Abstract
Enhanced levels of nuclear factor (NF)-κB-inducing kinase (NIK), an upstream kinase in the NF-κB pathway, have been implicated in the pathogenesis of chronic inflammation in diabetes. We investigated whether increased levels of NIK could induce skeletal muscle insulin resistance. Six obese subjects with metabolic syndrome underwent skeletal muscle biopsies before and six months after gastric bypass surgery to quantitate NIK protein levels. L6 skeletal myotubes, transfected with NIK wild-type or NIK kinase-dead dominant negative plasmids, were treated with insulin alone or with adiponectin and insulin. Effects of NIK overexpression on insulin-stimulated glucose uptake were estimated using tritiated 2-deoxyglucose uptake. NF-κB activation (EMSA), phosphatidylinositol 3 (PI3) kinase activity, and phosphorylation of inhibitor κB kinase β and serine-threonine kinase (Akt) were measured. After weight loss, skeletal muscle NIK protein was significantly reduced in association with increased plasma adiponectin and enhanced AMP kinase phosphorylation and insulin sensitivity in obese subjects. Enhanced NIK expression in cultured L6 myotubes induced a dose-dependent decrease in insulin-stimulated glucose uptake. The decrease in insulin-stimulated glucose uptake was associated with a significant decrease in PI3 kinase activity and protein kinase B/Akt phosphorylation. Overexpression of NIK kinase-dead dominant negative did not affect insulin-stimulated glucose uptake. Adiponectin treatment inhibited NIK-induced NF-κB activation and restored insulin sensitivity by restoring PI3 kinase activation and subsequent Akt phosphorylation. These results indicate that NIK induces insulin resistance and further indicate that adiponectin exerts its insulin-sensitizing effect by suppressing NIK-induced skeletal muscle inflammation. These observations suggest that NIK could be an important therapeutic target for the treatment of insulin resistance associated with inflammation in obesity and type 2 diabetes.
The proinflammatory transcription factor nuclear factor (NF)-κB remains sequestered in the cytoplasm by interacting with a group of inhibitory proteins, collectively referred to as inhibitor κB (IκB). Inflammatory signals cause phosphorylation and degradation of IκB, liberating NF-κB, which allows these transcription factors to translocate to the nucleus and activate inflammatory response genes. The upstream converging point for numerous signals leading to IκB phosphorylation and NF-κB activation is the IκB kinase (IKK) complex (formed by the regulatory IKKγ subunit and the two kinase subunits IKKα and IKKβ). Recent evidence suggests that serine phosphorylation of insulin receptor substrate-1 and subsequent skeletal muscle insulin resistance might be mediated by IKK-β (1).
The molecular events directly upstream of IKK activation are still not well defined, but biochemical analysis has demonstrated that mitogen-activated protein kinase kinase kinase 14/NF-κB-inducing kinase (NIK), a 110 kDa, TNF receptor associated factor-binding, Ser-Thr kinase, can act as a proximal inducer of IKK complex activity (2). Given its ability to activate IKKα and IKKβ, NIK has been extensively studied as an upstream kinase regulating NF-κB activation (3). We have reported increased NIK protein levels in type 2 diabetic mouse renal cortex and a subsequent increase in NF-κB-dependent proinflammatory gene expression (4). Mitogen-activated protein kinase kinase kinase kinase 4, another member of MAPK family, was shown to mediate TNF-α-induced insulin resistance in skeletal muscle and preadipocytes 3T3 cells (5). Effects of enhanced NIK expression on insulin resistance have not been studied. In the present study, we examined the effect of increased NIK expression on insulin-stimulated glucose uptake in skeletal muscle cells (L6 myotubes) in vitro. We also investigated whether adiponectin treatment attenuated NIK-induced inflammation and insulin resistance in these cells. Finally, we examined the effect of weight loss after bariatric surgery on skeletal muscle NIK in patients with metabolic syndrome.
Materials and Methods
Materials
αMEM and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum was from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD). Trypsin/EDTA was from Mediatech (Herndon, VA). Reagents for polyacrylamide gel electrophoresis were from Bio-Rad (Hercules, CA). [γ-32P]ATP and tritiated 2-deoxyglucose ([3H]2-DG) were obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Full-length adiponectin was obtained from Biovendor (Candler, NC).
Human subjects
We studied six (age, 38 ± 4 yr; body mass index, 48.5 ± 4.8 kg/m2; hemoglobin A1c, 6.0 ± 0.1%) nondiabetic morbidly obese participants with the metabolic syndrome, who underwent bariatric surgery (Roux-en-Y gastric bypass) for weight loss. Vastus lateralis muscle biopsies were obtained during surgery and 6 months after surgery and were used for determination of NIK protein. These studies were approved by the Baylor College of Medicine Institutional Review Board. Some of the results for the participants in this study have been published previously (6).
Experimental design
Eukaryotic expression vectors pRK-MycNIK or pRK-MycNIK kinase-dead dominant negative (NIKdn), encoding NIK Thr559Phe site mutant, were transfected into differentiated L6 myotubes (seventh to eight day of differentiation) using Lipofectamine and Plus reagent (Invitrogen). For experiments using adiponectin, 48 h after transfection, 2 μg/ml adiponectin (mouse recombinant trimeric HEK adiponectin; Biovendor) was added for 6 h. At the end of adiponectin treatment, cells were washed and insulin (100 nm) added for various time intervals as stated in respective experiments.
L6 cell tissue culture
Insulin-sensitive L6 skeletal muscle cells were obtained from Amira Klip (University of Toronto, Toronto, Canada). Stock myoblasts were grown and maintained in monolayer culture in αMEM containing 10% fetal bovine serum (vol/vol), 100 U/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 37 C until cells reached 40–50% confluence. To obtain fully differentiated myotubes, stock myoblasts were cultured in differentiation media (2% fetal bovine serum-αMEM and antibiotics). Cells were maintained in differentiation media for 7–8 d, with a medium change every 24–48 h.
[3H]2-DG uptake
[3H]2-DG uptake was determined as described previously (7). Briefly, myotubes were incubated for 20 min at 37 C in the presence or absence of 100 nm insulin, rinsed 2× in HEPES-buffered solution, and then incubated for 5 min in a HEPES-buffered solution containing 10 μm [3H]2-DG (0.5 μCi/ml [3H]2-DG). Cell-associated radioactivity was determined by scintillation counting (Beckman Instruments, Fullerton, CA).
Cell fractionation and EMSA
EMSA gel shift assay system was purchased from Promega (Madison, WI). Consensus NF-κB oligonucleotide was radiolabeled by T4 polynucleotide kinase as per manufacturer's protocol. Radiolabeled probe (50,000 cpm) was incubated with 5 μg of nuclear extract from NIK transfected and adiponectin-treated myotubes. Nuclear protein-DNA complexes were resolved by electrophoresis on 5% polyacrylamide gel under nondenaturating conditions in a Tris-borate/EDTA buffer and visualized by autoradiography.
Phosphatidylinositol 3 (PI3) kinase activity and serine-threonine kinase (Akt) immunoblots
For experiments requiring measurement of PI3 kinase activity and protein kinase B/Akt phosphorylation, myotubes were exposed to 100 nm insulin for 10 min at 37 C. PI3 kinase activity was assayed as described (7).
Statistical methods
All results are expressed as means ± sd. Both one- and two-way ANOVA tests were performed to evaluate overall group differences. This was followed by Tukey's post hoc test to determine pair-wise significance if the ANOVA test was significant. A Student's t test was performed for two-sample comparisons after checking for variance distribution via Levene's test. In human subjects, changes after weight loss in obese participants were compared using paired t tests. Linear regression analysis was used to examine the relationship between NIK and plasma adiponectin, AMP kinase (AMPK), and homeostatic model assessment-insulin resistance (HOMA-IR), respectively. In all cases, P < 0.05 was considered significant.
Results
Skeletal muscle NIK levels decrease after weight loss in patients with metabolic syndrome
As previously published (6), at 6 months after Roux-en-Y gastric bypass surgery, body weight (135.2 ± 13.8 to 100.3 ± 10.1 kg; P < 0.01), mean fasting plasma insulin (161.8 ± 10.4 to 84.7 ± 6.3 pmol/liter; P < 0.01), fasting plasma glucose (5.8 ± 0.3 to 4.7 ± 0.1 mmol/liter; P < 0.01), and insulin resistance (HOMA of insulin resistance) (6.0 ± 0.5 to 2.6 ± 0.2; P < 0.01) decreased significantly, whereas plasma adiponectin levels (6.5 ± 0.6 to 11.6 ± 0.9 μg/ml; P < 0.01) and skeletal muscle AMPK phosphorylation (P-AMPK/AMPK ratio) (0.47 ± 0.05 to 0.78 ± 0.13; P < 0.05) increased.
The increase in plasma adiponectin, enhanced skeletal muscle AMPK phosphorylation, and improvement in insulin sensitivity (HOMA-IR) was associated with a significant reduction in skeletal muscle NIK protein (P < 0.01) (Fig. 1). Taken collectively, before and after weight loss, skeletal muscle NIK correlated strongly and negatively with plasma adiponectin levels (R = −0.67, P < 0.01) and positively with insulin resistance (HOMA-IR) (R = 0.57, P < 0.05). Furthermore, skeletal muscle NIK tended to correlate negatively with skeletal muscle AMPK phosphorylation (R = −0.52, P = 0.08).
Fig. 1.
Skeletal muscle NIK levels decrease after weight loss after gastric bypass surgery in patients with metabolic syndrome. A, Western blottings of six subjects showing overall decline in NIK protein levels after weight loss. Tubulin used as loading control. B, Quantification of NIK pre and post weight loss. *, P < 0.01, pre vs. post weight loss. Pre, presurgery; post, postsurgery. Numerical numbers in the top panel blot indicate different subjects before surgery; a, same patient after surgery.
Mitogen-activated protein kinase kinase kinase 14/ NIK reduces insulin-mediated glucose uptake in L6 myotubes
To examine a role for NIK on insulin-stimulated glucose uptake in skeletal muscle cells, differentiated cultured L6 myotubes were transfected with NIK wild type (NIKwt) plasmid over a low concentration range (0.1–0.3 μg/ml). Western blottings of NIK protein are shown in Fig. 2A, lower panel, and a small but dose-dependent increase in NIK was observed. Forty-eight hours after transfection, cells were treated with 100 nm insulin for 20 min to stimulate glucose uptake. We observed an approximately 2-fold increase in net glucose uptake by insulin in these cells (Fig. 2, A and B), and a dose-dependent inhibition in insulin-stimulated glucose uptake was observed with increasing concentration of transfected NIK. Transfecting cells with empty vector did not affect insulin-stimulated glucose uptake. This experiment demonstrated that NIK can significantly reduce skeletal muscle glucose uptake.
Fig. 2.
Increased expression of NIK reduces insulin-stimulated 2-deoxyglucose (2DG) uptake. A, A dose-dependent decrease in insulin-stimulated 2DG uptake with increasing concentration of NIK. Upper panel shows 2DG uptake in the absence (solid bars) and presence (gray bars) of 100 nm insulin, whereas middle panel reports net insulin-stimulated 2DG uptake (insulin stimulated-unstimulated). Bottom panel shows a representative Western blotting of NIK expression probed using anti-Myc antibody (β-actin is loading control). B, Upper panel shows 2DG uptake in myotubes expressing NIKdn (0.2 μg/ml) or NIKwt (0.2 μg/ml) in presence and absence of insulin. Middle panel depicts net 2DG uptake. A representative Western blotting probed by anti-Myc antibody shows expression of NIKwt and NIKdn in the bottom panel. C, Adiponectin (2 μg/ml) restores NIK-induced loss of insulin sensitivity. Depiction of net change in 2DG uptake. Data represent mean ± sd of three independent experiments and were analyzed by one-way ANOVA followed by Tukey's post hoc test for significance between doses (A) and multiple treatment groups (B). *, P < 0.001 significantly different from unstimulated (−insulin) controls. †, P < 0.001 significantly different from NIK transfected samples. C, Controls.
Further, differentiated L6 myotubes were transfected with either NIKwt or kinase-dead NIKdn at 0.2 μg/ml, followed by measurements of insulin-stimulated glucose uptake. NIKwt reduced insulin sensitivity, whereas NIKdn, which was used at the same plasmid concentration but resulted in higher NIK protein levels (Fig. 2B, lower panel), did not significantly alter insulin-stimulated glucose uptake (Fig. 2C). These results indicate that the kinase activity of NIK is required for reducing insulin-stimulated glucose uptake.
Adiponectin prevents NIK-induced insulin resistance
Myotubes treated only with adiponectin showed a slight but insignificant increase in basal as well as insulin-stimulated glucose uptake (Fig. 2C). NIKwt transfection decreased insulin-stimulated glucose uptake as observed in the earlier experiments. However, in the presence of adiponectin, L6 myotubes preserved insulin sensitivity.
Adiponectin prevents NIK-induced inhibition of insulin-stimulated PI3 kinase activity and Akt phosphorylation
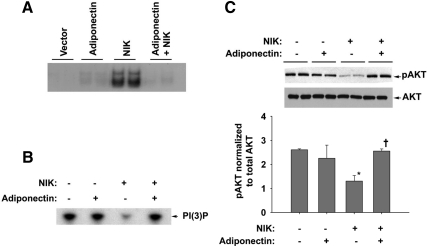
Figure 3A shows that NIK induced NF-κB activation, evidenced by NF-κB DNA binding, and before treatment of NIK-transfected myotubes with adiponectin completely attenuated NIK-induced increase in NF-κB binding. A significant decrease in insulin-stimulated PI3 kinase activity and PKB/Akt phosphorylation was observed with 0.2 μg/ml transfected NIK (Fig. 3, B and C, respectively). These NIK-induced changes were blocked with adiponectin pretreatment.
Fig. 3.
Adiponectin inhibits NIK-induced NF-κB activation and insulin-signaling pathway. A, Representative EMSA (out of three independent experiments) depicting NIK-induced increased NF-κB DNA binding that is blocked by adiponectin. B, Representative autoradiogram (out of three independent experiments) shows NIK-induced inhibition of PI3 kinase activity being restored by adiponectin pretreatment. C, Representative Western blotting normalized to respective total AKT and then expressed as fold change vs. control cells (lower panel). Cells transfected with empty vector serve as transfection control. Overall significance determined using one-way ANOVA. Significance between groups was analyzed by performing Tukey's post hoc test. *, P < 0.05 significantly different from control samples; †, P < 0.05 significantly different from NIK-transfected samples.
Discussion
The results of the present study demonstrate that NIK, a member of the MAPK family that plays a critical role in noncanonical NF-κB pathway activation, induced skeletal muscle insulin resistance in vitro. Furthermore, we demonstrate that adiponectin inhibits NIK-mediated skeletal muscle insulin resistance. We were motivated to perform these studies by our observation that skeletal muscle NIK levels decreased in association with increased plasma adiponectin levels, enhanced skeletal muscle AMPK phosphorylation, and improved insulin sensitivity after weight loss in obese patients with the metabolic syndrome.
NIK has been shown to be a potent proinflammatory kinase in a variety of cells and tissues. Thus, it is expected that increased NIK levels would be observed in inflammation-associated diseases, and recently, elevated NIK levels have been reported with multiple myeloma (8), rheumatoid arthritis (9), and diabetic nephropathy (4). Our observation that NIK is associated with skeletal muscle insulin resistance has implications for other inflammatory diseases that have an associated increased risk for insulin resistance.
Our observations that adiponectin inhibits NIK-mediated skeletal muscle inflammation and reductions in insulin-stimulated glucose uptake are novel. Specifically, we have shown that NIK-induced insulin resistance in L6 myotubes is associated with NF-κB nuclear translocation and DNA binding. Because a NF-κB consensus sequence was used for the EMSA and supershifts were not performed, we do not know precisely which NF-κB protein is responsible for the DNA binding shown in Fig. 3. Assuming RelA is the predominant NF-κB protein, this would imply that overexpressing NIK within the muscle cell activates both IKKα (used for noncanonical pathway activation) and IKKβ (used for canonical pathway activation) and that NIK-induced decreases in PI-3 kinase activity and Akt phosphorylation may result from IKKβ-mediated serine phosphorylation of insulin receptor substrate 1, as previously described (10). However, we cannot exclude the possibility that NIK negatively regulates PI3 kinase activity, either directly or indirectly through other kinases in an activation pathway that remains undefined. Previous work has shown that by suppressing the expression of another member of MAPK family, MAP4K4 protein in primary human skeletal muscle cells, TNF-α-induced insulin resistance can be prevented and MAP4K4 silencing prevented TNF-α-induced c-Jun N-terminal kinase and ERK phosphorylation (9).
Recent studies have shown that adiponectin is an antiinflammatory adipocytokine that suppresses TNF-α-mediated inflammatory responses in human aortic endothelial cells (11). Furthermore, Devaraj et al. (12) have shown that adiponectin dose dependently reduced C-reactive protein (CRP) mRNA and protein in human aortic endothelial cells. Adiponectin treatment significantly decreased NF-κB binding activity. Adiponectin also activated AMPK, resulting in decreased NF-κB activity and decreased CRP mRNA and protein, and these effects were mimicked by AICAR. Thus, adiponectin reduced CRP synthesis and secretion from human aortic endothelial cells via up-regulation of AMPK and down-regulation of NF-κB. Interestingly, similar findings were observed by them in rat primary hepatocytes. Adiponectin inhibits phagocytic activity and lipopolysaccharide-stimulated TNF-α production in macrophages (13). In addition, previous in vitro studies have shown that adiponectin suppresses lipopolysaccharide-induced NF-κB activation in pig primary adipocytes and 3T3-L1 adipocytes (14).
There are several mechanisms by which adiponectin enhances skeletal muscle insulin sensitivity. Decreased levels of adiponectin and adiponectin receptor 1 may have causal roles in skeletal muscle mitochondrial dysfunction and insulin resistance seen in type 2 diabetes (6, 15). Our results demonstrate the antiinflammatory effects of adiponectin in skeletal muscle and further suggest that adiponectin exerts its insulin-sensitizing effect by suppressing NIK-induced skeletal muscle inflammation. The precise molecular mechanisms by which adiponectin suppresses NIK-induced skeletal muscle inflammation remain to be studied.
Acknowledgments
We thank Dr. Amira Klip for providing the L6 cells.
Present address for V.S.: Bariatric and Metabolic Surgery Center, The Methodist Hospital, Houston, Texas 77030.
This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK079053 (to S.C. and R.G.T.), the American Diabetes Association Grant 1-06-CR-03 (to M.B.), and the Ron MacDonald Foundation at St Luke's Episcopal Hospital Grant 08RDM008 (to M.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Akt
- Serine-threonine kinase
- AMPK
- AMP kinase
- CRP
- C-reactive protein
- [3H]2-DG
- tritiated 2-deoxyglucose
- HOMA-IR
- homeostatic model assessment-insulin resistance
- IκB
- inhibitor κB
- IKK
- IκB kinase
- NF
- nuclear factor
- NIK
- NF-κB-inducing kinase
- NIKdn
- NIK kinase-dead dominant negative
- NIKwt
- NIK wild type
- PI3
- phosphatidylinositol 3.
References
- 1. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. 2005. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198 [DOI] [PubMed] [Google Scholar]
- 2. Häcker H, Karin M. 2006. Regulation and function of IKK and IKK-related kinases. Sci STKE 2006:re13. [DOI] [PubMed] [Google Scholar]
- 3. Xiao G, Harhaj EW, Sun SC. 2001. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell 7:401–409 [DOI] [PubMed] [Google Scholar]
- 4. Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG. 2006. Diabetes-induced activation of canonical and noncanonical nuclear factor-κB pathways in renal cortex. Diabetes 55:1252–1259 [DOI] [PubMed] [Google Scholar]
- 5. Austin RL, Rune A, Bouzakri K, Zierath JR, Krook A. 2008. siRNA-mediated reduction of inhibitor of nuclear factor-κB kinase prevents tumor necrosis factor-α-induced insulin resistance in human skeletal muscle. Diabetes 57:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holmes RM, Yi Z, De Filippis E, Berria R, Shahani S, Sathyanarayana P, Sherman V, Fujiwara K, Meyer C, Christ-Roberts C, Hwang H, Finlayson J, Dong LQ, Mandarino LJ, Bajaj M. 2011. Increased abundance of the adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif (APPL1) in patients with obesity and type 2 diabetes: evidence for altered adiponectin signalling. Diabetologia 54:2122–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinha S, Perdomo G, Brown NF, O'Doherty RM. 2004. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kB. J Biol Chem 279:41294–41301 [DOI] [PubMed] [Google Scholar]
- 8. Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM. 2010. Classical and/or alternative NF-κB pathway activation in multiple myeloma. Blood 115:3541–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noort AR, van Zoest KPM, Tas SW, Tak PP. 2010. Preferential expression of NF-κB-inducing kinase (NIK) in blood vessels of rheumatoid arthritis synovial tissue containing ectopic lymphoid neogenesis. J Transl Med 8(Suppl 1):P41 [Google Scholar]
- 10. Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. 2002. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kB kinase complex. J Biol Chem 277:48115–48121 [DOI] [PubMed] [Google Scholar]
- 11. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. 1999. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100:2473–2476 [DOI] [PubMed] [Google Scholar]
- 12. Devaraj S, Torok N, Dasu MR, Samols D, Jialal I. 2008. Adiponectin decreases C-reactive protein synthesis and secretion from endothelial cells: evidence for an adipose tissue-vascular loop. Arterioscler Thromb Vasc Biol 28:1368–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. 2008. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-α production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem 283:26850–26858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ajuwon KM, Spurlock ME. 2005. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol 288:R1220–R1225 [DOI] [PubMed] [Google Scholar]
- 15. Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. 2010. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 464:1313–1319 [DOI] [PubMed] [Google Scholar]