells were exposed to three different nitrosylating agents, processed and analyzed by q-oxPTPome. We detected nitrosylation of several PTPs (Figure 4H and Table S4), including Ptpn1 and Ptpn11 (Hsu and Meng, 2010; Li and Whorton, 2003). Consistent with the q-oxPTPome signals arising from nitrosylation, not oxidation, SRM signals for Ptpn23 and Ptpra D2 did not decrease (Figure 4H), and ROS levels remained unchanged (data not shown) in cells exposed to these agents.
Application of qPTPome and q-oxPTPome to cancer cells
ROS levels often increase in cancer (Liou and Storz, 2010). Although increased oxidation of individual PTPs has been reported in selected cancer cell lines (Lou et al., 2008), the global effects of excess ROS on PTP oxidation have not been assessed. As a proof of principle, we analyzed A431 (Figure 5A and Table S5) and NIH3T3 cells transformed with constitutively active Src (Src Y527F; Figure 5B and Table S5). Many PTPs were reversibly oxidized in both lines, with generally higher levels of oxidation in A431 cells. Notably, we detected ~20% oxidation of PTPN1 in A431 cells, remarkably similar to the 25% oxidation determined using a targeted MS method (Lou et al., 2008).
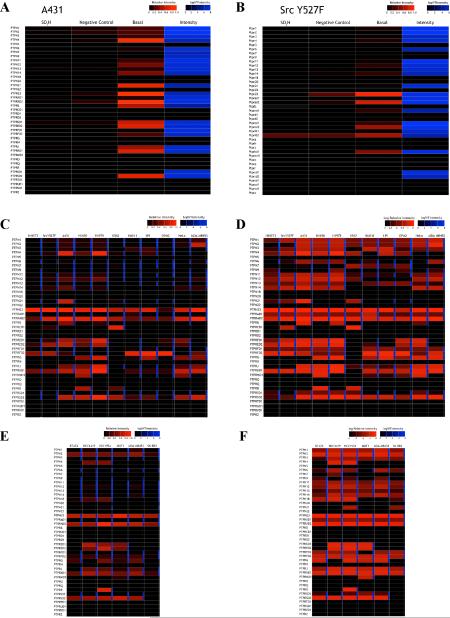
Figure 5. Cancer cells have unique PTP oxidation profiles.
Heat map showing basal PTP oxidation in A431 (A) and Src Y527F-transformed NIH3T3 (B) cells, measured by q-oxPTPome (n=2; 2 technical replicates each). “Negative control” represents cells that were lysed in the presence of DTT, treated with NEM followed by PV, and analyzed by LC-MS/SRM. (C) Basal PTP oxidation in cancer lines determined by q-oxPTPome (n=2; 2 technical replicates each). (D) Same as (C), but with scale altered to show PTPs that were oxidized to lower extents. (E) Basal PTP oxidation in HER2+ breast cancer lines measured by qoxPTPome (n=2; 2 technical replicates each). (F) Same as (E), but with scale altered to show PTPs that were oxidized to lower extents. Also, see Figure S4.
We compared PTP oxidation in A431 and Src Y527F cells with that in cell lines derived from several types of cancer (Figures 5C and D and Table S5). Global PTP oxidation was higher in Src Y527F cells, compared with non-transformed NIH3T3 cells. A431 and H1975 cells showed the highest levels of PTP oxidation and the highest levels of intracellular ROS (Figures S4B and C). Some PTP domains (e.g., PTPRA D2, PTPN23) were highly oxidized in all cancer cells tested; others (e.g., PTPN1, PTPN11) had lower and variable levels of basal oxidation in these cell lines.
We then compared PTP oxidation in six HER2+ breast cancer cell lines. Although some PTPs were differentially oxidized (Figures 5E and F and Table S5), there was less heterogeneity between these lines than between lines derived from different types of cancer (compare Figures 5C and E). Also, while there were some general trends in relative oxidation levels, each cancer line contained a unique profile of oxidized PTPs. Notably, the ROS levels in a given cell line and the extent of PTP oxidation did not correlate strongly (compare Figure 5 and Figures S4B and D).
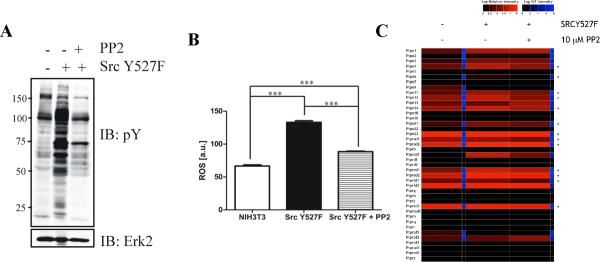
To ask if PTP oxidation was controlled by a mutant PTK, we treated SrcY527F cells with the Src kinase inhibitor, PP2. Global tyrosyl phosphorylation (Figure 6A) and ROS (Figure 6B) levels decreased following PP2 treatment, accompanied by an overall decrease in PTP oxidation (Figure 6C and Table S6). Nevertheless, some PTPs, (e.g., Ptpn3 and Ptpra D2) remained oxidized following PP2 treatment.
Figure 6. Reversibility of PTP oxidation in cancer cells following oncogene inhibition.
(A) Anti-phosphotyrosine immunoblot and (B) ROS levels of untransformed and Src Y527F cells treated with or without PP2, respectively. Data represent mean ± SEM (n=3; *** p<0.001, ANOVA with Bonferroni post-test). (C) Heat map showing effects of PP2 on levels of PTP oxidation in Src Y527F cells (n=2; 2 technical replicates each). * indicates PTPs with decreased oxidation following PP2 treatment.
Combining qPTPome with phosphotyrosine proteomics to suggest PTP substrates
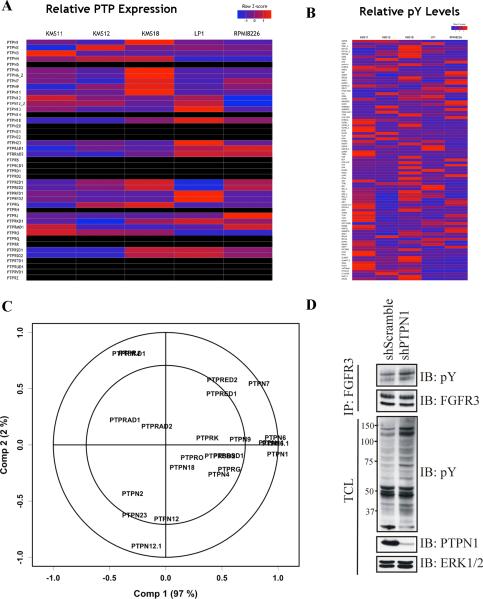
Finally, we asked if combining qPTPome with pY-proteomics could suggest PTP/substrate relationships. We used qPTPome to quantify PTP expression in several multiple myeloma (MM) cell lines (Figure 7A and Table S7) and measured 93 unique pY sites in the same cells by pY-proteomics (Figure 7B and Table S7). Using partial least squares regression (PLSR) analysis, we sought relationships between the levels of various pY-peptides and the expression of specific PTPs (see Experimental Procedures for details). The resultant model predicted relative pY-peptide levels well (Figure 7C and Table S7), with Component 1 accounting for 97% of the variance and Component 2 explaining another 2%. PTPN1 levels correlated most strongly with Component 1, with PTPN6 and PTPN11 also contributing significantly. PTPN7 explained most of the variation in Component 2, and contributed to Component 1.
Figure 7. Combining qPTPome and phosphotyrosine proteomics.
(A) Heat map showing relative PTP expression (based on z-score across all lines) measured by qPTPome in a panel of MM cell lines (n=2, 2 technical replicates each). Black box indicates PTPs that were not identified in any line. (B) Relative phosphotyrosine peptide levels (based on z-score across all MM lines) in the same cells (n=2). (C) Correlation loadings plot displaying results of the PLSR analysis. (D) Lysates from KMS11 cells stably infected with scrambled or PTPN1 shRNAs were immunoprecipated with FGFR3 antibodies and immunoblotted with anti-phosphotyrosine or -FGFR3 antibodies (top). Immunoblots of total cell lystate (TCL) with the indicated antibodies (bottom).
Overall, PTPN1, PTPN6, PTPN7 and PTPN11 levels could explain the majority of the variation for many of the pY sites analyzed. Interestingly, the site best explained by the first two components was from TYK2 (Table S7), a known PTP1B substrate (Myers et al., 2001). The relative pY levels of the FGFR3 activation loop phosphotyrosyl residue also were highly correlated with Components 1 and 2 (Table S7). Surprisingly, these correlations were positive: peptide phosphorylation increased as the PTP expression level increased (Table S7). Nevertheless, given the prominent role of PTPN1 in regulating tyrosyl phosphorylation of several other RTKs (Bourdeau et al., 2005), we tested the effects of a PTPN1 shRNA on one of these lines (KMS11) Remarkably, PTPN1 depletion led to increased tyrosyl phosphorylation of FGFR3 and several other proteins, including one with the molecular weight of TYK2 (Figure7D). These data suggest that, like TYK2, FGFR3 is probably a bona fide in vivo target of PTPN1.
DISCUSSION
By using proteomic methods that enable comprehensive analysis of the classical PTPome (Figure 1A), we identified the PTPs in multiple human, mouse and rat cell lines and tissues (Figure 2), including platelets (Figure 2D), cells in which RNA-based approaches have been of limited benefit. Our studies uncover significant post-translational regulation of PTP levels (Figure 2C) and unanticipated complexity in PTP oxidation evoked by exogenous and endogenous ROS in normal and neoplastic cells (Figures 4–6). Such differential PTP oxidation represents a new layer of complexity in signal transduction. Furthermore, combining our assay with pY-proteomics can help to identify PTP substrates (Figure 7).
We used SRM to quantify PTP expression because it is easily applied to tissues and cell lines, but our assay also accommodates other quantification methods (Yates et al., 2009). Additional variations could permit simultaneous identification of post-translational modifications (Figure S1): e.g., the oxPTP Ab could be used to isolate full length (denatured) PTPs, and phosphorylated tryptic peptides could be recovered (with TiO2 or anti-pY antibodies), followed by MS. The oxPTP Ab does not detect DUSPs, but by using more degenerate anti-Cys-SO3H hapten antibodies, analogous appproaches could monitor oxidation (and expression) of these (and other) cysteinyl proteins (Figure S1).
The lack of global methods to identify oxidized PTPs has hampered progress on redox regulation. Several other indirect approaches use an overall strategy similar to q-oxPTPome. The modified in-gel PTPase assay (Meng et al., 2002) has been the most successful, but it relies on immunodepletion for PTP identification, rarely detects RPTPs and is not quantitative. The modified cysteinyl-labeling assay isolates oxidized PTPs using iodoacetylpolyethylene oxide biotin (IAP-Biotin) (Boivin et al., 2010) or activity-based probes, such as α-bromobenzylphosphonate biotin (BBP-Biotin) (Kumar et al., 2004). Direct approaches using dimedone, which reacts selectively with SOH groups, represent a promising alternative (Poole and Nelson, 2008). Biotin-coupled dimedone probes (Nelson et al., 2010) and dimedone-specific antibodies (Seo and Carroll, 2009) are available. However, none of these approaches have been developed into a global MS assay for PTP oxidation. Recently, a modified IAP-Biotin probe was used to identify reactive cysteinyl residues by MS (Weerapana et al., 2010), but only three PTP-derived peptides were detected (from >1000 cysteine-containing species), and none corresponded to active site peptides. The high reactivity of IAP-Biotin with any reactive S− probably increases the background of abundant (non-PTP) proteins, which also could be problematic for dimedone-based approaches. These limitations are surmounted by q-oxPTPome, which uses a highly specific antibody to purify PTPs. Notably, q-oxPTPome cannot yet identify the PTPs oxidized upon growth factor stimulation, probably because only a small subset of PTPs is inactivated (under steady state conditions) by growth factor-evoked ROS (e.g., see (den Hertog et al., 2005; Rhee et al., 2000; Woo et al., 2010)). Similar obstacles confronted initial phosphoproteomic (including pY-proteomic) methods, prior to secondary enrichment strategies (Ahn et al., 2007; Pandey et al., 2002).
Our results add to growing evidence that ROS production and PTP oxidation are highly controlled in cells (Li et al., 2006; Woo et al., 2010). First, because our assay is quantitative, we can provide an upper bound on growth factor-induced increases in PTP oxidation (<5%). For such a small change to impact signaling (see Introduction), local control would be essential. Consistent with in vitro studies (Groen et al., 2005; Weibrecht et al., 2007), we find that PTPs also differ in relative oxidation sensitivity in cells. Surprisingly, though, the pattern of oxidized PTPs in cells stimulated by exogenous H2O2 differs from that evoked by increasing endogenous H2O2 levels (Figure 4). Furthermore, different cancer cells have distinct PTP oxidation profiles (Figure 5) that do not simply correlate with global ROS levels (Figure S4), again consistent with the idea that local ROS alterations are critical for altering tyrosyl phosphorylation events. Interestingly, transformation can have fairly durable effects on redox homeostasis, as oxidation of some PTPs cannot be reversed readily by oncogene inhibition (Figure 6). Further analyses of this phenomenom might shed light on key regulators of local ROS production/redox regulation.
Finally, qPTPome can be combined with pY-proteomics to suggest specific PTP substrates (Figure 7). We identified four PTPs that appeared to play a key role in regulating phosphotyrosyl protein homeostasis in MM cells. Two of these PTPs have been implicated in MM: PTPN6 is reportedly a tumor suppressor, whereas PTPN7 is over-expressed (Julien et al., 2011). PTPN11 is a known positive regulator of the RAS-MAPK pathway and is a bona fide oncogene (Chan et al., 2008). Although PTPN11 has not been implicated specifically in MM, it is required for the transformation of NIH3T3 cells expressing oncogenic FGFR3 (Agazie et al., 2003), a common MM oncogene. Moreover, PTPN1 depletion in KMS11 cells leads to FGFR3 hyper-phosphorylation and increased global phosphotyrosine levels (Figure 7D), suggesting that PTPN1 may be a key negative regulator of FGFR3. Unexpectedly, expression of PTPN1 (and the other three PTPs) correlates positively with tyrosyl phosphorylation of several peptides, yet knockdown causes increased phosphorylation of the same species. Conceivably, these apparently paradoxical findings reflect an attempt by MM cells to limit the effects of excess FGFR3 signaling by upregulating its negative regulators.
In any case, our results argue strongly that altered redox regulation provides an additional level of complexity to the cancer phenotype. Further refinement and application of qPTPome and q-oxPTPome should help delineate the contribution of ROS regulation of PTPs to normal and pathological cell signaling.
EXPERIMENTAL PROCEDURES
Cells and viral infection
Cell lines were cultured under standard conditions and treated with H2O2 (Bioshop) for 5 min or 100 μM BSO (Sigma) for 24 h, as indicated. Nitrosylation was induced by exposing cells for 30 min to 0.5 mM SNAP (Calbiochem) and L-cysteine (Sigma), GSNO (Alexis Biochemicals) and L-cysteine or CSNO (Sigma), each prepared as described (Li and Whorton, 2003). Src Y527F cells were provided by Dr. S. Courtneidge (Sanford-Burnham Medical Research Institute, USA) and treated with 10 μM PP2 (Calbiochem) for 4 h, when indicated. Generation of Ptpn11fl/KO cells and deletion of the floxed Ptpn11 allele are described in Supplemental Information. Lentiviruses expressing PTPN1 shRNA or a non-silencing control hairpin were generated by standard procedures and used to infect KMS11 cells. Human platelets were prepared by standard techniques (Pearce et al., 2004). For further details, see Supplemental Information.
Immunoblotting
Lysates were prepared in RIPA buffer containing a protease and phosphatase inhibitor cocktail, clarified, resolved by SDS-PAGE and analyzed by immunoblotting. For details, see Supplemental Information.
Quantitative real time PCR (qPCR)
RNA from brains of 10 week-old Ptprd+/+, Ptprd+/− and Ptprd−/− mice (C57BL/6J) was reverse transcribed and analyzed by Sybr green-based qPCR (Sigma), according to the manufacturer's instructions. For details, see Supplemental Information.
ROS levels
Cells (80% confluence) were serum-starved overnight and treated as indicated. Plates were washed with PBS (2×), trypsinized, incubated for 5 min with 300 nM DCF-DA (Invitrogen) at 37°C and washed immediately with ice-cold PBS (3×). Mean fluorescence intensity (MFI) was quantified by flow cytometry (FACs Calibur).
qPTPome
Cells or tissues were homogenized in 50 mM Hepes, pH 7.4, 150 mM NaCl, 10% glycerol, 1% NP40, containing 10 mM DTT and a protease and phosphatase inhibitor cocktail. Lysates were clarified at 16,100×g for 15 min at 4°C. Supernatants were rotated in the dark at room temperature (RT) for 30 min., desalted by gel filtration into 20 mM Hepes, pH 7.4, treated with 100 μM PV (Huyer et al., 1997), and rotated at 4°C for 1 h in the dark. Proteins were denatured in SDS (0.5%) and EDTA (5 mM) at 95°C for 5 min, diluted 5-fold into NP40 buffer (20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 1% NP-40, 2 mM EDTA) and immunoprecipitated overnight at 4°C with the oxPTP Ab (mouse monoclonal anti-oxidized PTP active site antibody, clone 335636 [R&D Systems]; 5μg Ab/mg protein)) and 40μL of packed protein G Sepharose beads.
For MS, samples were incubated in 9M urea/4.5 mM DTT at 60°C for 30 min, cooled to RT, treated with 10 mM iodoacetamide (IAM) for 15 min in the dark, diluted to a final concentration of 2 M urea, 20 mM HEPES, pH 8.0, and digested overnight at RT in 10 μg/mL TPCK-trypsin (Pierce). IAM prevents incomplete digestion due to disulfide bonds and ensures alkylation of other cysteines (besides the catalytic cysteine) found in some PTP active site peptides. Peptides were purified on a C18 column (Waters) by following the manufacturer's instructions, lyophilized, resuspended in 1.4 mL peptide IP buffer (50 mM Hepes, pH 7.4, 50 mM NaCl) and incubated for 30 min on a shaker at RT. Insoluble matter was removed by centrifugation at 2,000×g. Supernatants were collected, and PTP-SO3H active site peptides were isolated by immunoprecipitation with the oxPTP Ab. Immunoprecipitates were washed in 1 mL peptide IP buffer (3×), follwed by 1 mL HPLC grade H2O (3×), eluted by incubating with 80 uL 0.15% trifluoroacetic acid for 10 min at RT (3×), dried and analyzed by MS. The Ptpn11 AQUA peptide (QESIVDAGPVVVHCSO3HSAGIGR; six 13C and four 15N), was purchased from Thermo Scientific.
q-oxPTPome
q-oxPTPome buffer (50 mM Hepes, pH 6.5, 150 mM NaCl, 10% glycerol, 1% NP40) was degassed overnight at 30–35 mmHg, transferred into a sealed round-bottom flask equilibrated with N2, purged with N2 for 1 h, transferred to an anaerobic workstation (ESBE Invivo2 400), and treated with 10 mM NEM (Sigma), 100 μg/mL catalase (Calbiochem) and a protease and phosphatase inhibitor cocktail. Cells were washed with ice-cold degassed PBS, transferred to the workstation and lysed in q-oxPTPome buffer. Lysates were incubated for 1 h at 4°C in the dark, and homogenates were clarified at 16,100×g for 15 min at 4°C. Supernatants were collected in the anaerobic workstation, and the buffer exchanged by gel filtration into degassed 20 mM Hepes, pH 7.4. Desalted lysates were treated with 10 mM DTT and rotated in the dark for 30 min at RT. After exchanging the buffer to remove DTT, samples were treated with 100 μM PV, rotated at 4°C for 1 h in the dark and analyzed by immunoprecipitation or MS. The DTT step is included to reduce all reversibly oxidized PTPs; omitting this step prevents PV from accessing the PTP active site and limits PTP oxidation to the SO3H state.
Measurement of all PTP oxidation states
The total signal for each PTP is provided by qPTPome, while q-oxPTPome determines the cumulative fraction of each PTP in the SOH and SO3H states. To measure the proportion of each PTP in the SO3H state, H2O2-treated cells were lysed in the presence of DTT and analyzed by SRM. The difference between the q-oxPTPome and SO3H signal provides the fraction of PTPs in the SOH state. Because the SO2H state is not converted to the SO3H state by PV, the difference in signal between cells subjected to qPTPome in unstimulated and H2O2-stimulated cells provides the fraction of each PTP in the SO2H state. If PTPs are not converted to the SO2H state, the intensities from these two procedures should be the same; any difference corresponds to the fraction of each PTP converted to the SO2H state. Finally, the fraction of active PTPs is the difference between the qPTPome and q-oxPTPome plus SO2H signals, respectively (see Figure 4D).
LC-MS/MS
LC/MS, peptide identification, and SRM were performed by using standard techniques and procedures. Samples were prepared for pY-proteomics as described (St-Germain et al., 2009). See Supplemental Information for details.
Statistical analysis
Data are presented as the mean ± SEM. Statistical significance was determined using Student's t-test (paired or unpaired) or one-way ANOVA, as appropriate. If ANOVA was significant, individual differences were evaluated using Bonferroni post-test. Statistical analyses were performed using GraphPad Prism 5, with p<0.05 considered significant.
Given the multivariate multiple linear regression problem: Y=XB+ε (1), where X is an I × K predictor matrix of PTPs and Y is an I × J response matrix of pY sites with both X and Y measured in five (I) different MM cell lines, PLSR was implemented in the R (version 1.40) package PLS (Mevik and Wehrens, 2007) to fit a solution to (1) using the NIPALS algorithm. PLSR attempts to predict Y from X and describe their common structure by simultaneously decomposing the predictor and response variables into a common set of orthogonal factors and specific loadings: X=TPT; Y=UCT~=TBCT, where T is the score matrix, P is the loading matrix, B is a diagonal matrix of regression weights, and C is a weight matrix. T and U are chosen such that their covariance is maximal. “Leave-one-out” cross-validation was used to determine the optimal number of components to use in the regression. Prior to fitting the model, we excluded those PTPs and pY sites where there were two or fewer measurements across the five cell lines.
Supplementary Material
HIGHLIGHTS
Developed proteomic approach to monitor classical PTP expression
Adaptation of the method allows quantification of the fraction of each oxidized PTP
Different cancer cell lines contain unique PTP oxidation profiles
Method can be combined with phosphotyrosine proteomics to suggest PTP substrates
ACKNOWLEDGEMENTS
We thank Dr. Petr Heneberg for qPCR and Dr. Suzanne Trudel (Ontario Cancer Institute/Princess Margaret Hospital) for the MM cell lines. This work was supported by National Institutes of Health Grant R37CA49152 (B.G.N.), the Human Frontiers of Science Program Grant RGP0039/2009-C (B.G.N.), the Canadian Institutes of Health Research (CIHR; M.F.M.), the Canadian Cancer Society Research Institute (M.F.M.), the Swedish Research Council (A.O.) and the EU-funded PTPNET Research Training Network (A.O.). Additional support was provided by the Ontario Ministry of Health and Long Term Care and the Princess Margaret Hospital Foundation. B.G.N. and M.F.M. are Canada Research Chairs, Tier 1. Y.A.S. is a British Heart Foundation (BHF) Intermediate Research Fellow (FS/08/034/25085) and J.M. is a BHF Post Doctoral Fellow (PG/07/034/22775). R.K. and I.S.H. are the recipients of graduate fellowships from the CIHR and the Natural Sciences and Engineering Council of Canada, respectively. Arne Östman is a co-inventor of the monoclonal oxidized PTP active site antibody and receives royalties for sales of the antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agazie YM, Movilla N, Ischenko I, Hayman MJ. The phosphotyrosine phosphatase SHP2 is a critical mediator of transformation induced by the oncogenic fibroblast growth factor receptor 3. Oncogene. 2003;22:6909–6918. doi: 10.1038/sj.onc.1206798. [DOI] [PubMed] [Google Scholar]
- Ahn NG, Shabb JB, Old WM, Resing KA. Achieving in-depth proteomics profiling by mass spectrometry. ACS Chem Biol. 2007;2:39–52. doi: 10.1021/cb600357d. [DOI] [PubMed] [Google Scholar]
- Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, Olsen OH, Jansen PG, Andersen HS, Tonks NK, Moller NP. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin B, Yang M, Tonks NK. Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Sci Signal. 2010;3:pl2. doi: 10.1126/scisignal.3137pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau A, Dube N, Tremblay ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Current opinion in cell biology. 2005;17:203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Burridge K, Nelson A. An in-gel assay for protein tyrosine phosphatase activity: detection of widespread distribution in cells and tissues. Anal Biochem. 1995;232:56–64. doi: 10.1006/abio.1995.9961. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008;27:179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- Chen CY, Willard D, Rudolph J. Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry. 2009;48:1399–1409. doi: 10.1021/bi801973z. [DOI] [PubMed] [Google Scholar]
- den Hertog J, Groen A, van der Wijk T. Redox regulation of protein-tyrosine phosphatases. Arch Biochem Biophys. 2005;434:11–15. doi: 10.1016/j.abb.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Denu JM, Dixon JE. Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- Elschenbroich S, Kislinger T. Targeted proteomics by selected reaction monitoring mass spectrometry: applications to systems biology and biomarker discovery. Molecular bioSystems. 2010;7:292–303. doi: 10.1039/c0mb00159g. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Current opinion in cell biology. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras MC, Zhang YL, Kharitidi D, Barr AJ, Knapp S, Tremblay ML, Pause A. HD-PTP is a catalytically inactive tyrosine phosphatase due to a conserved divergence in its phosphatase domain. PLoS One. 2009;4:e5105. doi: 10.1371/journal.pone.0005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen A, Lemeer S, van der Wijk T, Overvoorde J, Heck AJ, Ostman A, Barford D, Slijper M, den Hertog J. Differential oxidation of protein-tyrosine phosphatases. The Journal of biological chemistry. 2005;280:10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- Gross S, Blanchetot C, Schepens J, Albet S, Lammers R, den Hertog J, Hendriks W. Multimerization of the protein-tyrosine phosphatase (PTP)-like insulin-dependent diabetes mellitus autoantigens IA-2 and IA-2beta with receptor PTPs (RPTPs). Inhibition of RPTPalpha enzymatic activity. The Journal of biological chemistry. 2002;277:48139–48145. doi: 10.1074/jbc.M208228200. [DOI] [PubMed] [Google Scholar]
- Gstaiger M, Aebersold R. Applying mass spectrometry-based proteomics to genetics, genomics and network biology. Nat Rev Genet. 2009;10:617–627. doi: 10.1038/nrg2633. [DOI] [PubMed] [Google Scholar]
- Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. The Journal of biological chemistry. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- Julien SG, Dube N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nat Rev Cancer. 2011;11:35–49. doi: 10.1038/nrc2980. [DOI] [PubMed] [Google Scholar]
- Kumar S, Zhou B, Liang F, Wang WQ, Huang Z, Zhang ZY. Activity-based probes for protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2004;101:7943–7948. doi: 10.1073/pnas.0402323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiry P, Torkamani A, Schork NJ, Hegele RA. Kinase mutations in human disease: interpreting genotype-phenotype relationships. Nat Rev Genet. 2010;11:60–74. doi: 10.1038/nrg2707. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. The Journal of biological chemistry. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Whorton AR. Regulation of protein tyrosine phosphatase 1B in intact cells by S-nitrosothiols. Arch Biochem Biophys. 2003;410:269–279. doi: 10.1016/s0003-9861(02)00696-3. [DOI] [PubMed] [Google Scholar]
- Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, Tonks NK, Meng TC. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. Febs J. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. The Journal of biological chemistry. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Mevik BH, Wehrens R. The pls Package: Principal Component and Partial Least Squares Regression in R. Journal of Statistical Software. 2007;18:1–24. [Google Scholar]
- Mizuno K, Hasegawa K, Katagiri T, Ogimoto M, Ichikawa T, Yakura H. MPTP delta, a putative murine homolog of HPTP delta, is expressed in specialized regions of the brain and in the B-cell lineage. Mol Cell Biol. 1993;13:5513–5523. doi: 10.1128/mcb.13.9.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. The Journal of biological chemistry. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- Nelson KJ, Klomsiri C, Codreanu SG, Soito L, Liebler DC, Rogers LC, Daniel LW, Poole LB. Use of dimedone-based chemical probes for sulfenic acid detection methods to visualize and identify labeled proteins. Methods Enzymol. 2010;473:95–115. doi: 10.1016/S0076-6879(10)73004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- Pandey A, Blagoev B, Kratchmarova I, Fernandez M, Nielsen M, Kristiansen TZ, Ohara O, Podtelejnikov AV, Roche S, Lodish HF, et al. Cloning of a novel phosphotyrosine binding domain containing molecule, Odin, involved in signaling by receptor tyrosine kinases. Oncogene. 2002;21:8029–8036. doi: 10.1038/sj.onc.1205988. [DOI] [PubMed] [Google Scholar]
- Pearce AC, Senis YA, Billadeau DD, Turner M, Watson SP, Vigorito E. Vav1 and vav3 have critical but redundant roles in mediating platelet activation by collagen. The Journal of biological chemistry. 2004;279:53955–53962. doi: 10.1074/jbc.M410355200. [DOI] [PubMed] [Google Scholar]
- Persson C, Kappert K, Engstrom U, Ostman A, Sjoblom T. An antibody-based method for monitoring in vivo oxidation of protein tyrosine phosphatases. Methods. 2005;35:37–43. doi: 10.1016/j.ymeth.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Persson C, Sjoblom T, Groen A, Kappert K, Engstrom U, Hellman U, Heldin CH, den Hertog J, Ostman A. Preferential oxidation of the second phosphatase domain of receptor-like PTP-alpha revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2004;101:1886–1891. doi: 10.1073/pnas.0304403101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picotti P, Bodenmiller B, Mueller LN, Domon B, Aebersold R. Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell. 2009;138:795–806. doi: 10.1016/j.cell.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxidants & redox signaling. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, Greenfield EA, Salgia R, Griffin JD. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. The Journal of biological chemistry. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- Seo YH, Carroll KS. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc Natl Acad Sci U S A. 2009;106:16163–16168. doi: 10.1073/pnas.0903015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- St-Germain JR, Taylor P, Tong J, Jin LL, Nikolic A, Stewart, Ewing RM, Dharsee M, Li Z, Trudel S, et al. Multiple myeloma phosphotyrosine proteomic profile associated with FGFR3 expression, ligand activation, and drug inhibition. Proc Natl Acad Sci USA. 2009;106:20127–20132. doi: 10.1073/pnas.0910957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Tanner JJ, Parsons ZD, Cummings AH, Zhou H, Gates KS. Redox regulation of protein tyrosine phosphatases: structural and chemical aspects. Antioxidants & redox signaling. 2010;15:77–97. doi: 10.1089/ars.2010.3611. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- Walther TC, Mann M. Mass spectrometry-based proteomics in cell biology. J Cell Biol. 2010;190:491–500. doi: 10.1083/jcb.201004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MB, Bachovchin DA, Mowen K, Baker D, Cravatt BF. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature. 2010;468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibrecht I, Bohmer SA, Dagnell M, Kappert K, Ostman A, Bohmer FD. Oxidation sensitivity of the catalytic cysteine of the protein-tyrosine phosphatases SHP-1 and SHP-2. Free Radic Biol Med. 2007;43:100–110. doi: 10.1016/j.freeradbiomed.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Yates JR, Ruse CI, Nakorchevsky A. Proteomics by mass spectrometry: approaches, advances, and applications. Annu Rev Biomed Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wolf-Yadlin A, Ross PL, Pappin DJ, Rush J, Lauffenburger DA, White FM. Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules. Mol Cell Proteomics. 2005;4:1240–1250. doi: 10.1074/mcp.M500089-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Wang Y, Dixon JE. Dissecting the catalytic mechanism of protein-tyrosine phosphatases. Proc Natl Acad Sci USA. 1994;91:1624–1627. doi: 10.1073/pnas.91.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.