Abstract
Although thiazolidinediones (TZD) effectively improve hyperglycemia and increase adiponectin, a proinsulin-sensitizing adipokine, they also increase adipogenesis via peroxisome proliferator-activated receptor (PPAR)γ induction, which may be undesirable. Recent safety concerns about some TZD have prompted the search for next generation agents that can enhance glycemic control and adiponectin independent of PPARγ or adipogenesis. Reminiscent of TZD action, a human adenovirus, adenovirus 36 (Ad36), up-regulates PPARγ, induces adipogenesis, and improves systemic glycemic control in vivo. We determined whether this effect of Ad36 requires PPARγ and/or adipogenesis. Glucose uptake and relevant cell signaling were determined in mock-infected or human adenoviruses Ad36 or Ad2-infected cell types under the following conditions: 1) undifferentiated human-adipose-tissue-derived stem cells (hASC), 2) hASC differentiated as adipocytes, 3) hASC in presence or absence of a PPARγ inhibitor, 4) NIH/3T3 that have impaired PPARγ expression, and 5) PPARγ-knockout mouse embryonic fibroblasts. Mouse embryonic fibroblasts with intact PPARγ served as a positive control. Additionally, to determine natural Ad36 infection, human sera were screened for Ad36 antibodies. In undifferentiated or differentiated hASC, or despite the inhibition, down-regulation, or the absence of PPARγ, Ad36 significantly enhanced glucose uptake and PPARγ, adiponectin, glucose transporter 4, and glucose transporter 1 protein abundance, compared with mock or Ad2-infected cells. This indicated that Ad36 up-regulates glucose uptake and adiponectin secretion independent of adipogenesis or without recruiting PPARγ. In humans, natural Ad36 infection predicted greater adiponectin levels, suggesting a human relevance of these effects. In conclusion, Ad36 provides a novel template to metabolically remodel human adipose tissue to enhance glycemic control without the concomitant increase in adiposity or PPARγ induction associated with TZD actions.
According to the latest National Diabetes Factsheet released by the Centers for Disease Control (http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf), the United States alone has about 26 million individuals with diabetes mellitus (DM), and even more (79 million) with prediabetes (pre-DM). Hence, better treatment and prevention approaches are urgently needed. Adipose tissue is increasingly recognized for its contribution to systemic glycemic control and offers a drug discovery target (1). Excessive adiposity is strongly associated with impaired glycemic control, which can be effectively improved by reducing excess body fat. Yet, paradoxically, adipogenesis, which involves the induction of adipogenic genes, including peroxisome proliferator-activated receptor (PPAR)γ, has long been recognized to enhance insulin-dependent glucose uptake (2), whereas impaired adipogenesis is associated with insulin resistance (3). PPARγ, a member of the nuclear hormone receptor super family, differentiates adipocyte precursors to mature adipocytes and also plays a pivotal role in influencing adipose tissue glucose uptake (4). Although both of its closely related forms, PPARγ1 and PPARγ2, are present in adipose tissue (5), adipocyte differentiation is stimulated primarily by PPARγ2 (6).
In adipose tissue, induction of PPARγ increases adipogenesis, up-regulates adiponectin (a proinsulin-sensitizing adipokine) and glucose transporter (Glut)1 and Glut4 (cellular Glut responsible for glucose uptake), and enhances glucose uptake (7). Conversely, PPARγ down-regulation impairs adipogenesis, adiponectin abundance, and insulin-stimulated glucose uptake (7). PPARγ agonists induce adipocyte differentiation and enhance cellular glucose uptake (8, 9), antidiabetic agents thiazolidinediones (TZD) being the most notable example (10). By acting as ligands for PPARγ, TZD increase adiponectin and improve glycemic control. Importantly, the improvement in adipose tissue metabolic profile by the TZD does not require fat loss, which is very appealing, given the limited success of long-term weight loss and weight maintenance approaches. Instead, due to the activation of PPARγ, TZD promote adipogenesis, leading to increased adiposity (11).
Although the effects of TZD on adiponectin and glycemic control are very attractive, adipogenesis they promote by PPARγ activation may be undesirable. In fact, Larsen et al. (11) question that “… effect of long term treatment on weight gain after TZD treatment is unknown and it may be questioned whether the use of these ‘adipogenic compounds’ is appropriate considering that excess body fat is almost a prerequisite for the development of type 2 diabetes.” In addition, cardiovascular and other adverse effects of some agents from the TZD class of drugs have been reported recently (12, 13) that appear to be mediated by PPARγ. Therefore, ideally, the next generation of antidiabetic agents should improve glycemic control without fat loss and improve adipose tissue metabolic profile independent of PPARγ. In fact, several compounds that either completely or partially bypass PPARγ activation or its downstream signaling events are under investigation for promoting glucose uptake (14, 15). Our previous work indicated that adenovirus 36 (Ad36), a human adenovirus, may offer a template to identify and develop such therapeutic targets.
Ad36 was first isolated from a human fecal sample (16). It is among 51 known human adenovirus serotypes and belongs to the subgroup D of adenoviruses (16). In general, human adenoviruses are mainly associated with infections of the respiratory or gastrointestinal tract or conjunctiva, but such symptoms associated with Ad36 infection are not reported. The action of Ad36 on host metabolism appears strikingly similar to that of the TZD in some, but not all, aspects. In adipocyte progenitors, Ad36 up-regulates PPARγ and its target genes, including adiponectin, and induces commitment, differentiation, and lipid accumulation (17–19). Moreover, Ad36 increases basal or insulin-stimulated glucose uptake in adipose tissue, adipocyte progenitors, and myoblasts (20, 21), actions that are similar to those of the TZD. In chow- animals, like TZD, experimental Ad36 infection increases adiposity (22–25) yet improves their glycemic control (24, 25).
On the other hand, our recent data also underscored some differences in action between Ad36 and TZD. Particularly in the presence of a high-fat (HF) diet, TZD improve glycemic control but also promote lipid storage in various organs, including liver (26, 27), probably via PPARγ. In contrast, in HF-fed mice (60% fat diet), Ad36 infection does not increase adiposity, or PPARγ expression in adipose tissue or liver (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org), compared with uninfected mice. However, Ad36 lastingly increases adiponectin abundance, improves glycemic control, and interestingly, reduces hepatic lipid accumulation in these mice (25). These differences suggested that up-regulation of PPARγ and adipogenesis could be uncoupled from Ad36-induced improvement in glycemic control.
Overall, it appears that in vivo, Ad36 increases adipose tissue glucose uptake and enhances adiponectin abundance, which in turn increases glucose uptake by skeletal muscle and reduces hepatic glucose output (25). Thus, enhancing the metabolic profile of adipose tissue appears central to Ad36-induced improvement in systemic glycemic control. This valuable property of Ad36 could be exploited to design novel antidiabetic approaches that would modulate adipose tissue metabolically but without a concomitant increase in adiposity associated with TZD. To further assess the potential of Ad36 to alter adipose tissue metabolic profile in humans, this study determined whether Ad36 1) directly and separately enhances metabolic profile of human primary adipocyte progenitors as well as adipocytes; 2) enhances cellular glucose uptake and adiponectin abundance, independent of PPARγ; and 3) is associated with greater adiponectin levels in humans.
Materials and Methods
Experimental methods are outlined. Detailed assays and techniques are described under techniques and assays section. Human adipose tissues for the isolation of human-adipose-tissue-derived stem cells (hASC) were obtained after approval by the Pennington Biomedical Research Center (PBRC)'s Institutional Review Board Protocol PBRC23040 (experiments 1, 2, and 3a). A human study approved by the PBRC Institutional Review Board and listed on the ClinicalTrials.gov website (NCT00219440) was used to obtain serum samples for Ad36 antibody testing (experiment 4).
Experiment 1. Glucose uptake by Ad36 in preadipocytes
At 70% confluence, human preadipocytes-hASC were infected either with media (mock, n = 6) or with 3.8 multiplicity of infection (MOI) of Ad36 (n = 6) or Ad2 (n = 6). Basal and insulin-stimulated glucose uptake was determined on d 1, 3, and 5 postinfection (pi). Proteins were harvested and stored at −80 C until used for Western blotting (WB) (see techniques and assays).
Experiment 2. Glucose uptake by Ad36 in adipocytes
hASC were differentiated to form adipocytes (see techniques and assays). Nine days later, the adipocytes were infected either with media (mock, n = 6) or with 5 MOI of Ad36 (n = 6) or Ad2 (n = 6). Basal and insulin-stimulated glucose uptake was determined 72 h pi. Proteins were harvested and stored at −80 C until used for WB (see techniques and assays).
Experiment 3. Role of PPARγ in Ad36-induced glucose uptake
Contribution of PPARγ was determined by measuring Ad36-induced glucose uptake and adipogenesis in relative or complete absence of PPARγ as follows.
Experiment 3a. Chemical inhibition of PPARγ
Confluent hASC were exposed to adipogenic media to induce differentiation (see techniques and assays), with or without 10 μm GW9662, a specific inhibitor of PPARγ2 (28). Inhibition of PPARγ by GW9662 was verified and an effective dose determined as described in Supplemental data. Media were renewed every 3 d for up to 6 d, after which the adipocytes were infected with either media (mock, n = 12) or with 5 MOI of Ad36 (n = 12) or Ad2 (n = 12). Basal and insulin-stimulated glucose uptake was determined 72 h pi. Proteins were harvested and stored at −80 C until used for WB (see techniques and assays).
Experiments 3b and 3c. Impaired PPARγ expression and absence of PPARγ
Experiment 3b determined whether Ad36 will increase glucose uptake in NIH/3T3 cells, which are murine fibroblasts, with low PPARγ expression (6). 3T3-L1-coxsackie and adenovirus receptor (CAR) cells, which are murine embryonic fibroblasts with intact PPARγ that overexpress “coxsackie virus adenovirus receptor” for ensuring adenoviral entry, were used as a positive control.
Experiment 3c determined glucose uptake by Ad36 in the absence of PPARγ by using murine PPARγ−/− embryonic fibroblasts [mouse embryonic fibroblasts (MEF)].
In both experiments, 3b and 3c, 70% confluent cells were infected with either media (mock, n = 6) or with 5 MOI of Ad36 (n = 6) or Ad2 (n = 6). Basal glucose uptake was determined 72 h pi for CAR and NIH/3T3 cells and 24 h pi for MEF. Protein and RNA were harvested and stored at −80 C until used for WB or real-time quantitative PCR (qRT-PCR) assay.
Experiment 3d. Lipid accumulation
To determine lipid accumulation, confluent CAR, NIH/3T3, and MEF cells were exposed to differentiation media and incubated for 14 d. In a separate set, 70% confluent NIH/3T3 and MEF cells were infected with media (control, n = 6), 3.8 MOI of Ad36 (n = 6) or Ad2 (n = 6), and incubated for 12 d. Cells were stained with lipid specific dye Oil Red O (ORO) as described (18). Phase contrast images were collected using a Zeiss Axiovert 200 inverted microscope with an AxioCam HRc digital camera (Zeiss, Oberkochen, Germany).
Replicates
The time course of glucose uptake (experiment 1) was repeated twice. Experiments 3a, 3b, and 3c were repeated at least three separate times for glucose uptake assays. Each time, six or more replicates were included for each group. Three replicates for a group were used for WB.
Experiment 4. Adiponectin in humans naturally infected with Ad36
Appropriately stored archived baseline serum samples obtained from subjects participating in a clinical trial were tested in a blinded fashion for neutralizing antibodies to Ad36. The subjects had provided informed consent. Baseline demographic, anthropometric, laboratory, and body composition data were gathered as described previously (29). Serum samples were screened for Ad36 antibodies by using serum neutralization assay as described (25, 30), and the differences in HbA1c levels of this cohort based on Ad36 antibody status were reported previously (21), which showed significantly better glycemic control in Ad36 seropositive subjects. Here, we compared the seropositive and seronegative groups for serum adiponectin levels measured by the PBRC Clinical Laboratory.
Techniques and assays
Viruses
Ad36 and Ad2 were obtained from American Type Culture Collection (catalog nos. VR913 and VR846, respectively; Manassas, VA). Ad36 was plaque purified and propagated in A549 cells (human lung cancer cell line) as described (22, 23).
Isolation of hASC
hASC were isolated from liposuction aspirates as previously described (31, 32). Briefly, the stromo-vascular fraction was resuspended in DMEM/F-12 (HyClone, Logan, UT) plus 10% fetal bovine serum (FBS) with antibiotics/antimycotics and plated at a density of 0.156 ml of tissue digest/cm2 as passage 0 and used for experiments within passages 2–4.
Cell culture
Subconfluent hASC were maintained in six-well tissue culture plates in DMEM/F-12 (HyClone) and NIH/3T3 cells, MEF, or CAR cells were maintained in DMEM (no. 1995-065; Invitrogen, Carlsbad, CA), containing 10% FBS at 37 C in an atmosphere of 5% CO2.
Induction of adipogenic differentiation
hASC.
Two days after confluence, cells were treated with differentiation induction medium (0.5 mm methylisobutylxanthine, 1 mm dexamethasone, 1 mm insulin, 1 m pantothenate, 33 mm roziglitazone, and 66 mm biotin) in DMEM/F-12 (HyClone) and 10% FBS. Medium was renewed every 3 d for up to 9 d.
CAR, NIH/3T3, or MEF.
Cells were treated with differentiation induction medium (0.5 mm methylisobutylxanthine, 1 mm dexamethasone, and 1 mm insulin) in DMEM (Cellgro, Mediatech, Inc., Manassas, VA) and 10% FBS. Two days later, differentiation medium was replaced with maintenance media containing 0.25 mm insulin and renewed every 2 d.
Infection of cells
At confluency, culture media were removed, and cells were incubated for 1 h with 100 μl/cm2 media (mock infection) or media plus Ad36 or Ad2 (3.8 MOI/cell, unless noted otherwise). After infection, media and virus were replaced with respective media plus 10% FBS and antimycotics/antibiotics.
Glucose uptake assay
The cells were incubated overnight in serum-free medium, then washed twice with PBS, followed by the addition of saline (for basal glucose uptake) or 100 nmol/liter insulin (for insulin-stimulated glucose uptake) and incubated for 15 min at 37 C. Uptake of [3H]2-deoxy-D-glucose was determined as described (21, 33). Data are expressed as picomoles 2-deoxyglucose per milligram protein. The protein content was determined with a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL). Each assay included five or more replicates.
Western blot analyses
WB on polyvinylidene fluoride membranes were probed with antibodies toward PPARγ (no. 7273; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), Glut4 (no. 35826; Abcam, Cambridge, MA; no. 1282; R&D Systems, Minneapolis, MN), Glut1 (no. 1603; Santa Cruz Biotechnology, Inc.), adiponectin (nos. 3604 and 3608; Chemicon, Bedford, MA), hexon-Ad36 (anti-Ad36 rabbit polyclonal antibody, 1:3000), or hexon-Ad2 (anti-Ad2 rabbit polyclonal antibody, 1:5000), followed by secondary antibody conjugated with horseradish peroxidase. Signals were detected by enhanced chemiluminescence and quantitated using AlphaEaseFC analyzer software. Signals were normalized to glyceraldehydes phosphate dehydrogenase (GAPDH) (no. 4300; Ambion, Austin, TX) abundance.
Membrane and cytosol protein fractions for determining Glut4 translocation were isolated using the compartmental protein extraction kit (catalog no. 2145; Millipore, Bedford, MA).
Real-time quantitative PCR
The relative expressions of E4orf1 of Ad36 and Ad2 were determined by qRT-PCR as described (18). Briefly, mRNA was extracted from CAR, NIH/3T3, and MEF cells, using the RNeasy Mini kit (no. 74101; QIAGEN, Valencia, CA). Residual DNA was eliminated using deoxyribonuclease-I (no. 18068-015; Invitrogen). Total RNA (1 μg) was reverse transcribed to cDNA using iscript cDNA synthesis kit (no. 170-8890; Bio-Rad, Hercules, CA). PCR Core System II (no. M7665; Promega, Madison, WI) was used for the amplification of cDNA. cDNA template (100 ng) was amplified (ABI PRISM 7900HT, Applied Biosystems, Carlsbad, CA) by qPCR using the ΔΔCt method. Primer sequences were as follows.
Ad36E4orf1 forward: 5′-CCCTCGCGGACATACAAAA-3′,
Ad36E4orf1 reverse: 5′-GCCGGGAGAAGACATGATCTC-3′,
Ad36E4orf1 probe: 5′-/56-FAM/TGC/TGCTCT/ZEN/TTAACCACACGGACCA/3IABkFQ/-3′;
Ad2E4orf1 forward: 5′-CCTAGGCAGGAGGGTTTTTC-3′,
Ad2E4orf1 reverse: 5′-ATAGCCCGGGGGAATACATA-3′;
Mouse GAPDH forward primer: 5′-GATGCTAAATGGGCAGAAGC-3′,
Mouse GAPDH reverse primer: 5′-CTGGCCCTCATAGCACACTT-3′.
No template control and RNA from uninfected cells were used as negative control, and RNA from 3T3-L1 cells expressing Ad36E4orf1 was used as positive control.
Immunofluorescence
To visualize viral proteins, hASC were processed as described (20). hASC were costained with anti-Ad36 or anti-Ad2 rabbit polyclonal antibody, followed by goat antirabbit Alexa Fluor 594 (Invitrogen) secondary antibody, and 4′,6-diamidino-2-phenylindole (Invitrogen) for experiment 1 or with boron-dipyrromethene (Invitrogen) for experiment 2. Images were acquired on a Zeiss Axioplan-2 (Everest) using LD Plan Neofluar (×20 objective) and Photometrics Cool Snap HQ camera. All images were processed using ImageJ software (National Institutes of Health, Bethesda, MD).
Confirmation of infection
In hASC, the infection with appropriate viruses (Ad36 or Ad2) was determined by screening for viral proteins using WB. Murine cells do not strongly support adenoviral protein formation; therefore, infection of CAR, NIH/3T3, and MEF cells was confirmed by the presence of E4orf1 mRNA for Ad36 or Ad2, using qRT-PCR.
Statistical analysis
Differences between multiple groups were determined by one-way ANOVA followed by Tukey's (P < 0.01). Change in PPARγ abundance between 0 and 10 μm GW9662 (see figure 3C), for each group, was determined by Student's t test (P < 0.01). Ad36 positive and negative human subjects were also compared using Student's t test.
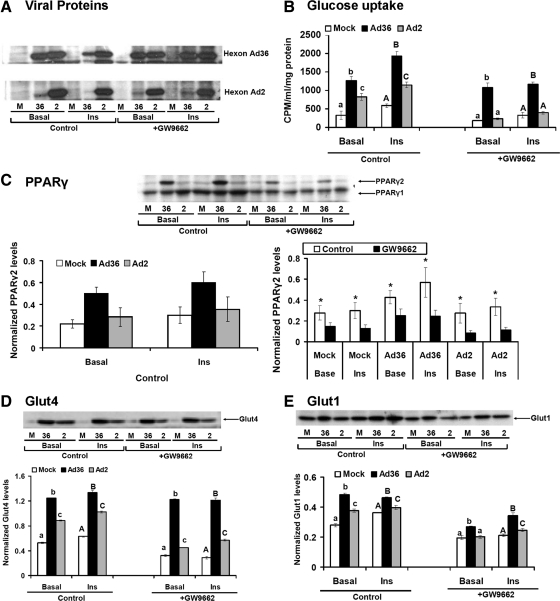
Fig. 3.
Ad36 up-regulates glucose uptake despite chemical inhibition of PPARγ. Confluent hASC were exposed to adipogenic media to induce differentiation as described in techniques and assays, with or without 10 μm GW9662, a specific inhibitor of PPARγ2. Six days later, the cells were infected either with media (mock, n = 12) or with 5 MOI of Ad36 (n = 12) or Ad2 (n = 12). Basal and insulin-stimulated glucose uptake assay was determined 72 h later. A, WB showing viral proteins in Ad36 and Ad2-infected cells and their absence in mock group. B, Basal and insulin-stimulated glucose uptake in absence or presence of GW9662. C–E, PPARγ, Glut4, and Glut1 abundance determined by WB (n = 3 replicates per group per protein); mean ± sd. Alphabets denote statistical significance. Groups denoted with different alphabets differ significantly from each other (P < 0.01). Upper case or lower case alphabets are used for separate comparisons of clusters. *, P < 0.01 compared with GW9662. CPM, Counts per minute; M, mock; INS, insulin.
Results
Experiment 1. Ad36 increases glucose uptake and adipogenesis in human preadipocytes
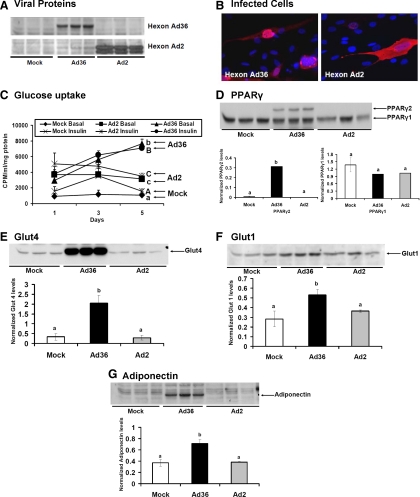
Successful infection of undifferentiated cells by Ad36 or Ad2 and the absence of infection in the mock group were confirmed by the presence of appropriate viral proteins (Fig. 1, A and B). A time course of basal and insulin-stimulated glucose uptake showed continued and significantly greater increase after Ad36 infection, compared with mock or Ad2 infections (Fig. 1C). The adipocyte progenitors used were not differentiated, which may explain their lack of response to insulin-stimulated glucose uptake in the mock-infected group. As expected, the preadipocytes did not express PPARγ2 at baseline (data not shown). But, as previously observed (17–19, 34), Ad36 induces preadipocyte differentiation over time, as indicated by significantly greater PPARγ2 abundance 5 d pi (Fig. 1D). Concurrently, Ad36 significantly increased protein abundance of Glut1 and Glut4 and adiponectin (Fig. 1, E–G), which probably contributes to the up-regulation of glucose uptake by Ad36. Thus, Ad36 may recruit adipocyte progenitors from human adipose tissue, to enhance glucose uptake and adiponectin abundance. Considering that the up-regulation of PPARγ, adiponectin, Glut, and glucose uptake is associated with adipogenesis (35, 36), the effect of Ad36 may be secondary to adipogenesis it induces in preadipocytes. By directly infecting mature adipocytes, experiment 2 below determined whether Ad36 increases glucose uptake without adipogenesis.
Fig. 1.
Ad36 increases glucose uptake in human preadipocytes. At 70% confluence, human preadipocytes (hASC) were infected either with media (mock, n = 6) or with 3.8 MOI of Ad36 (Ad36 n = 6) or Ad2 (n = 6) as described in techniques and assays. A, WB showing viral proteins in Ad36 and Ad2-infected cells and their absence in mock group. B, Immunohistochemistry images showing 4′,6-diamidino-2-phenylindole (DAPI)-stained (blue) cell nuclei and Ad36 or Ad2 hexon protein antibody (red). C, A time course of basal and insulin-stimulated glucose uptake determined on d 1, 3, and 5 pi. D–G, PPARγ, Glut4, Glut1, and adiponectin abundance determined by WB (n = 3 replicates per group per protein). Alphabets denote statistical significance; mean ± sd. Groups denoted with different alphabets differ significantly from each other (P < 0.01). Upper case or lower case alphabets are used for separate comparisons of clusters. For instance, in C, the lower case alphabets denote a comparison of basal glucose uptake in mock, Ad36, and Ad2 groups, and the upper case alphabets denote the comparison between insulin-stimulated conditions. CPM, Counts per minute.
Experiment 2. Ad36 increases glucose uptake in human adipocytes
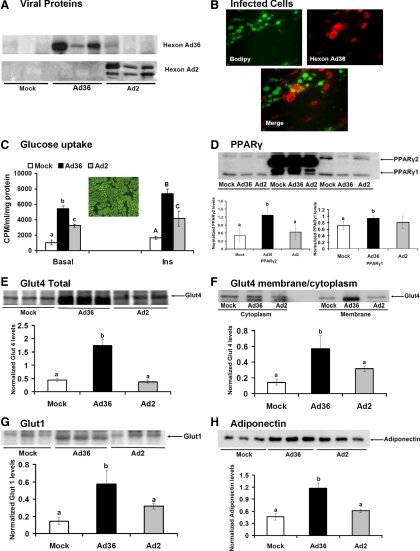
We hypothesized that in addition to recruiting adipocyte progenitors to improve glucose disposal, Ad36 infection of mature adipocytes will favorably modify their metabolic profile. Successful infection of cells by Ad36 or Ad2 and the absence of infection in the mock group were confirmed by the presence of appropriate viral proteins (Fig. 2, A and B). Almost 100% of the hASC were differentiated adipocytes (Fig. 2C). In these adipocytes, Ad36 significantly increased basal and insulin-stimulated glucose uptake compared with mock or Ad2-infected cells (Fig. 2C). Although all groups expressed PPARγ1 and PPARγ2, Ad36-infected hASC showed significantly greater abundance of the proteins (Fig. 1D). The increase in glucose uptake by Ad36 may be mediated via the up-regulation of Glut4 abundance and membrane translocation, and greater Glut1 abundance (Fig. 2, E–G). Ad36 significantly increased adiponectin abundance (Fig. 2H), which suggested a metabolic remodeling of mature adipocytes by Ad36.
Fig. 2.
Ad36 increases glucose uptake in human adipocytes. hASC were exposed to adipogenic media to induce differentiation. Nine days later, the adipocytes were infected either with media (mock, n = 6) or with 5 MOI of Ad36 (Ad36 n = 6) or Ad2(n = 6) as described in techniques and assays. A, WB showing viral proteins in Ad36 and Ad2-infected cells and their absence in mock group. B, Immunohistochemistry images showing boron-dipyrromethene-stained (green) cells indicative of lipid accumulation and Ad36 hexon protein antibody (red). Merged image shows viral infection of adipocytes. C, Basal and insulin-stimulated glucose uptake determined 72 h pi. Inset image shows that most cells were adipocytes, when infected. D–H, PPARγ, Glut4, membrane translocation of Glut4, Glut1, and adiponectin abundance determined by WB (n = 3 replicates per group per protein). Alphabets denote statistical significance; mean ± sd. Groups denoted with different alphabets differ significantly from each other (P < 0.01). Upper case or lower case alphabets are used for separate comparisons of clusters. CPM, Counts per minute; INS, insulin.
Mature adipocytes may or may not need PPARγ to maintain their differentiation, but they require it for glucose uptake (7, 37). Hence, the concomitant up-regulation of PPARγ2 and glucose uptake by Ad36 in adipocytes suggested the requirement of PPARγ2 for Ad36-induced glucose uptake, which was tested in experiment 3.
Experiment 3. PPARγ is not required for Ad36-induced up-regulation of glucose uptake or adiponectin
The role of PPARγ in Ad36-induced glucose uptake was determined in hASC and MEF in multiple ways.
Experiment 3a. Chemical inhibition of PPARγ
Preliminary experiment used 0, 1, or 10 μm GW9662, a specific PPARγ inhibitor (28), to determine the effective dose. Compared with the uninduced hASC, those treated with differentiation inducer media increased PPARγ2, glucose uptake. and adipogenic differentiation (Supplemental Fig. 2, A–C), which were all attenuated by GW9662 treatments (Supplemental Fig. 2). This showed that at 10 μm concentration, GW9662 is an effective inhibitor of PPARγ2 and that the cellular glucose uptake tracked PPARγ2 expression. Subsequent experiments used 10 μm GW9662 to test whether Ad36 will increase glucose uptake when PPARγ2 is inhibited. The presence of viral infection in cells in absence or in presence of insulin and with and without GW9662 was confirmed (Fig. 3A). Compared with the mock or Ad2-infected groups, Ad36 significantly increased glucose uptake in the absence or in presence of GW9662 (Fig. 3B). In the absence of GW9662, Ad36 significantly up-regulated PPARγ2 abundance (Fig. 3C). Treatment with the inhibitor reduced PPARγ2 abundance in all groups (by 50% or more) (Fig. 3C). However, it did not reduce Ad36-induced abundance of Glut4 or Glut1 (Fig. 3, D and E).
Experiment 3b. Impaired expression of PPARγ
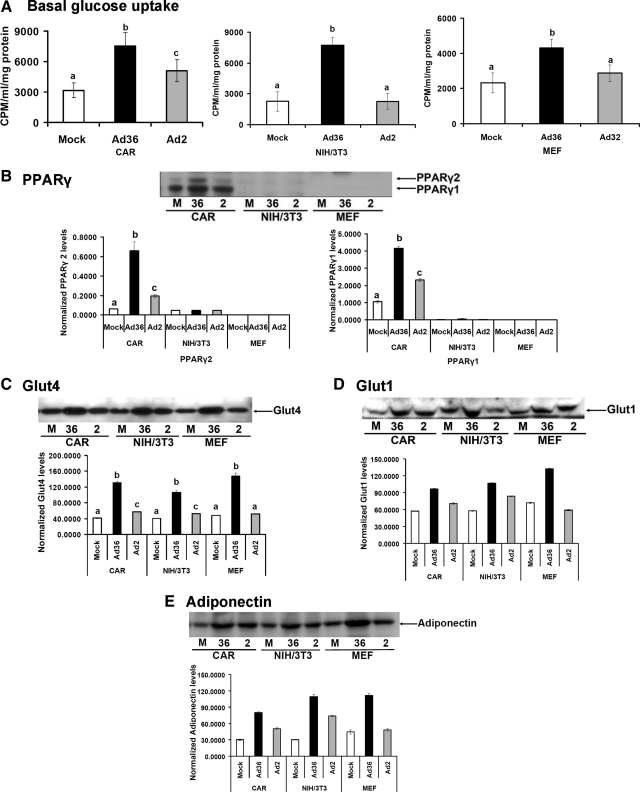
Infection of cells by Ad36 or Ad2 was confirmed by detecting the expression of viral mRNA (E4orf1 gene) in respective cells, by qRT-PCR for experiments 3b and 3c (data not shown). As expected, Ad36 significantly increased basal glucose uptake and the abundance of PPARγ, Glut4, Glut1, and adiponectin in 3T3-L1 preadipocytes (CAR cells) (Fig. 4, A–E). NIH/3T3 cells did not express much PPARγ2 or PPARγ1 (Fig. 4B). However, Ad36 significantly increased basal glucose uptake (Fig. 4A) and Glut4, Glut1, and adiponectin abundance in these cells (Fig. 4, B–E), which are PPARγ2 target genes.
Fig. 4.
Ad36 up-regulates glucose uptake or adiponectin despite the impaired or absent PPARγ expression. This experiment determined whether Ad36 will increase glucose uptake in CAR cells, which have intact PPARγ, in NIH/3T3 cells, which have low PPARγ expression, and in murine PPARγ−/− embryonic fibroblasts (MEF). Each cell type (70% confluent) was infected either with media (mock, n = 6) or with 5 MOI of Ad36 (n = 6) or Ad2 (n = 6) as described in techniques and assays. A, Basal glucose uptake was determined 72 h pi for CAR and NIH/3T3 cells and 24 h pi for MEF. B–E, PPARγ, Glut4, Glut1, and adiponectin abundance determined by WB (n = 3 replicates per group per protein); mean ± sd. Alphabets denote statistical significance. Groups denoted with different alphabets differ significantly from each other (P < 0.01). CPM, Counts per minute; M, mock.
Experiment 3c. Absence of PPARγ expression
PPARγ−/− MEF most rigorously tested the ability of Ad36 to up-regulate glucose uptake in the absence of PPARγ. Ad36, but not Ad2, robustly increased basal glucose uptake and Glut4, Glut1, and adiponectin, in the absence of PPARγ (Fig. 4, A–E). However, the dependence of Ad36 on PPARγ to induce adipogenesis was indicated by the absence of cell differentiation in NIH/3T3 or PPARγ−/− MEF cells (Fig. 5). These data showed that Ad36 can increase glucose uptake and adiponectin, without recruiting PPARγ and adipogenesis. Ability to up-regulate adiponectin in the absence of PPARγ may be particularly important for improving glycemic control in humans.
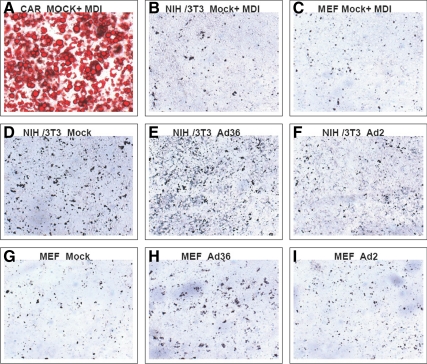
Fig. 5.
Ad36 does not increase lipid accumulation in NIH/3T3 and MEF cells. Confluent 3T3-L1, NIH/3T3, and MEF cells were exposed to differentiation media and incubated for 14 d. In a separate set, 70% confluent NIH/3T3 and MEF cells were infected with media [control group (n = 6)], 3.8 MOI of Ad36 (n = 6), or Ad2 (n = 6) and incubated for 12 d. Cells were stained ORO. Phase contrast images were collected using a Zeiss Axiovert 200 inverted microscope with an AxioCam HRc digital camera (magnification, ×10). A–C, MDI-treated 3T3-L1, NIH/3T3, and MEF cells, respectively. As a positive control, 3T3-L1 shows strong adipogenic differentiation as indicated by ORO staining. No lipid accumulation was observed in NIH/3T3 and MEF cells, despite MDI treatment. D–I, No lipid accumulation observed, indicating a lack of adipogenic differentiation. MDI, Methyl-isobutyl-xanthine, dexamethasone, insulin.
Experiment 4. Natural Ad36 infection in humans predicts greater adiponectin
Adiponectin is an insulin-sensitizing protein secreted by the adipose tissue (38, 39). Despite an over abundance of adipose tissue, serum adiponectin levels are low in obesity and even lower in obese subjects with type 2 DM (40, 41). Increases in adiponectin can contribute to better glycemic control. Serum from a total of 48 subjects was screened, and six had Ad36 antibodies, indicative of a past Ad36 infection (21). This cohort showed similar age, body mass index (BMI), body weight, and body fat percentage between Ad36 positive and Ad36 negative subjects. However, the Ad36 positive subjects had better glycemic control as indicated by significantly lower HbA1c levels (21). A comparison of these two groups showed that despite a similar BMI and adiposity, the Ad36-infected group (one male/five females) had about 1.5-fold greater (P < 0.02) serum adiponectin levels (9.2 ± 4.5 vs. 6.0 ± 2.8 μg/ml) compared with Ad36 seronegative group (15 males/27 females).
Due to only one male in Ad36 positive group, statistically adjusting the results for gender was difficult. Instead, we reanalyzed the data only for females. They were all obese without significant differences in their BMI (35.1 ± 5.54 vs. 37.4 ± 5.88 kg/m2; P = 0.42), adiponectin levels were greater for Ad36 positive (n = 5) vs. Ad36 negative (n = 27) groups (9.84 ± 4.7 vs. 6.4 ± 3.1 μg/ml; P = 0.042), even though the Ad36+ females were older (66.4 ± 8.2 yr) when compared with the Ad36 negative women (56.6 ± 9.9 yr; P = 0.028). Thus, the higher adiponectin levels in Ad36 positive group were not attributable to preponderance of females in the group. Although a greater sample size will further strengthen the observations, this association agrees well with the robust adiponectin up-regulation induced by Ad36 in experimentally infected mice (25) and in cells from experiments 1–3 of this study.
Discussion
Beneficial effects of Ad36 on glucose metabolism in vivo have been reported. In chow-fed animals, Ad36 increases adiposity, yet improves glycemic control (24, 25). However, in HF-fed mice, without increasing adiposity, Ad36 significantly and lastingly improved hyperglycemia and increased adipose tissue abundance of adiponectin (25). Despite the HF diet in these mice, Ad36 increases the expression of liver adiponectin receptors, the phosphorylation of liver AMP-activated protein kinase, an adiponectin target, and reduces hepatic lipid accumulation (25), a known effect of adiponectin (42, 43). This suggests that Ad36 metabolically remodels adipose tissue despite a HF diet and does not require fat loss to improve glycemic control. If harnessed, this property could have practical benefits, given the wide spread prevalence of HF intake and the challenges in maintaining long-term weight loss in humans. As a first step, we needed to identify the cells of adipose tissue targeted by Ad36.
Given that the differentiation of preadipocytes to mature adipocytes increases glucose uptake (2), it was unclear if Ad36 targets only preadipocytes, which differentiate and increase glucose uptake, or it can even recruit mature adipocytes. We now report that Ad36 individually remodels metabolism in human preadipocytes as well as mature adipocytes. A direct effect in modulating glucose uptake of mature adipocytes is particularly important. It indicates that Ad36 can enhance the metabolic profile of existing, mature adipocytes, without requiring adipogenesis to recruit new fat cells. This contrasts with the effect of TZD, which mainly enhance the adipose tissue metabolic profile by promoting the development of new, smaller adipocytes (44). This Ad36 property is highly useful for understanding metabolic engineering of existing adipose tissue.
Mechanistic studies have indicated that Ad36 induces commitment, differentiation, and lipid accumulation of adipocyte progenitors (17–19, 45) and increases cellular glucose uptake (20, 21, 45). The concomitant up-regulation of adipogenesis and glucose disposal by Ad36 in adipocyte progenitors and mature adipocytes strongly implicated a role of PPARγ, a master regulator of adipogenesis (46) and systemic insulin sensitivity (4). Indeed, Ad36 robustly up-regulates PPARγ in adipose tissue and adipocyte progenitors (17–19, 21). However, the interdependence of Ad36-induced induction of PPARγ, adipogenesis, glucose uptake, and adiponectin abundance was unclear. To test the dependence of Ad36-induced up-regulation of adiponectin and glucose uptake on PPARγ, multiple approaches were used to down-regulate PPARγ. We used GW9662, a selective, irreversible, and effective PPARγ antagonist (28), in hASC, NIH3T3 fibroblasts, which have impaired PPARγ expression (6), or PPARγ-deleted MEF (46). All approaches confirmed that Ad36 increases glucose uptake and adiponectin abundance, despite PPARγ depletion, a particularly attractive property, considering that adipocytes require PPARγ for Glut1 and Glut4-mediated glucose uptake (7). On the other hand, Ad36 could not induce adipogenesis without PPARγ. The results indicated that it may be possible to favorably alter adipose tissue metabolism, without inducing PPARγ and the consequential adipogenesis, an important and valuable deviation from the action of the TZD. Nugent et al. (47) have also expressed a similar possibility to dissociate the effects of PPARγ on adipogenesis and glucose uptake. Ad36 may provide a template to exogenously reengineer the metabolic profile of adipose tissue, without increasing adiposity.
Considering that adiponectin is a target gene of PPARγ, the ability of Ad36 to up-regulate adiponectin even in absence of PPARγ is particularly remarkable and valuable. Phosphatidylinositol 3-kinase (PI3K) is a strong promoter of adiponectin secretion (48). Ad36 robustly up-regulates PI3K signaling (20, 21, 25). It is likely that Ad36 increases adiponectin via the activation of PI3K pathway. Up-regulation of adiponectin has important metabolic benefits. The currently known key strategies to increase adiponectin include weight loss or PPARγ activation via the TZD (49). Therefore, the potential of Ad36 to increase adiponectin without weight loss and independent of PPARγ may offer an attractive model to design therapeutic targets.
The human relevance of the interaction of Ad36 infection and adiponectin was determined in a post hoc screening of human sera. It is interesting that humans naturally infected with Ad36 had significantly greater adiponectin levels and may explain the better glycemic control reported in such subjects (21, 25). The congruence of the results from cell studies and human data suggests that Ad36 may increase adiponectin in humans. Also, it conceptually supports the human relevance of using Ad36 to reveal pathways to improve glycemic control in humans.
To further pursue this promising approach, additional insight about the mechanism is required. Ad2 was used to assess the specificity of the effect of Ad36. In vivo, Ad2 is nonadipogenic and does not significantly improve glycemic control (25, 50). In this study, Ad2 had some effect on increasing cellular glucose uptake but a minimal effect at most on PPARγ, adiponectin, or Glut. Also, Ad2 had no effect on glucose uptake when PPARγ was depleted. Therefore, it appears that some increase in cellular glucose uptake induced by Ad2 is a nonspecific response to infection. However, Ad36 modulates glucose metabolism through targeting specific pathways.
It should be noted that our long-term goal is not to use Ad36 infection for improving glycemic control but to exploit its properties to develop more effective and targeted strategies for managing impaired glycemic control. This approach is not without precedence; unique properties of microbes have been used for developing drugs for important diseases, antibiotics being the most known example. Similarly, harnessing certain properties of viruses for beneficial purposes has been creatively used for several years, including the use of bactericidal properties of a bacteriophage virus (51), the oncolytic ability of a mutant adenovirus (52), or the use of Herpes simplex virus and several other viruses for the treatment of cancers (53). We expect to use Ad36 proteins to uncover novel approaches to improve glycemic control by exogenously manipulating host proteins. Such viral protein(s) that is(are) necessary and sufficient for Ad36-induced glucose disposal need(s) to be identified. Identifying such a candidate protein would narrow the focus to interactions between the candidate protein and host cell signaling.
Taken together, the studies to date show that a natural Ad36 infection predicts better glycemic control in humans, independent of age, sex, or adiposity, and the experimental Ad36 infection of animals increases adiposity, yet improves glycemic control in animals. Increased glucose uptake by adipose tissue and skeletal muscle, increased adiponectin, and reduced hepatic glucose output seem to contribute to systemic glycemic control in Ad36-infected animals. In vitro studies identified that Ad36 induces PPARγ and adipogenesis and concomitantly increases cellular glucose uptake via a rat sarcoma-mediated, PI3K-dependent up-regulation of Glut. Here, we have shown that it may be possible for an exogenous agent to recruit adipocyte progenitors as well as mature adipocytes and reengineer their metabolic profile to improve glycemic control and that this may be done without recruiting PPARγ. Future studies of Ad36 proteins may offer a template to improve metabolic profile of adipose tissue in humans without requiring weight loss or without increasing adiposity.
Acknowledgments
We thank assistance of Dr. Gang Yu and Xiying Wu in isolating hASC from lipoaspirates; Dr. Bruce Spiegelman (Dana-Farber Cancer Institute, Boston, MA) for providing PPARγ−/− MEF; and Drs. James Wade, Ann Reilley, Michael Teague, and their office staffs and patients for the donation of their time and lipoaspirate tissues to this study.
This work was supported in part by the National Institutes of Health Grant R-01 DK066164 and the American Diabetes Association Grant 1-09-IN-13 (to N.V.D.). Isolated hASC, provided by J.M.G. of the Molecular Mechanisms Core of the Pennington Biomedical Research Center Clinical Nutrition Research Unit, was supported by NIH Grant 1P30 DK072476. This work used Genomics core facilities at the Pennington Biomedical Research Center that are supported in part by Center of Biomedical Research Excellence (NIH P20-RR021945) and Nutrition Obesity Research Center (NIH 1P30-DK072476) center grants.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ad36
- Adenovirus 36
- BMI
- body mass index
- CAR
- 3T3-L1-coxsackie and adenovirus receptor
- DM
- diabetes mellitus
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehydes phosphate dehydrogenase
- Glut
- glucose transporter
- hASC
- human-adipose-tissue-derived stem cells
- HF
- high fat
- MEF
- mouse embryonic fibroblast
- MOI
- multiplicity of infection
- PBRC
- Pennington Biomedical Research Center
- PI3K
- phosphatidylinositol 3-kinase
- ORO
- Oil Red O
- pi
- postinfection
- PPAR
- peroxisome proliferator-activated receptor
- qRT-PCR
- real-time quantitative PCR
- TZD
- thiazolidinedione
- WB
- Western blotting.
References
- 1. Nawrocki AR, Scherer PE. 2005. Keynote review: the adipocyte as a drug discovery target. Drug Discov Today 10:1219–1230 [DOI] [PubMed] [Google Scholar]
- 2. Hauner H, Röhrig K, Spelleken M, Liu LS, Eckel J. 1998. Development of insulin-responsive glucose uptake and GLUT4 expression in differentiating human adipocyte precursor cells. Int J Obes Relat Metab Disord 22:448–453 [DOI] [PubMed] [Google Scholar]
- 3. Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS, Cam MC, Cushman SW, Smith U. 2004. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun 317:1045–1051 [DOI] [PubMed] [Google Scholar]
- 4. Spiegelman BM. 1998. PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes 47:507–514 [DOI] [PubMed] [Google Scholar]
- 5. Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. 1995. Structural organization of mouse peroxisome proliferator-activated receptor γ (mPPARγ) gene: alternative promoter use and different splicing yield two mPPARγ isoforms. Proc Natl Acad Sci USA 92:7921–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tontonoz P, Hu E, Spiegelman BM. 1994. Stimulation of adipogenesis in fibroblasts by PPARγ 2, a lipid-activated transcription factor. Cell 79:1147–1156 [DOI] [PubMed] [Google Scholar]
- 7. Liao W, Nguyen MT, Yoshizaki T, Favelyukis S, Patsouris D, Imamura T, Verma IM, Olefsky JM. 2007. Suppression of PPAR-γ attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 293:E219–E227 [DOI] [PubMed] [Google Scholar]
- 8. Choi SS, Cha BY, Iida K, Lee YS, Yonezawa T, Teruya T, Nagai K, Woo JT. 2011. Artepillin C; as a PPARγ ligand; enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biochem Pharmacol 81:925–933 [DOI] [PubMed] [Google Scholar]
- 9. Choi SS, Cha BY, Lee YS, Yonezawa T, Teruya T, Nagai K, Woo JT. 2009. Magnolol enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Life Sci 84:908–914 [DOI] [PubMed] [Google Scholar]
- 10. Staels B, Fruchart JC. 2005. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 54:2460–2470 [DOI] [PubMed] [Google Scholar]
- 11. Larsen TM, Toubro S, Astrup A. 2003. PPARγ agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord 27:147–161 [DOI] [PubMed] [Google Scholar]
- 12. Semenkovich CF. 2005. TZDs and diabetes: testing the waters. Nat Med 11:822–824 [DOI] [PubMed] [Google Scholar]
- 13. Lipscombe LL, Gomes T, Lévesque LE, Hux JE, Juurlink DN, Alter DA. 2007. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA 298:2634–2643 [DOI] [PubMed] [Google Scholar]
- 14. Knouff C, Auwerx J. 2004. Peroxisome proliferator-activated receptor-γ calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev 25:899–918 [DOI] [PubMed] [Google Scholar]
- 15. Mukherjee R, Hoener PA, Jow L, Bilakovics J, Klausing K, Mais DE, Faulkner A, Croston GE, Paterniti JR., Jr 2000. A selective peroxisome proliferator-activated receptor-γ (PPARγ) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol 14:1425–1433 [DOI] [PubMed] [Google Scholar]
- 16. Wigand R, Gelderblom H, Wadell G. 1980. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol 64:225–233 [DOI] [PubMed] [Google Scholar]
- 17. Pasarica M, Mashtalir N, McAllister EJ, Kilroy GE, Koska J, Permana P, de Courten B, Yu M, Ravussin E, Gimble JM, Dhurandhar NV. 2008. Adipogenic human adenovirus Ad-36 induces commitment, differentiation and lipid accumulation in human adipose-derived stem cells. Stem Cells 26:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rathod MA, Rogers PM, Vangipuram SD, McAllister EJ, Dhurandhar NV. 2009. Adipogenic cascade can be induced without adipogenic media by a human adenovirus. Obesity 17:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers PM, Fusinski K, Rathod MA, Loiler S, Pasarica M, Shaw M, Kilroy G, Sutton G, McAllister E, Mashtalir N, Gimble J, Holland T, NV D. 2007. Human adenovirus Ad-36 induces adipogenesis via its E4 orf-1 gene. Int J Obesity 32 397–406 [DOI] [PubMed] [Google Scholar]
- 20. Wang ZQ, Cefalu WT, Zhang XH, Yu Y, Qin J, Son L, Rogers PM, Mashtalir N, Bordelon JR, Ye J, Dhurandhar NV. 2008. Human adenovirus type 36 enhances glucose uptake in diabetic and non-diabetic human skeletal muscle cells independent of insulin signaling. Diabetes 57:1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers PM, Mashtalir N, Rathod MA, Dubuisson O, Wang Z, Dasuri K, Babin S, Gupta A, Markward N, Cefalu WT, Dhurandhar NV. 2008. Metabolically favorable remodeling of human adipose tissue by human adenovirus Ad-36. Diabetes 57:2321–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. 2000. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord 24:989–996 [DOI] [PubMed] [Google Scholar]
- 23. Dhurandhar NV, Israel BA, Kolesar JM, Mayhew G, Cook ME, Atkinson RL. 2001. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord 25:990–996 [DOI] [PubMed] [Google Scholar]
- 24. Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL, MohanKumar S, MohanKumar PS, Markward N, Dhurandhar NV. 2006. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity 14:1905–1913 [DOI] [PubMed] [Google Scholar]
- 25. Krishnapuram R, Dhurandhar EJ, Dubuisson O, Kirk-Ballard H, Bajpeyi S, Butte N, Sothern MS, Larsen-Meyer E, Chalew S, Bennett B, Gupta AK, Greenway FL, Johnson W, Brashear M, Reinhart G, Rankinen T, Bouchard C, Cefalu WT, Ye J, Javier R, Zuberi A, Dhurandhar NV. 2011. A template to improve glycemic control without reducing adiposity or dietary fat. Am J Physiol Endocrinol Metab 300:E779–E789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuda O, Stankova B, Tvrzicka E, Hensler M, Jelenik T, Rossmeisl M, Flachs P, Kopecky J. 2009. Prominent role of liver in elevated plasma palmitoleate levels in response to rosiglitazone in mice fed high-fat diet. J Physiol Pharmacol 60:135–140 [PubMed] [Google Scholar]
- 27. Fernandes-Santos C, Evangelista Carneiro R, de Souza Mendonca L, Barbosa Aguila M, Mandarim-de-Lacerda CA. 2009. Rosiglitazone aggravates nonalcoholic Fatty pancreatic disease in C57BL/6 mice fed high-fat and high-sucrose diet. Pancreas 38:e80–e86 [DOI] [PubMed] [Google Scholar]
- 28. Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG. 2002. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41:6640–6650 [DOI] [PubMed] [Google Scholar]
- 29. Gupta AK, Smith SR, Greenway FL, Bray GA. 2009. Pioglitazone treatment in type 2 diabetes mellitus when combined with portion control diet modifies the metabolic syndrome. Diabetes Obes Metab 11:330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, Augustus AS. 2005. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes 29:281–286 [DOI] [PubMed] [Google Scholar]
- 31. Dubois S, Halvorsen Y, Ravussin E, Gimble J. 2005. Primary stromal cell culture from adipose tissue. From liposuction to needle biopsy. Adipocytes 1:139–144 [Google Scholar]
- 32. Sen A, Lea-Currie YR, Sujkowska D, Franklin DM, Wilkison WO, Halvorsen YD, Gimble JM. 2001. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem 81:312–319 [DOI] [PubMed] [Google Scholar]
- 33. Klip A. 2009. The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab 34:481–487 [DOI] [PubMed] [Google Scholar]
- 34. Rathod MA, Rogers PM, Dhurandhar NV. 2006. Human adenovirus Ad-36 infection induces differentiation and replication of preadipocytes. Obes Rev 7:138 [Google Scholar]
- 35. Farmer SR. 2005. Regulation of PPARγ activity during adipogenesis. Int J Obes 29 (Suppl 1):S13–S16 [DOI] [PubMed] [Google Scholar]
- 36. Fu Y, Luo N, Klein RL, Garvey WT. 2005. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46:1369–1379 [DOI] [PubMed] [Google Scholar]
- 37. Tamori Y, Masugi J, Nishino N, Kasuga M. 2002. Role of peroxisome proliferator-activated receptor-γ in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 51:2045–2055 [DOI] [PubMed] [Google Scholar]
- 38. Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. 2001. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 108:1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ravussin E. 2002. Adiponectin enhances insulin action by decreasing ectopic fat deposition. Pharmacogenomics J 2:4–7 [DOI] [PubMed] [Google Scholar]
- 40. You T, Yang R, Lyles MF, Gong D, Nicklas BJ. 2005. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab 288:E741–E747 [DOI] [PubMed] [Google Scholar]
- 41. Pajvani UB, Scherer PE. 2003. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep 3:207–213 [DOI] [PubMed] [Google Scholar]
- 42. Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. 2006. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J Biol Chem 281:2654–2660 [DOI] [PubMed] [Google Scholar]
- 43. Shen Z, Liang X, Rogers CQ, Rideout D, You M. 2010. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 298:G364–G374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McLaughlin TM, Liu T, Yee G, Abbasi F, Lamendola C, Reaven GM, Tsao P, Cushman SW, Sherman A. 2010. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity 18:926–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vangipuram SD, Yu M, Tian J, Stanhope KL, Pasarica M, Havel PJ, Heydari AR, Dhurandhar NV. 2007. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes 31:87–96 [DOI] [PubMed] [Google Scholar]
- 46. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. 1999. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617 [DOI] [PubMed] [Google Scholar]
- 47. Nugent C, Prins JB, Whitehead JP, Savage D, Wentworth JM, Chatterjee VK, O'Rahilly S. 2001. Potentiation of glucose uptake in 3T3-L1 adipocytes by PPARγ agonists is maintained in cells expressing a PPARγ dominant-negative mutant: evidence for selectivity in the downstream responses to PPARγ activation. Mol Endocrinol 15:1729–1738 [DOI] [PubMed] [Google Scholar]
- 48. Pereira RI, Leitner JW, Erickson C, Draznin B. 2008. Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3′-kinase. Life Sci 83:638–643 [DOI] [PubMed] [Google Scholar]
- 49. Boden G, Cheung P, Mozzoli M, Fried SK. 2003. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism 52:753–759 [DOI] [PubMed] [Google Scholar]
- 50. Whigham LD, Israel BA, Atkinson RL. 2006. Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am J Physiol Regul Integr Comp Physiol 290:R190–R194 [DOI] [PubMed] [Google Scholar]
- 51. Hanlon GW. 2007. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int J Antimicrob Agents 30:118–128 [DOI] [PubMed] [Google Scholar]
- 52. Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373–376 [DOI] [PubMed] [Google Scholar]
- 53. Crompton AM, Kirn DH. 2007. From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets 7:133–139 [DOI] [PubMed] [Google Scholar]