Abstract
We test the hypothesis that 12-hydroperoxyeicosatetraenoic acid (12(s)-HPETE) and 12-hydroxyeicosatetraenoic acid (12-HETE) perfused into the renal pelvis increase afferent renal nerve activity (ARNA) and suppress renin release in rats fed a low-salt (LS) diet via activation of the transient receptor potential vanilloid type 1 (TRPV1) expressed in renal sensory nerves. 12(s)-HPETE or 12-HETE given into the left renal pelvis dose-dependently increased ARNA, which was abolished by AMG9810, a selective TRPV1 antagonist, or by RP67580, a selective neurokinin 1 receptor antagonist, in normal salt or LS-treated rats. 12(s)-HPETE, 12-HETE, or substance P perfused into the left renal pelvis suppressed plasma angiotensin I (Ang I) levels in LS rats, which was abolished by AMG9810 or attenuated by ipsilateral renal denervation (RD). 12(s)-HPETE or 12-HETE increased release of substance P and calcitonin gene-related peptide from the ipsilateral kidney, which was abolished by AMG9810 but not RP67580, RD, or RP67580 plus RD. Immunofluorescence staining showed that TRPV1-positive nerve fibers located in the renal cortex, medulla, and pelvis, and that the sympathetic nerve marker, neuropeptide Y, but not neurokinin 1 receptors expressed in the juxtaglomerular region colocalized with renin. Thus, our data show that 12(s)-HPETE and 12-HETE enhance ARNA and substance P/calcitonin gene-related peptide release but suppress renin activity in LS rats, and these effects are abolished when TRPV1 is blocked. These results indicate that TRPV1 mediates 12(s)-HPETE and 12-HETE action in the kidney in such a way that dysfunction in TRPV1 may lead to disintegrated regulation of renin and renal function.
The transient receptor potential vanilloid type 1 (TRPV1), a ligand-gated cation channel, may be activated by various chemical or physical stimuli including vanilloid compounds, noxious heat, lipid metabolites, or proton (1, 2). TRPV1 channels are primarily expressed in sensory neurons housed in dorsal root ganglia and sensory nerve terminals of unmyelinated C-fibers or thinly myelinated Aδ-fibers that innervate a number of organs/tissues including the lung, heart, kidney, and blood vessel (3–9). The renal pelvis, pelvi-ureteric junction, and ureter are heavily innervated by TRPV1-positive sensory nerves located between the layers of smooth muscles and epithelia (10, 11). Activation of TRPV1 expressed in sensory nerves leads to the release of sensory neuropeptides including substance P (SP) and calcitonin gene-related peptide (CGRP), and SP subsequently activates the neurokinin 1 (NK1) receptors located in sensory nerves resulting in an increase in afferent renal nerve activity (ARNA) that would inhibit contralateral sympathetic nerve activity (i.e. efferent renal nerve activity, ERNA) and renal excretory function via the reno-renal reflex (12, 13).
SP and CGRP, colocalized in sensory nerve fibers in the kidney, may have direct or indirect effects on renin release (14). Whereas SP appears to inhibit renin release in the kidney (15), CGRP is found to stimulate renin secretion in humans or in isolated rat renal juxtaglomerular cells (16). Activation of sympathetic nerves, which have been shown to innervate the juxtaglomerular cells (15), leads to renin release (17). TRPV1 expressed in the kidney has been shown to be activated by hypertonic saline perfused into the renal pelvis, indicative of a key role of TRPV1 in the regulation of sodium and water homeostasis (18, 19). Moreover, degeneration of TRPV1-positive sensory nerves by neonatal treatment with capsaicin (CAP) impairs salt-induced suppression of plasma renin activity (19), suggesting that TRPV1 may mediate salt-dependent renin regulation albeit direct evidence is lacking.
12-Hydroperoxyeicosatetraenoic acid (12(s)-HPETE), which is an immediate metabolic product of arachidonic acid and 12-lipoxygenase (12-LOX), shares three-dimensional structural similarity with CAP, a selective TRPV1 agonist (20). 12(s)-HPETE and its stable metabolite, 12-hydroxyeicosatetraenoic acid (12-HETE), evoke TRPV1 currents in both sensory neurons and TRPV1-transfected human embryonic kidney 293 cells with the former as a more potent TRPV1 agonist (21). In sensory neurons, 12(s)-HPETE induced activation of TRPV1 mediates bradykinin-induced inflammation and cannabinoid type 1 receptor-induced increases in intracellular Ca2+ concentrations (22, 23). Moreover, TRPV1 contributes to 12(s)-HPETE-derived protection against myocardial ischemia/reperfusion injury (24). However, it is unclear whether 12(s)-HPETE and 12-HETE may regulate activity of sensory nerves innervating the kidney to modulate renal excretory function via activation of TRPV1 in vivo.
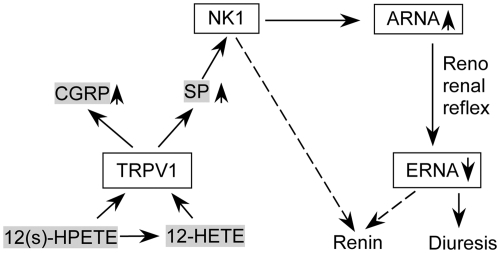
As a potential endogenous TRPV1 agonist, 12-HETE is a long-lived substance in circulation, and circulating 12-HETE distributed to the kidney is much higher than in other organs (25), with its concentration at 65 pg/mg protein in the outer cortex of renal slices (26), 1.2 ng/mg creatinine level in urine from normal rats (27), and significantly elevated in urine under pathological conditions (26, 27). 12-HETE perfused into the isolated kidney or incubated with renal slices suppresses renin release (28, 29). The lipoxygenase inhibitor given acutely or chronically that reduces plasma 12-HETE levels increases plasma renin activity, with the chronic setting independence of changes in blood pressure (26). However, the molecular mechanism(s) underlying 12-HETE-mediated renin regulation is largely unknown. The present study tests the hypotheses that 1) 12(s)-HPETE or 12-HETE perfused into the left renal pelvis enhances ipsilateral ARNA and contralateral diuresis via activation of TRPV1 and downstream NK1 receptors owing to SP release upon TRPV1 activation; 2) ipsilateral renal denervation abolishes 12(s)-HPETE- or 12-HETE-induced contralateral diuresis; 3) 12(s)-HPETE or 12-HETE perfused into the renal pelvis suppresses renin activity via activation of TRPV1; and 4) TRPV1-mediated renin inhibition is attributed to, at least in part, NK1 activation or ERNA suppression via the reno-renal reflex (Fig. 1).
Fig. 1.
Illustration showing potential mechanisms for 12(s)-HPETE- and 12-HETE-dependent renin regulation. Solid lines represent activation and dash lines depict suppression. Activation of TRPV1 channels by 12(s)-HPETE or 12-HETE perfused into the renal pelvis causes the release of CGRP and SP. SP subsequently activates NK1 receptors to increase ARNA. Enhanced ARNA suppresses ERNA via reno-renal reflex, resulting in decreases in renin release and increases in urine excretion. Activation of NK1 in distal tubules may inhibit renin via tubuloglomerular feedback.
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee of Michigan State University. Male Wistar rats (Charles River Laboratories, Wilmington, MA) at 5 wk of age were randomly assigned to a normal sodium (NS) diet (0.5% of Na+ by weight, Harlan Teklad, Madison, WI) or a low salt (LS) diet (0.15% of Na+ by weight, Harlan Teklad) group and treated for 3 wk. All rats drank water ad libitum throughout the experiment. Both NS- and LS-treated rats were used in dose-response studies, whereas only LS rats were used for studies determining key effects of 12(s)-HPETE and 12-HETE as specified below.
Surgical procedures
Rat anesthesia was achieved by giving 50 mg/kg pentobarbital sodium ip and maintained with 10 mg/kg · h of this drug given iv at 50 μl/min via polyethylene catheters (PE50) inserted into the left jugular veins. To continuously monitor mean artery pressure (MAP) during experiments, left carotid arteries were cannulated with catheters (PE50) and connected to a Statham 231 pressure transducer coupled to a Gould 2400s recorder (Gould Instrument Systems, Valley View, OH). Two catheters (PE50) were placed into both sides of the ureters with their tips in the renal pelvis via midline incision for urine collection. The catheters were inserted 1–2 mm after their tips reached the renal hilum. The drugs were perfused at 20 μl/min, a rate that did not change renal pelvis pressure (12), into the left renal pelvis via a MD-2000 microdialysis tube (inner diameter, 0.18/outer diameter, 0.22 mm; BASi, West Lafayette, IN) placed inside of the PE50 catheter with its tip 1–2 mm out of the PE50 catheter in the renal pelvis. Through a left flank incision, renal nerves were separated at the angle between the abdominal aorta and the renal artery and placed on the bipolar stainless steel electrode with a stereoscopic dissecting microscope. After the renal nerve activity was verified using its pulse synchronous rhythmicity with the heartbeat, the nerve fibers were transected, and the distal part was attached to the electrode with Kwik-Cast and Kwik-Sil (World Precision Instruments, Sarasota, FL). The nerve activity signals were filtered at 1000-Hz high-frequency cutoff and 100-Hz low-frequency cutoff, amplified × 20,000 by a Grass model P511 AC Amplifier and recorded with a Gould 2400s recorder (Gould Instrument Systems). The voltage integration was used to analyze renal nerve activity. The postdeceased renal activity was recorded as background and subtracted from all values, respectively. ARNA was shown in percent of its basal value. To acutely denervate the left kidney, nerve fibers were sectioned and the renal artery was painted with 10% phenol diluted in ethanol.
Perfusion protocols and contralateral urine flow rate (Uflow) determination
All experiments were started about 1.5 h after the surgery. The renal pelvis perfusion was performed in two 3-min periods for ARNA recording experiments, and a 3-min period followed by a 30-min period for plasma collection experiments (12). AMG9810 (10−6 m) (Sigma-Aldrich, St. Louis, MO), a selective TRPV1 antagonist, 10−4 m RP67580 (Tocris), a selective NK1 antagonist, or vehicle was perfused within the first 3-min period, followed by the 3-min perfusion with 4 × 10−6 m CAP (Sigma-Aldrich), a selective TRPV1 agonist, 1.5 × 10−8 m SP (Sigma-Aldrich), 1.5 × 10−7 mCGRP (Sigma-Aldrich), 10−15 m 12(s)-HPETE (BioMol international), or 10−10 m 12-HETE (Sigma-Aldrich). Vehicle was perfused in the first period in cases in which one of the drugs was perfused alone during the second period. The doses and protocols used were based on previous studies showing effective stimulation or blockade of ARNA and/or Uflow (12, 18, 33, 35). ARNA recorded before the first perfusion period was shown as the basal value, during the second 3-min perfusion period as the treatment value, and 10 min after the perfusion as the recovery value. Urine from contralateral kidneys was collected for 30 min before the first perfusion period and 30 min starting at the beginning of the second perfusion period in LS rats. Uflow was expressed as per gram of kidney weight per min (μl/g · min).
Angiotensin I (Ang I) levels in plasma from ipsilateral or contralateral kidney veins
Blood samples collected from left and right renal veins in tubes containing 10 mg/ml EDTA and 500 KIU/ml aprotinin were centrifuged at 5000 × g for 20 min at 4 C. Plasma was collected and stored at −80 C without incubating at 37 C in the presence of peptidase inhibitors, and thus the results reflect postsampling in vitro condition without interfering angiotensin generation and destruction. Before performing RIA with the Ang I RIA kit (rat RIA kit; Peninsula Laboratories, Inc., San Carlos, CA) to assess the renin activity, plasma was purified with C-18 columns, eluted with 60% acetonitrile in 0.1% trifluoroacetic acid at 4 C, and dried. The dry samples were dissolved in the RIA buffer and incubated with primary antibody, 125I-labeled peptide, and secondary antibody at 4 C.
SP and CGRP release in urine from the ipsilateral kidney
Urine from the left kidney was collected from a subset of LS-treated animals that were treated with various drugs using the same protocols as above without being subjected to ARNA recording. Urine samples were purified and SP and CGRP levels were detected with the use of the RIA kits (Peninsula Laboratories, Inc.) as described previously (12, 18). Concentrations of SP and CGRP were normalized by the kidney weight.
Immunofluorescence staining
For fixation, anesthetized rats were tanscardially perfused with 4% paraformaldehyde. Kidneys were removed, embedded in tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC), and sectioned into 10 μm. After being blocked with 5% BSA+0.3% Triton X-100 for 30 min, sections were incubated with guinea pig-anti-TRPV1 (1:500, Millipore Corp., Bedford, MA), rabbit-anti-NK1 (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit-antineuropeptide Y (NPY) (1:100, Santa Cruz Biotechnology) and/or goat-antirenin (1:400, Santa Cruz Biotechnology) antiserum with 2% BSA at 4 C overnight. After washing, sections were incubated with donkey-antiguinea pig Rhodamine Red X (RRX)-labeled IgG (1:100), donkey-antirabbit RRX-labeled IgG (1:50), or donkey-antigoat fluorescein isothiocyanate-labeled IgG (1:50, Jackson ImmunoResearch) for 2 h. Sections were washed, mounted with antifade medium (Vector Laboratories, Inc., Burlingame, CA), and viewed under the microscope.
Statistics analysis
All data were shown as means ± se. One-way ANOVA followed with Tukey-Kramer multiple comparison tests were used to analyze the differences between groups. Differences were considered statistically significant at P < 0.05.
Results
MAP was monitored throughout the following experiments via left carotid arteries, which were not altered by renal pelvis perfusion of 12(s)-HPETE or 12-HETE in the presence or absence of AMG9810 or RP67580 at any given doses in NS- or LS-treated rats (data not shown). These results were consistent with our previous data, which showed that MAP was not altered by the increase in ARNA when TRPV1 is activated by renal pelvis perfusion of its agonists (34, 35).
Effects of 12(s)-HPETE and 12-HETE on ARNA in NS-treated rats
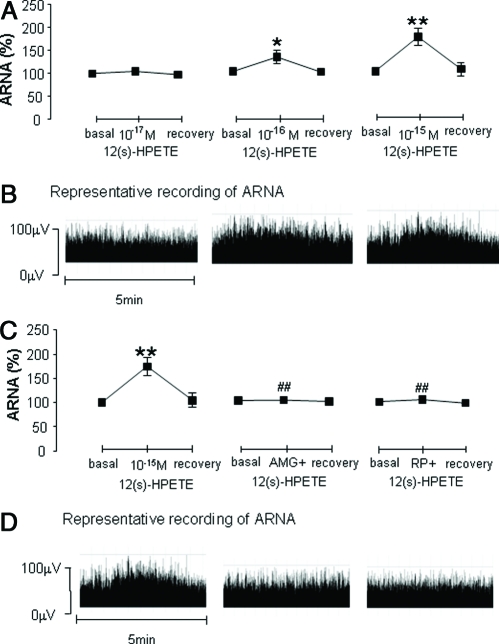
12(s)-HPETE (10−17 m, 10−16 m, or 10−15 m) perfused into the left renal pelvis in NS-treated rats increased ipsilateral ARNA in a dose-dependent manner (Fig. 2, A and B), and 10−15 m12(s)-HPETE was chosen for the rest of the experiments because of robust ARNA responses. 10−6 mAMG9810, a selective TRPV1 antagonist, or 10−4 mRP67580, a selective NK1 antagonist, abolished 10−15 m12(s)-HPETE-triggered increases in ARNA (Fig. 2, C and D).
Fig. 2.
Ipsilateral ARNA in response to 12(s)-HPETE with or without 10−6 mAMG9810 or 10−4 m RP67580 perfused into the left renal pelvis of rats fed an NS diet (n = 5–7 in each group). A, ARNA induced by 10−17, 10−16, and 10−15 m12(s)-HPETE given into the renal pelvis. C, ARNA induced by 10−15 m12(s)-HPETE alone or with AMG9810 or RP67580. B and D, Representative recording of ARNA in each group. *, P < 0.05; and **, P < 0.01 vs. basal value of each group. ##, P < 0.01 vs. 12(s)-HPETE alone group.
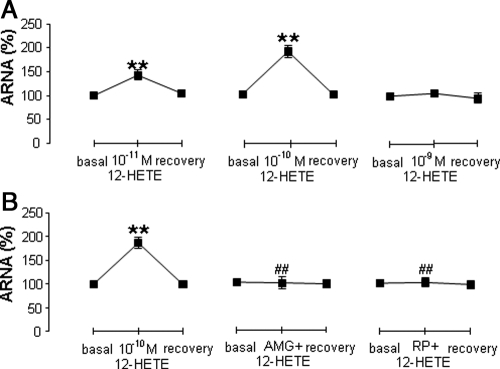
10−11mand 10−10 mbut not 10−9 m12-HETE activated ipsilateral ARNA (Fig. 3A), and 10−10 m12-HETE was used for the rest of the experiments given its effectiveness in increasing ARNA. 10−10 m12-HETE-induced increases in ARNA were blocked by 10−6 mAMG9810 or 10−4 m RP67580 (Fig. 3B).
Fig. 3.
Ipsilateral ARNA in response to 12-HETE with or without 10−6 m AMG9810 or 10−4 m RP67580 perfused into the left renal pelvis of rats fed an NS diet (n = 5–7 in each group). A, ARNA activated by 10−11, 10−10, and 10−9 m 12-HETE given into the renal pelvis. B, ARNA induced by 10−10 m 12-HETE alone or with AMG9810 or RP67580. **, P < 0.01 vs. basal value; and ##, P < 0.01 vs. 12(s)-12-HETE alone group.
Effects of 12(s)-HPETE and 12-HETE on ARNA in LS-treated rats
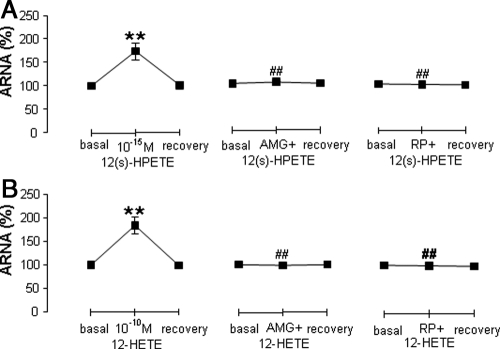
Ipsilateral ARNA was increased by 10−15 m12(s)-HPETE perfused into the left renal pelvis in LS-treated rats (Fig. 4A), and AMG9810 or RP67580 abolished 12(s)-HPETE-induced increases in ARNA (Fig. 4A). Similarly, 10−10 m12-HETE increased ipsilateral ARNA (Fig. 4B), which was blocked by AMG 9810 or RP67580 in LS-treated rats (Fig. 4B).
Fig. 4.
Ipsilateral ARNA in response to 10−15 m 12(s)-HPETE or 10−10 m 12-HETE with or without 10−6 m AMG9810 or 10−4 m RP67580 perfused into the left renal pelvis of rats fed a LS diet (n = 6 in each group). A, ARNA induced by 12(s)-HPETE alone or with AMG 9810 or RP67580. B, ARNA induced by 12-HETE alone or with AMG9810 or RP67580. **, P < 0.01 vs. basal value; and ##, P < 0.01 vs. 12(s)-HPETE or 12-HETE alone group.
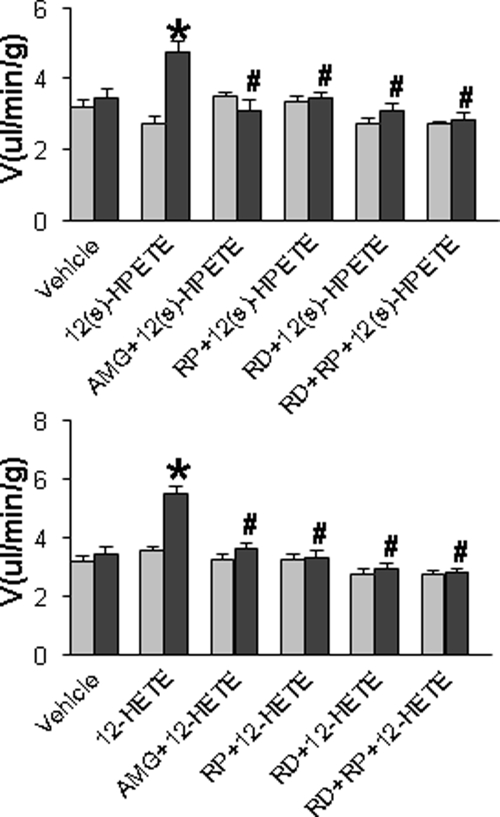
Effects of 12(s)-HPETE and 12-HETE on contralateral Uflow in LS-treated rats
Contralateral Uflow was examined to assess contralateral diuresis induced by the reno-renal reflex after activation of ipsilateral ARNA. Both 12(s)-HPETE and 12-HETE perfused into the left renal pelvis increased contralateral urinary excretion, which was abolished by AMG9810, RP67580, ipsilateral renal denervation, or RP67580 perfused into the renal denervated rats (Fig. 5).
Fig. 5.
Contralateral Uflow in response to 10−15 m 12(s)-HPETE or 10−10 m 12-HETE with or without renal denervation (RD), 10−6 m AMG 9810 or 10−4 m RP67580 perfused into the left renal pelvis of rats fed a LS diet (n = 5–7 in each group). *, P < 0.05 vs. before treatment value; #, P < 0.05 vs. 12(s)-HPETE or 12-HETE alone group.
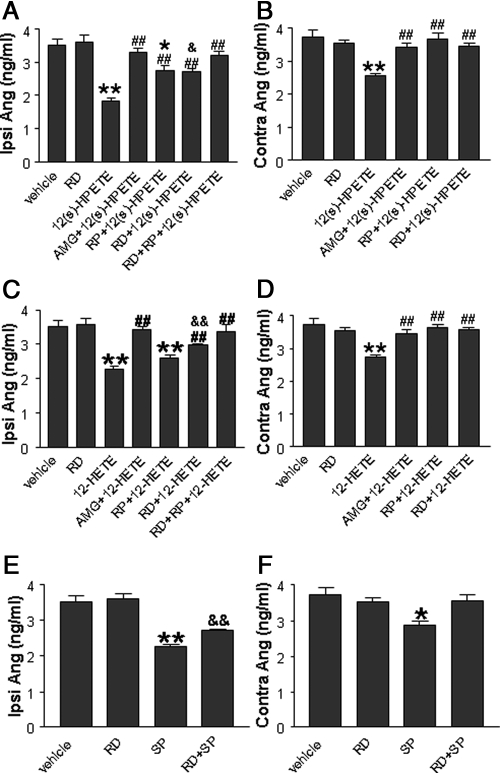
Effects of 12(s)-HPETE and 12-HETE on ipsilateral or contralateral Ang I levels in LS-treated rats
Ang I levels in plasma from the ipsilateral or contralateral renal vein were examined in LS-treated rats. Neither ipsilateral nor contralateral Ang I levels were affected by ipsilateral renal denervation compared with the vehicle group (Fig. 6). Ipsilateral Ang I levels were reduced by 12(s)-HPETE, which was abolished by AMG9810 or RP67580 plus renal denervation but only attenuated by RP67580 or renal denervation alone (Fig. 6A). Contralateral Ang I levels were also reduced by 12(s)-HPETE (Fig. 6B), although the magnitude of the reduction was smaller compared with that of the ipsilateral kidney (P < 0.05, Fig. 6, A and B). 12(s)-HPETE-induced decreases in contralateral Ang I levels were abolished by AMG9810, RP67580, or renal denervation (Fig. 6B).
Fig. 6.
Plasma levels of Ang I from the ipsilateral or contralateral renal vein in response to 10−15 m 12(s)-HPETE or 10−10 m 12-HETE with or without renal denervation (RD), 10−6 m AMG 9810, or 10−4 m RP67580 perfused into the left renal pelvis of rats fed a LS diet (n = 5–7 in each group). A and C, Ipsilateral plasma Ang I level induced by 12(s)-HPETE or 12-HETE alone or with AMG 9810 or RP67580 in intact or denervated rats. B and D, contralateral plasma Ang I level induced by 12(s)-HPETE or 12-HETE alone or with AMG9810 or RP67580 in intact or denervated rats. E, Ipsilateral plasma Ang I level induced by SP in intact or denervated rats. F, Contralateral plasma Ang I level induced by SP in intact or denervated rats. *, P < 0.05; or **, P < 0.01 vs. vehicle-treated group; #, P < 0.05; or ##, P < 0.01 vs. 12(s)-HPETE- or 12-HETE-treated groups; and &, P < 0.05; or &&, P < 0.01 vs. renal denervation (RD) group.
Similarly, 12-HETE perfused into the left renal pelvis reduced ipsilateral Ang I levels (Fig. 6C), which was abolished by AMG9810 or RP67580 plus renal denervation and attenuated by RP67580 or renal denervation alone (Fig. 6C). 12-HETE decreased contralateral Ang I levels, and the magnitude of the reduction was smaller compared with that of the ipsilateral kidney (P < 0.05; Fig. 6, C and D). AMG9810, RP67580, or renal denervation abolished 12-HETE-induced decreases in contralateral Ang I levels (Fig. 6D).
The effects of SP on Ang I levels were also examined. Ipsilateral Ang I levels were reduced by SP with or without renal denervation in LS-treated rats, and the reduction was greater with intact renal innervation (Fig. 6E). SP also reduced contralateral Ang I levels albeit the magnitude of the reduction was smaller compared with that of the ipsilateral kidney (P < 0.05; Fig. 6, E and F), and SP-induced decreases in contralateral Ang I levels were abolished by renal denervation (Fig. 6F).
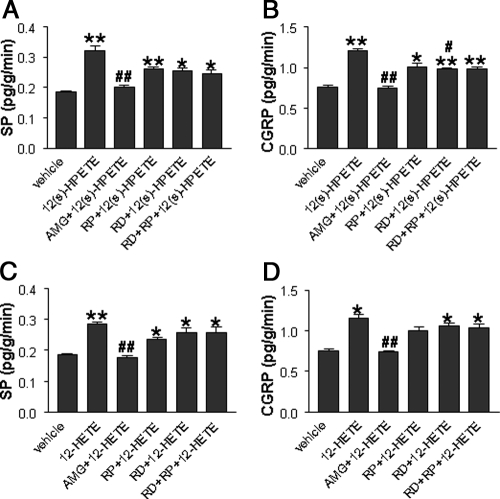
Effects of 12(s)-HPETE and 12-HETE on ipsilateral SP and CGRP Release in LS-treated rats
RIA was performed to assess the levels of SP and CGRP released into urine obtained from the ipsilateral kidney. Both SP and CGRP release was enhanced by 12(s)-HPETE compared with the vehicle, and 12(s)-HPETE-induced increases in SP and CGRP were abolished by AMG9810 (Fig. 7, A and B) but not by RP67580, renal denervation, or PR67580 plus renal denervation (Fig. 7, A and B). Similarly, 12-HETE perfused into the renal pelvis increased SP and CGRP release into urine, which was abolished by AMG 9810 but not by RP67580, renal denervation, or RP67580 plus renal denervation (Fig. 7, C and D).
Fig. 7.
Level of SP and CGRP release into urine from the ipsilateral kidney in response to 10−15 m 12(s)-HPETE- or 10−10 m 12-HETE with or without renal denervation (RD), 10−6m AMG9810, or 10−4 m RP67580 perfused into the left renal pelvis of rats fed a LS diet (n = 5–7 in each group). *, P < 0.05; or **, P < 0.01 vs. vehicle-treated group; #, P < 0.05; or ##, P < 0.01 vs. 12(s)-HPETE- or 12-HETE-treated groups.
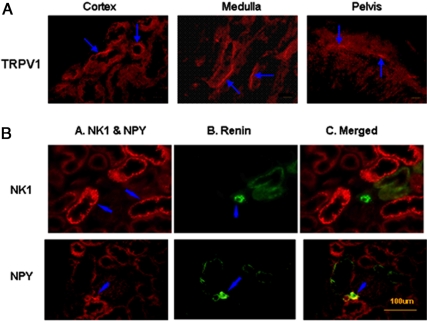
Localization of TRPV1, NK1, NPY, and renin in the kidney
Figure 8A shows TRPV1-positive nerve fibers were located in the renal cortex, medulla, and pelvis, providing anatomical evidence supporting the results obtained from functional studies reported above. Figure 8B shows that sympathetic neural peptide NPY, but not the NK1 receptor, was found around the arteries and juxtaglomerular apparatus area colocalized with renin.
Fig. 8.
Immunofluorescence staining of TRPV1 (panel A) in the renal cortex, medulla, and renal pelvis, as well as NK1, NPY, and renin (panel B) in the renal cortex (scale 100 μm). The negative controls in which anti-TRPV1, anti-NK1, anti-NPY, or antirenin removed were devoid of staining (data not shown). TRPV1, NK1, and NPY were labeled with red fluorescence (arrows), and renin was labeled with green fluorescence (arrows). The double staining of NK1 or NPY with renin was shown in yellow (arrows).
Discussion
Concentrations of 12-HETE, the stable metabolite product of 12(s)-HPETE, are much higher in the kidney than in other organs (25). Urinary 12-HETE levels have been shown to be increased in essential hypertensive individuals and in diabetic rats (30, 31). Renal pelvis perfusion used in the present study renders renal sensory nerves exposed to perfused compounds similarly, in terms of molecules and concentrations, to that found in urine, permitting us to study the importance of perfused compounds, i.e. 12-HETE and 12(s)-HPETE, in the kidney.
In extrarenal tissues, 12(s)-HPETE contributes to several pathophysiological processes via activation of TRPV1 (23, 24, 32). 12(s)-HPETE or CAP elicits long-term synaptic depression that is abolished by ablation of TRPV1 expressed in hippocampal interneurons in the brain (32). 12(s)-HPETE-induced activation of TRPV1 expressed in sensory C-fibers protects against myocardial ischemia/reperfusion injury via triggering SP and CGRP release that subsequently causes coronary vasodilation (24). Increased 12(s)-HPETE levels by bradykinin-induced activation of the 12-LOX pathway in dorsal root ganglia cause inflammatory pain via stimulating TRPV1 (22). Our data show that 12(s)-HPETE or 12-HETE perfused into the renal pelvis increases ARNA, which is abolished by blockade of TRPV1 or NK1 receptors. These data indicate that 12(s)-HPETE and 12-HETE activate TRPV1 in the renal pelvis, resulting in SP release that subsequently activates NK1 receptors to increase ARNA. This notion is supported by previous evidence showing that CAP-induced increases in ARNA are mediated by NK1 activation resulting from SP release (12).
It has been shown that 12-HETE perfused into the isolated kidney from the renal artery increases water and electrolyte excretion via the increases in glomerular filtration rate (GFR) (29). Similarly, CAP perfused into the isolated kidney in vitro or into the renal pelvis in vivo increases GFR, urinary volume, and sodium excretion and the release of SP and CGRP (34, 35). Moreover, CAP-induced increases in GFR and renal excretory function are abolished by blockade of TRPV1 or simultaneous blockade of NK1 and CGRP receptors (34, 35). The results in this study show that blockade of TRPV1 or NK1 receptors abolishes 12(s)-HPETE- or 12-HETE-induced increases in urine excretion, indicating that 12(s)-HPETE and 12-HETE perfused into the renal pelvis activate the TRPV1-NK1 pathway to increase renal excretory function possibly via enhancing the GFR. Although urinary sodium excretion was not measured in the present study, our previous results showed that the increase in urinary sodium excretion always paralleled the increase in urinary water excretion when TRPV1 is activated (34, 35). Thus, it is likely that 12(s)-HPETE and 12-HETE perfused into the renal pelvis may also increase urinary sodium excretion via activation of the TRPV1.
12(s)-HPETE and 12-HETE produced by the 12-LOX pathway in renal vascular tissues are potent inhibitors of renin release (36), which impose negative feedback regulation on renin secretion from juxtaglomerular cells (37). Likewise, 12-HETE perfused into the isolated kidneys via the renal artery reduces renin release in rats fed a NS diet (29). 12-HETE has an exaggerated inhibitory effect on renin activity in diabetic rats, resulting in reduced renin activity in these animals (38). High-salt intake enhances the inhibitory tone of renin, possibly by increasing renal cortex 12(s)-HPETE and 12-HETE production, and LOX inhibitors given acutely or chronically to these rats decrease 12-HETE levels and increase plasma renin activity independently of changes in blood pressure (26). Furthermore, high-salt-induced suppression of plasma renin activity is impaired whereas CAP-sensitive sensory nerves are degenerated (19), indicating that TRPV1-positive sensory nerves may play an inhibitory role in regulating renin activity. To provide direct evidence that TRPV1 is involved in 12(s)-HPETE- and 12-HETE-mediated inhibition of renin release induced by LS treatment, rats fed a LS diet were used in the present study. The data show that 12(s)-HPETE or 12-HETE perfused into the renal pelvis suppresses LS-induced renin release without changing MAP, and blockade of TRPV1 abolishes the effect of 12(s)-HPETE and 12-HETE. These data indicate that TRPV1-positive sensory nerves mediate 12(s)-HPETE- and 12-HETE-induced inhibition of renin release.
Activation of TRPV1 in sensory nerves leads to SP release that activates NK1 receptors to increase ARNA, resulting in subsequent suppression of sympathetic nerve activity (12, 39). It is known that SP inhibits whereas activation of β-1-adrenoceptors by norepinephrine released from sympathetic fibers innervating the juxtaglomerular apparatus stimulates renin release in the kidney (15, 17, 40, 41). The data in this study show that 12(s)-HPETE or 12-HETE perfused into the renal pelvis increases SP and CGRP release, which is abolished by blockade of TRPV1. Additionally, blockade of the NK1 receptors impairs the ability of 12(s)-HPETE or 12-HETE to suppress LS-induced renin release, indicating that activation of the NK1 receptors by SP mediates, at least in part, the inhibitory role of 12(s)-HPETE or 12-HETE in renin release. Furthermore, exogenous SP perfused into the renal pelvis suppresses LS-induced renin release, and this effect of SP in the contralateral kidney is abolished by renal denervation. These results indicate that SP-induced decreases in contralateral renin release are mediated by the suppression of sympathetic nerve activity resulting from SP activation of NK1 receptors in sensory nerves via reno renal reflex.
TRPV1-positive sensory nerves have been found heavily innervating the renal pelvis (11, 12, 42), a finding consistent with the results obtained in the present study. These data provide anatomical evidence supporting the notion that activation of TRPV1 by 12(s)-HPETE and 12-HETE in the urine or renal tissue interstitial fluid may affect renal function by increased ARNA. Furthermore, two structurally distinct subtypes of sympathetic axon are located in the juxtaglomerular region of afferent arterioles to control renin release (43, 44). Our immunofluorescence results show that NPY-positive sympathetic nerves, but not NK1 receptors, are found in the juxtaglomerular region where renin is produced, suggesting that 12(s)-HPETE and 12-HETE may inhibit renin release via reflex suppression of sympathetic nerve activity after enhancement of ARNA by 12(s)-HPETE and 12-HETE.
Perspectives
12(s)-HPETE and 12-HETE, potent endogenous TRPV1 agonists, are highly concentrated in the kidney and further elevated in disease states (20, 21, 25, 45). Although 12(s)-HPETE and 12(s)-HETE have been implicated as key players in the pathological process in diseases including diabetes and hypertension (28, 30, 31), the molecular mechanism(s) mediating the effect of 12(s)-HPETE and 12(s)-HETE remains to be defined. The data in the present study provide direct evidence that TRPV1 may serve as the receptor for 12(s)-HPETE and 12(s)-HETE in the kidney to regulate renin activity and renal function (Fig. 1). Once better models and tools are developed, future studies focused on elucidating the physiological relevance of endogenous 12(s)-HPETE and 12(s)-HETE in the regulation of renin activity and renal function by the use of inhibitors of the arachidonic pathway, including cyclooxygenase and LOX inhibitors, may expand our understanding of the role of the HPETE-TRPV1 pathway in renal physiology. It follows that targeting TRPV1 or its downstream NK1 receptors in the kidney may modulate 12(s)-HPETE and 12(s)-HETE effects and thus prevent or treat disorders associated with disregulation of 12(s)-HPETE and 12(s)-HETE such as diabetes and hypertension.
Acknowledgments
We thank Shuang Q. Yu (Department of Medicine, Michigan State University, East Lansing, Michigan) for his excellent technical assistance in immunohistological staining of TRPV1 in the kidney.
This work was supported by National Institutes of Health Grants HL-57853, HL-73287, and DK67620.
Disclosure Summary: None of the authors have anything to disclose.
Footnotes
- Ang I
- Angiotensin I
- ARNA
- afferent renal nerve activity
- CAP
- capsaicin
- CGRP
- calcitonin gene-related peptide
- ERNA
- efferent renal nerve activity
- GFR
- glomerular filtration rate
- 12-HETE
- 12-hydroxyeicosatetraenoic acid
- 12-(s)-HPETE
- 12-hydroperoxyeicosatetraenoic acid
- 12-LOX
- 12-lipoxygenase
- LS
- low salt
- MAP
- mean artery pressure
- NK1
- neurokinin 1
- NPY
- neuropeptide Y
- NS
- normal sodium
- PE
- polyethylene
- RD
- renal denervation
- RRX
- Rhodamine Red X
- SP
- substance P
- TRPV1
- transient receptor potential vanilloid type 1
- Uflow
- urine flow rate.
References
- 1. Julius D, Basbaum AI. 2001. Molecular mechanisms of nociception. Nature 413:203–210 [DOI] [PubMed] [Google Scholar]
- 2. Pedersen SF, Owsianik G, Nilius B. 2005. TRP channels: an overview. Cell Calcium 38:233–252 [DOI] [PubMed] [Google Scholar]
- 3. Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. 2000. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA 97:3655–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo A, Vulchanova L, Wang J, Li X, Elde R. 1999. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11:946–958 [DOI] [PubMed] [Google Scholar]
- 5. De Schepper HU, De Winter BY, Van Nassauw L, Timmermans JP, Herman AG, Pelckmans PA, De Man JG. 2008. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J Physiol 586:5247–5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahluwalia A, Vallance P. 1997. Evidence for functional responses to sensory nerve stimulation of rat small mesenteric veins. J Pharmacol Exp Ther 281:9–14 [PubMed] [Google Scholar]
- 7. Akerman S, Kaube H, Goadsby PJ. 2003. Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol 140:718–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhong B, Wang DH. 2008. N-Oleoyldopamine, a novel endogenous capsaicin-like lipid, protects the heart against ischemia-reperfusion injury via activation of TRPV1. Am J Physiol 295:H728–H735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kollarik M, Undem BJ. 2002. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rolle U, Brylla E, Tillig B. 1999. Immunohistochemical detection of neuronal plexuses and nerve cells within the upper urinary tract of pigs. BJU Int 83:1045–1049 [DOI] [PubMed] [Google Scholar]
- 11. Feng NH, Lee HH, Shiang JC, Ma MC. 2008. Transient receptor potential vanilloid type 1 channels act as mechanoreceptors and cause substance P release and sensory activation in rat kidneys. Am J Physiol 294:F316–F325 [DOI] [PubMed] [Google Scholar]
- 12. Xie C, Sachs JR, Wang DH. 2008. Interdependent regulation of afferent renal nerve activity and renal function: role of transient receptor potential vanilloid type 1, neurokinin 1, and calcitonin gene-related peptide receptors. J Pharmacol Exp Ther 325:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kopp UC, Smith LA, DiBona GF. 1985. Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am J Physiol 249:F507–F517 [DOI] [PubMed] [Google Scholar]
- 14. Hua XY, Theodorsson-Norheim E, Lundberg JM, Kinn AC, Hokfelt T, Cuello AC. 1987. Co-localization of tachykinins and calcitonin gene-related peptide in capsaicin-sensitive afferents in relation to motility effects on the human ureter in vitro. Neuroscience 23:693–703 [PubMed] [Google Scholar]
- 15. Ganong WF, Porter JP, Bahnson TD, Said SI. 1984. Peptides and neurotransmitters that affect renin secretion. J Hypertens 2:75–82 [PubMed] [Google Scholar]
- 16. Kurtz A, Muff R, Born W, Lundberg JM, Millberg BI, Gnädinger MP, Uehlinger DE, Weidmann P, Hökfelt T, Fischer JA. 1988. Calcitonin gene-related peptide is a stimulator of renin secretion. J Clin Invest 82:538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aoi W, Henry DP, Weinberger MH. 1976. Evidence for a physiological role of renal sympathetic nerves in adrenergic stimulation of renin release in the rat. Circ Res 38:123–125 [PubMed] [Google Scholar]
- 18. Zhu Y, Xie C, Wang DH. 2007. TRPV1-mediated diuresis and natriuresis induced by hypertonic saline perfusion of the renal pelvis. Am J Nephrol 27:530–537 [DOI] [PubMed] [Google Scholar]
- 19. Wang DH, Zhao Y. 2003. Increased salt sensitivity induced by impairment of sensory nerves: is nephropathy the cause? J Hypertens 21:403–409 [DOI] [PubMed] [Google Scholar]
- 20. Suh YG, Oh U. 2005. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des 11:2687–2698 [DOI] [PubMed] [Google Scholar]
- 21. Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. 2000. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97:6155–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. 2002. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99:10150–10155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim SR, Bok E, Chung YC, Chung ES, Jin BK. 2008. Interactions between CB(1) receptors and TRPV1 channels mediated by 12-HPETE are cytotoxic to mesencephalic dopaminergic neurons. Br J Pharmacol 155:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sexton A, McDonald M, Cayla C, Thiemermann C, Ahluwalia A. 2007. 12-Lipoxygenase-derived eicosanoids protect against myocardial ischemia/reperfusion injury via activation of neuronal TRPV1. FASEB J 21:2695–2703 [DOI] [PubMed] [Google Scholar]
- 25. Clouet P, Niot I, Bouchard P, Gree R, Lellouche JP, Beaucourt JP, Fonlupt P, Duperray B, Bezard J, Lagarde M. 1991. Distribution of tritium labeled 12(S) hydroxy-eicosatetraenoic acid (12-HETE) in the rat. Prostaglandins 42:39–45 [DOI] [PubMed] [Google Scholar]
- 26. Stern N, Nozawa K, Kisch E, Tuck ML, Golub M, Eggena P, Knoll E. 1996. Tonic inhibition of renin secretion by the 12 lipoxygenase pathway: augmentation by high salt intake. Endocrinology 137:1878–1884 [DOI] [PubMed] [Google Scholar]
- 27. Ma J, Natarajan R, LaPage J, Lanting L, Kim N, Becerra D, Clemmons B, Nast CC, Surya Prakash GK, Mandal M, Adler SG. 2005. 12/15-lipoxygenase inhibitors in diabetic nephropathy in the rat. Prostaglandins, leukotrienes, and essential fatty acids 72:13–20 [DOI] [PubMed] [Google Scholar]
- 28. Antonipillai I, Nadler J, Horton R. 1988. Angiotensin feedback inhibition on renin is expressed via the lipoxygenase pathway. Endocrinology 122:1277–1281 [DOI] [PubMed] [Google Scholar]
- 29. Quilley CP, McGiff JC. 1990. Isomers of 12-hydroxy-5,8,10,14-eicosatetraenoic acid reduce renin activity and increase water and electrolyte excretion. J Pharmacol Exp Ther 254:774–780 [PubMed] [Google Scholar]
- 30. Quintana LF, Guzmán B, Collado S, Clària J, Poch E. 2006. A coding polymorphism in the 12-lipoxygenase gene is associated to essential hypertension and urinary 12(S)-HETE. Kidney Int 69:526–530 [DOI] [PubMed] [Google Scholar]
- 31. Hatley ME, Srinivasan S, Reilly KB, Bolick DT, Hedrick CC. 2003. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem 278:25369–25375 [DOI] [PubMed] [Google Scholar]
- 32. Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. 2008. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57:746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu Y, Wang Y, Wang DH. 2005. Diuresis and natriuresis caused by activation of VR1-positive sensory nerves in renal pelvis of rats. Hypertension 46:992–997 [DOI] [PubMed] [Google Scholar]
- 34. Li J, Wang DH. 2008. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol Res 57:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu Y, Wang DH. 2008. Segmental regulation of sodium and water excretion by TRPV1 activation in the kidney. J Cardiovasc Pharmacol 51:437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antonipillai I, Nadler JL, Robin EC, Horton R. 1987. The inhibitory role of 12- and 15-lipoxygenase products on renin release. Hypertension 10:61–66 [DOI] [PubMed] [Google Scholar]
- 37. Antonipillai I. 1990. 12-lipoxygenase products are potent inhibitors of prostacyclin-induced renin release. Proc Soc Exp Biol Med 194:224–230 [DOI] [PubMed] [Google Scholar]
- 38. Antonipillai I, Jost-Vu E, Natarajan R, Nadler J, Horton R. 1995. Renin response to 12-hydroxyeicosatetraenoic acid is increased in diabetic rats. Diabetes 44:321–325 [DOI] [PubMed] [Google Scholar]
- 39. Ma MC, Huang HS, Chen CF. 2002. Impaired renal sensory responses after unilateral ureteral obstruction in the rat. J Am Soc Nephrol 13:1008–1016 [DOI] [PubMed] [Google Scholar]
- 40. Gullner HG, Bartter FC. 1979. Participation of substance P in the control of renin release. Life Sci 24:2449–2454 [DOI] [PubMed] [Google Scholar]
- 41. Kopp U, Aurell M, Nilsson IM, Ablad B. 1980. The role of beta-1-adrenoceptors in the renin release response to graded renal sympathetic nerve stimulation. Pflugers Arch 387:107–113 [DOI] [PubMed] [Google Scholar]
- 42. Chen Y, Hoover DB. 1995. Autoradiographic localization of NK1 and NK3 tachykinin receptors in rat kidney. Peptides 16:673–681 [DOI] [PubMed] [Google Scholar]
- 43. Luff SE, Hengstberger SG, McLachlan EM, Anderson WP. 1992. Distribution of sympathetic neuroeffector junctions in the juxtaglomerular region of the rabbit kidney. J Auton Nerv Syst 40:239–253 [DOI] [PubMed] [Google Scholar]
- 44. Matsumura Y, Kawazoe S, Ichihara T, Shinyama H, Kageyama M, Morimoto S. 1988. Stimulatory effects of neuronally released norepinephrine on renin release in vitro. Am J Physiol 255:F614–F620 [DOI] [PubMed] [Google Scholar]
- 45. Sasaki M, Hori MT, Hino T, Golub MS, Tuck ML. 1997. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens 10:371–378 [PubMed] [Google Scholar]